Selective geranylation of biflavonoids by Aspergillus terreus aromatic prenyltransferase (AtaPT)†
Kangping
Xu
a,
Can
Yang
a,
Yuanyuan
Xu
a,
Dan
Li
a,
Shumin
Bao
a,
Zhenxing
Zou
a,
Fenghua
Kang
a,
Guishan
Tan
ab,
Shu-Ming
Li
 c and
Xia
Yu
c and
Xia
Yu
 *a
*a
aSchool of Pharmaceutical Sciences, Central South University, Changsha, Hunan 410013, People's Republic of China. E-mail: xyu226@csu.edu.cn
bXiangya Hospital of Central South University, Changsha 410008, People's Republic of China
cInstitut für Pharmazeutische Biologie und Biotechnologie, Philipps-Universität Marburg, Robert-Koch Straße 4, 35037 Marburg, Germany
First published on 20th November 2019
Abstract
Prenylation increases the bioactivity of flavonoids. Herein, we report the first examples of regioselective enzymatic geranylation of biflavonoids using Aspergillus terreus aromatic prenyltransferase (AtaPT). For biflavonoids 1–3 dimerized through a diphenyl linkage, geranylation occurs at the hydrogen bond involving C5′′–OH group, which is less chemically accessible than other OH groups in the molecule. This study would be referential for developing green synthetic solutions for prenylated biflavonoids.
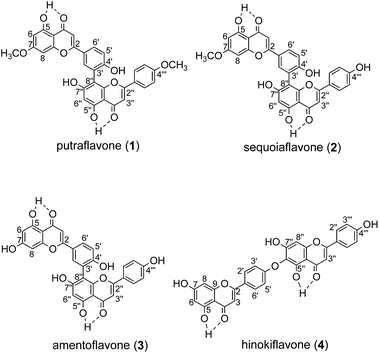
Biflavonoids are formed by dimerization of flavonoid moieties and they are rich in structural diversity due to the different flavonoid options and attachment modes. Many biflavonoids possess pharmacological properties1,2 such as anti-inflammatory,3 anti-oxidative,4 anti-viral, amyloid β aggregation inhibitory,5 peripheral blood vessel dilatational and anti-tumor activities.6 Prenylation is an important diversification that is widely found in various natural products, such as flavonoids, indole alkaloids, coumarins, etc.7 Introduction of prenyl side chains generally increases the lipophilicity of the backbone compounds, which can penetrate the cell membrane more easily and achieve increased interaction with the target protein. Thereby, prenylation often leads to enhanced bioactivities.8–10 However, isoprenyl-biflavonoids are very rare in nature, which is in sharp contrast to the structural diversity of natural biflavonoids.11 Regular chemical synthesis of prenylated biflavonoids is difficult due to steric hindrance, poor chemo-selectivity, etc. In particular, prenylation of C5–OH and C5′′–OH is absent in nature, possibly due to their intramolecular hydrogen bond with the neighboring keto groups (Fig. 2).
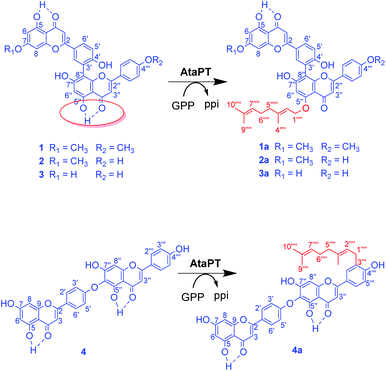
In nature, prenylation processes are usually catalyzed by highly specific prenyltransferases, using prenyl donors such as dimethylallyl pyrophosphate (DMAPP) or geranyl pyrophosphate (GPP).12 In the last decade, progress has been made in enzymatic transfer of prenyl moieties onto a variety of flavonoids. Several prenyltransferases have been reported to catalyze geranylation of flavonoids. Importantly, they are water soluble and can be easily expressed as recombinant proteins in well-developed bacterial hosts such as Escherichia coli. Successful examples for geranylation include NphB from Streptomyces,13 CsPT3 from Cannabis sativa14 and AtaPT (Aspergillus terreus aromatic prenyltransferase).15 They catalyze the C6, C8, C3′ or 7-OH geranylation of several flavonoids (Fig. 1). Among them, AtaPT was reported to catalyze geranylation of flavonoids with the highest promiscuity.15,16 For most prenylation reactions towards flavonoids by AtaPT, instead of a dominating product with prenylation at a desired position, mixtures of products modified at different positions were observed.15
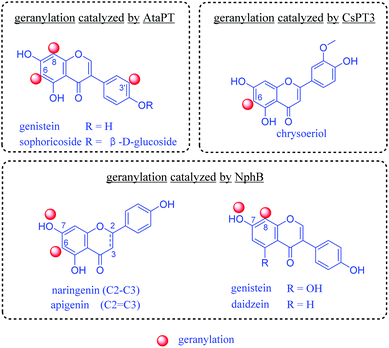 | ||
| Fig. 1 Previous studies on enzymatic geranylation towards flavonoids.13–15 | ||
Unlike the well-studied flavonoid monomer prenylation, enzymatic prenylation of biflavonoids is new to science. Developing enzymatic tools that can prenylate biflavonoids could be an important solution for the generation of isoprenyl derivatives of biflavonoids. In the present work, we report examples of selective geranylation of biflavonoids by AtaPT, including O-geranylation at the hydrogen bond involving C5′′–OH group in several biflavonoids dimerized through a diphenyl linkage, and C-geranylation at C3′′′ for a biflavonoid that is dimerized through an ether linkage.
The recombinant C-terminal His6-tagged AtaPT was expressed using plasmid pCDFDuet-ataPT in Escherichia coli BL21(DE3) cells and was purified to homogeneity (Fig. S1†). Several natural biflavonoids were incubated with AtaPT in the presence of GPP at 37 °C for 16 h. Negative control assays were performed with inactivated AtaPT by boiling for 20 min. HPLC analysis was used to monitor product formation. Among the tested substrates, several natural biflavonoids (Fig. 2), including putraflavone (1), sequoiaflavone (2) amentoflavone (3) and hinokiflavone (4), were found to give new products in the presence of GPP. In each of these assays, a new peak, corresponding to 1a–4a, was observed under the described conditions (Fig. 3). Biflavonoids 1–3, which are dimerized through a diphenyl linkage, are better accepted than 4 (dimerized through an ether linkage). The percent conversions were determined as 18.6%, 45.3%, 21.2% and 8.7% for 1, 2, 3 and 4, respectively.
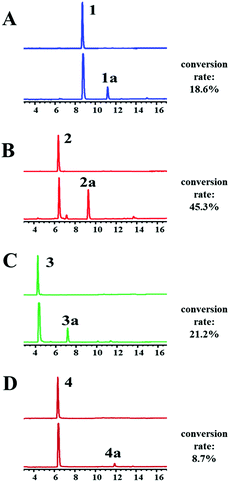 | ||
| Fig. 3 HPLC chromatograms of incubation mixtures of 1–4 with his6-tagged AtaPT. Detection was carried with a diode array detector for absorption at 254 nm. | ||
For structure elucidation, the four enzymatic products 1a–4a were isolated via semi-preparative HPLC and subjected to NMR and HR-ESI-MS analyses (Fig. S2–S27 and Tables S1–S5†). NMR analyses of 1a, 2a and 4a were performed in DMSO-d6, while those of 3a were performed in both acetone-d6 and DMSO-d6. HR-ESI-MS (Fig. S2–S5 and Table S1†) indicated the presence of one geranyl moiety each in the enzyme products by showing molecular masses that are 136 Daltons larger than those of the respective substrates.
In the 1H NMR spectra of the products 1a and 2a, the signals at δH 4.59–4.60 (2H, d, H-1′′′′), 5.46 (1H, t, H-2′′′′), 1.74 (3H, s, H-4′′′′), 2.06–2.11 (4H, m, H-5′′′′ and H-6′′′′), 5.11–5.12 (1H, t, H-7′′′′), 1.65 (3H, s, H-9′′′′), and 1.58–1.59 (3H, s, H-10′′′′) clearly revealed the presence of a geranyl moiety in their structures (Fig. S10 and S15†). The resonance of H-1′′′′ in the range of 4.59–4.60 ppm proved the regular attachment of the geranyl moiety to an aromatic oxygen atom in the products 1a and 2a.17 Substrates 1 and 2 are of the same backbone and they only differ in the methylation pattern at two phenol groups. Compounds 1a and 2a retained all of the 12 aromatic 1H signals in the corresponding substrates 1 and 2, proving unambiguously the regular attachment of the geranyl moiety to an oxygen atom.
Clear correlations were observed in the NOESY spectrum (Fig. S14†) between δH 4.59 (2H, d, H-1′′′′) and δH 6.25 (1H, s, H-6′′) proving the geranylation at C5′′–OH or C7′′–OH at the ortho-position of H-6′′, which was also revealed by the correlations in the HMBC spectra from H-1′′′′ and H-6′′ to the same O-substituted aromatic carbon at δC 158.5 ppm (Fig. S12†). Normally, the 13C NMR signal for the hydrogen bond involving C4![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C4′′
O and C4′′![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in biflavonoids appears at approximately δC 182 ppm, as shown for substrates 1–4.18–20 In comparison, the signal of C4′′
O in biflavonoids appears at approximately δC 182 ppm, as shown for substrates 1–4.18–20 In comparison, the signal of C4′′![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in product 1a was shifted upfield to δC 176.0 ppm, which was conducted by the removal of the de-shielding effect from the hydrogen bond between C5′′–OH and C4′′
O in product 1a was shifted upfield to δC 176.0 ppm, which was conducted by the removal of the de-shielding effect from the hydrogen bond between C5′′–OH and C4′′![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, confirming the geranylation at C5′′–OH in the product 1a (Scheme 1).
O, confirming the geranylation at C5′′–OH in the product 1a (Scheme 1).
In the NOESY spectrum of the product 2a (Fig. S19†), correlations between δH 4.60 (2H, d, H-1′′′′) and δH 6.31 (2H, s, H-6 and H-6′′) indicated the geranylation at C5–OH/C7–OH at the ortho-position of H-6 or C5′′–OH/C7′′–OH besides H-6′′. As observed for 1a, the signal of C4′′![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the product 2a was also detected at δC 176.0 ppm, confirming the geranylation at C5′′–OH in the product 2a (Scheme 1).
O in the product 2a was also detected at δC 176.0 ppm, confirming the geranylation at C5′′–OH in the product 2a (Scheme 1).
Similar to 1a and 2a, the 1H NMR spectra of the product 3a also clearly revealed the regular attachment of the geranyl moiety to an aromatic oxygen atom, with signals (obtained in acetone-d6 or DMSO-d6, Fig. S20 and S21†) at δH 4.57–4.70 (2H, d, H-1′′′′), 5.46–5.56 (1H, t, H-2′′′′), 1.73–1.77 (3H, s, H-4′′′′), 2.06–2.14 (4H, m, H-5′′′′ and H-6′′′′), 5.12–5.13 (1H, t, H-7′′′′), 1.65–1.66 (3H, s, H-9′′′′), and 1.59–1.60 (3H, s, H-10′′′′). The appearance of only one hydrogen bonding proton at 13.0 ppm (Fig. S20†) indicates the geranylation at the hydrogen bond involving C5–OH or C5′′–OH other than the normal phenol hydroxyl group. NOESY correlation (Fig. S22†) between δH 4.57 (2H, d, H-1′′′′) and 6.16 (1H, s, H-6′′) other than δH 6.05 (1H, s, H-6) proves unambiguously the geranylation at C5′′–OH.
The signals of a regular geranyl group also appeared in the 1H NMR spectrum of the product 4a (Fig. S23†). The chemical shifts of the terminal methylene protons in the geranyl group are at approximately 3.31 ppm, suggesting the attachment of the geranyl moiety to an aromatic carbon atom instead of an oxygen atom.17 In comparison with two AA′BB′ ring systems in substrate 4,18 signals for only one AA′BB′ ring system at δH 8.02 (2H, d, H-2′ and H-6′) and 7.03 (2H, d, H-3′ and H-5′), with an additional ABX ring system at δH 7.79 (1H, H-2′′′), 6.96 (1H, H-5′′′) and 7.81 (1H, H-6′′′), were observed for 4a, indicating the attachment of the geranyl moiety in the ABX ring system in 4a. Cross peaks of δH 3.31 (2H, H-1′′′′) to δC 128.7 (C-3′′′) and 159.2 (C-4′′′) confirmed the ortho-position of the C-geranyl moiety to C4′′′–OH. Therefore, geranylation has taken place at C-3′′′ in ring B′. As shown in Fig. S9,† all the aromatic rings in 4a could be connected by analyzing the NOESY spectrum, and the attachment of the geranyl moiety at C-3′′′ at ring B′ was unambiguously proved (Scheme 1).
Enzymatic kinetic parameters including the Michaelis constants (KM) and turnover numbers (Kcat) were determined for the AtaPT catalyzing reactions by the Hanes–Woolf analysis. Comparable KM values for 2 and 3 were determined at 0.13 and 0.14 mM, respectively. 4 revealed a higher KM value at 0.18 mM, while that of 1 was found to be 0.10 mM. The turnover number of 2 was 1.4–2.6-fold higher than those of 1, 3 and 4. Therefore, AtaPT showed much higher catalytic efficiency towards 2 than other substrates with the constant Kcat/KM at 277 M−1 s−1, while the corresponding values for 1, 3 and 4 were 150, 71 and 61 M−1 s−1, respectively (Table 1).
| Substrate | K m [mM] | K cat [s−1] | K cat/KM [M−1 s−1] |
|---|---|---|---|
| Putraflavone (1) | 0.10 | 0.015 | 150.0 |
| Sequoiaflavone (2) | 0.13 | 0.036 | 276.9 |
| Amentoflavone (3) | 0.14 | 0.010 | 71.4 |
| Hinokiflavone (4) | 0.18 | 0.011 | 61.1 |
In this work, we have studied AtaPT for its substrate promiscuity towards several biflavonoids (1–4), and have successfully prepared four new geranylated biflavonoid derivatives (1a–4a). Interestingly, AtaPT revealed different regio- and chemo-selectivities towards these substrates. In compounds 1–3, 4–6 hydroxyl groups may participate in the O-geranylation reaction, among which 7–OH and 4′′′–OH are most chemically active, while 5–OH and 5′′–OH are much inert. Interestingly, using AtaPT as a catalyst, O-geranylation happened selectively at the C-5′′ position of substrates 1a–3a. The C-5′′ hydroxyl group in 1–3 is stabilized by an intramolecular hydrogen bond with the C-4′′ carbonyl group, which leads to difficulty in undergoing the selective substitution reaction for chemical synthesis. Therefore, selective C-5′′ geranylation to compound 1–3 is a tough task for organic chemists. In contrast, C-geranylation at C-3′′′ is selectively involved in the formation of compound 4a. Noticeably, the substrate 4 is formed by a different dimerization mode of two flavonoids, where the C4′–O–C6′′ ether linkage is involved. This interesting switching on of the regio-selectivity and C-/O-chemo-selectivity suggests two different binding modes for these two subclasses of biflavonoids. As the first report on the selective enzymatic prenylation of biflavonoids, our results indicate that AtaPT could be a potential tool in geranylated derivatives of biflavonoids.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21708049 and 21877129), the Natural Science Foundation of Hunan Province (No. 2018JJ3630), the Postgraduates Innovation Program of Central South University (No. 1053320184056) and the Open Sharing Fund for the Large-scale Instruments and Equipments of Central South University. We thank Dr Guogen Liu and Dr Hongping Long for obtaining the NMR and HR-ESI-MS spectra.Notes and references
- H. P. Kim, H. Park, K. H. Son, H. W. Chang and S. S. Kang, Arch. Pharmacal Res., 2008, 31, 265–273 CrossRef CAS PubMed.
- S. Yu, H. Yan, L. Zhang, M. Shan, P. Chen, A. Ding and S. F. Li, Molecules, 2017, 22, 299 CrossRef.
- N. Sirimangkalakitti, L. D. Juliawaty, E. H. Hakim, I. Waliana, N. Saito, K. Koyama and K. Kinoshita, Bioorg. Med. Chem. Lett., 2019, 29, 1994–1997 CrossRef CAS.
- E. O. Farombi, I. A. Adedara, B. O. Ajayi, O. R. Ayepola and E. E. Egbeme, Basic Clin. Pharmacol. Toxicol., 2013, 113, 49–55 CrossRef CAS.
- N. Sirimangkalakitti, L. D. Juliawaty, E. H. Hakim, I. Waliana, N. Saito, K. Koyama and K. Kinoshita, Bioorg. Med. Chem. Lett., 2019, 29, 1994–1997 CrossRef CAS.
- H. Yao, B. Chen, Y. Zhang, H. Ou, Y. Li, S. Li, P. Shi and X. Lin, Molecules, 2017, 22, 325 CrossRef.
- K. Yazaki, K. Sasaki and Y. Tsurumaru, Phytochemistry, 2009, 70, 1739–1745 CrossRef CAS.
- B. Botta, A. Vitali, P. Menendez, D. Misiti and G. D. Monache, Curr. Med. Chem., 2005, 12, 713–739 CrossRef CAS PubMed.
- X. Chen, E. Mukwaya, M. S. Wong and Y. Zhang, Pharm. Biol., 2014, 52, 655–660 CrossRef CAS.
- O. Wesolowska, J. Gasiorowska, J. Petrus, B. Czarnik-Matusewicz and K. Michalak, Biochim. Biophys. Acta, 2014, 1838, 173–184 CrossRef CAS.
- A. Saelee, S. Phongpaichit and W. Mahabusarakam, Nat. Prod. Res., 2015, 29, 1884–1888 CrossRef CAS.
- J. Winkelblech, A. Fan and S. M. Li, Appl. Microbiol. Biotechnol., 2015, 99, 7379–7397 CrossRef CAS.
- T. Kumano, S. B. Richard, J. P. Noel, M. Nishiyama and T. Kuzuyama, Bioorg. Med. Chem., 2008, 16, 8117–8126 CrossRef CAS PubMed.
- K. A. Rea, J. A. Casaretto, M. S. Al-Abdul-Wahid, A. Sukumaran, J. Geddes-McAlister, S. J. Rothstein and T. A. Akhtar, Phytochemistry, 2019, 164, 162–171 CrossRef CAS PubMed.
- R. Chen, B. Gao, X. Liu, F. Ruan, Y. Zhang, J. Lou, K. Feng, C. Wunsch, S. M. Li, J. Dai and F. Sun, Nat. Chem. Biol., 2017, 13, 226–234 CrossRef CAS.
- B. Gao, R. Chen, X. Liu, J. Dai and F. Sun, Acta Crystallogr., Sect. F: Struct. Biol. Commun., 2015, 71, 889–894 CrossRef CAS PubMed.
- H. X. Zou, X. Xie, X. D. Zheng and S. M. Li, Appl. Microbiol. Biotechnol., 2011, 89, 1443–1451 CrossRef CAS.
- P. Coulerie, M. Nour, A. Maciuk, C. Eydoux, J. C. Guillemot, N. Lebouvier, E. Hnawia, K. Leblanc, G. Lewin, B. Canard and B. Figadere, Planta Med., 2013, 79, 1313–1318 CrossRef CAS.
- A. I. Suarez, M. B. Diaz, F. D. Monache and R. S. Compagnone, Fitoterapia, 2003, 74, 473–475 CrossRef CAS.
- K. R. Markham, C. Sheppard and H. Geiger, Phytochemistry, 1987, 26, 3335–3337 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ob02296a |
| This journal is © The Royal Society of Chemistry 2020 |