One-step assembly of alkoxypyrroloindolines via iodine-catalyzed alkoxycyclization of indole derivatives†
Yan
Li‡
,
Juan
Guo‡
,
Xunbo
Lu
and
Fangrui
Zhong
 *
*
Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), 1037 Luoyu road, Wuhan 430074, China. E-mail: chemzfr@hust.edu.cn; Fax: +(0)86-(0)27-87543632
First published on 15th November 2019
Abstract
Herein we report an iodine-catalyzed alkoxycyclization of tryptamine derivatives under mild reaction conditions. This method distinguished itself by providing a catalytic, one-step assembly of diversely functionalized C3a-alkoxypyrroloindolines as well as dihydrofuran and lactone fused indolines. Mechanistic studies suggest that an ionic pathway is operative and this probably accounts for the diastereospecificity of all isolated cycloadducts.
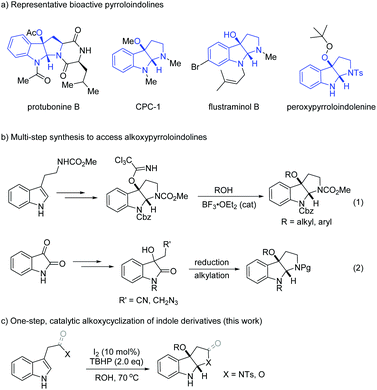
Ring-fused indolines are essential structural frameworks spread across a wide array of natural alkaloids.1 Amongst those, the pyrroloindoline skeletons are well known for their strong bioactivity profiles.2 A subset of molecules of this family are the ones oxygenated at the C3a position wherein the importance of the substituent diversity is underlined in various known bioactive natural products and synthetic pharmaceuticals (Scheme 1a).3
In this context, synthetic methodologies towards C3a-oxygenated pyrroloindolines have been pursued intensively. Tryptophan derivatives are easily accessible precursors for assembling the pyrroloindoline ring systems through oxidative cyclization processes.4 This approach has been established to site-selectively oxygenate the C3a carbon with hydroxyl-, acetoxyl, peroxyl and other groups in a single step.5 However, the preparation of alkoxylpyrroloindolines remains a challenging task probably due to the incompatibility with protic reactants (i.e., alcohols) in an ionic process or the difficulty in homolytic cleavage of the O–H bond with a high bond dissociation energy (BDE is ca.105 kcal mol−1) to form an alkoxyl radical.6 Therefore, an indirect multi-step approach is typically applied to access alkoxylpyrroloindolines. In this regard, Chisholm and co-workers disclosed an efficient protocol through Lewis acid catalyzed displacement of C3a-trichloroacetimidate pyrroloindoline, which was accessed in three steps starting from protected tryptamines (Scheme 1b, (1)).7 In addition, isatins have also been frequently utilized as reactive precursors to derive 3-hydroxyoxindoles bearing a nitrile or azido group, followed by reductive cyclization and alkylation to furnish alkoxylpyrroloindolines (Scheme 1b, (2)).8 Unlike other C3a oxygenated pyrroloindolines,5 the catalytic one-step assembly of the alkoxy counterpart is still unknown. In our related studies on catalytic synthesis of indole derivatives,9 recently we discovered that the radical cyclization of tryptamines under TBAI/TBHP conditions efficiently led to C3a-peroxypyrroloindolenines.9a Interestingly, the use of alcoholic solvents would interrupt the peroxycyclization event and consequently give rise to C3a-alkoxylpyrroloindolines (Scheme 1c). Pleasingly, the protocol is also applicable for the intramolecular cyclization of tryptophol and indole-3-acetic acid, thus allowing access to indolines fused with tetrahydrofuran and lactone.10 While there are limited synthetic methods to obtain these skeletons, this study provides a direct catalytic approach to generate frameworks with such molecular complexity.
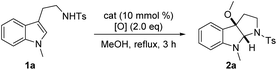
We initiated our investigations by performing the oxidative cyclization of a model substrate tryptamine sulfonamide 1a with TBAI/TBHP in reflux MeOH in air. Pleasingly, these conditions led to the isolation of methoxypyrroloindoline 2a in decent yield (Table 1, entry 1). The unbalanced mass was primarily due to other reaction pathways including the known C3a-peroxycyclization.9a Stimulated by this outcome, we further examined the effect of other iodine catalysts. Molecular iodine displayed superior performance than TBAI, NaI and MePPh3I (entries 2–4). Notably, no cyclization occurred when either the catalyst or TBHP was obviated (entries 5 and 6), indicating the incompetency of molecular oxygen as a terminal oxidant. Subsequent evaluation of others (e.g., CHP, H2O2 and mCPBA) revealed that peroxides were somewhat effective (entries 7–9), starkly contrasting PhI(OAc)2 that virtually arrested the methoxycyclization event to furnish C3a-acetylpyrroloindoline (entry 10). In addition, conducting the reaction in diluted MeOH was beneficial, affording a slightly higher isolated yield of 2a (70%, entry 11). In fact, this transformation did not absolutely necessitate external thermal activation; the desired cyclization also took place smoothly at room temperature with marginally the same yield (67%), but at the expense of reaction time (entry 12). Of note, for this process the aqueous TBHP (70% solution in water) was found to be superior to the anhydrous one, thus adding to the practicality of this protocol (entry 13).
| Entry | Catalyst | [O] | Yieldb (%) |
|---|---|---|---|
| a Reactions were performed with 1a (0.2 mmol), catalyst (0.02 mmol), and oxidant (0.4 mmol) in MeOH (8.0 mL) for 3 hours. b Isolated yield. c 12.0 mL MeOH was used. d At room temperature for 16 hours. e TBHP (∼5.5 mol L−1 in decane) was used. CHP = cumyl hydroperoxide; TBHP = tert-butyl hydroperoxide (70% solution in water); mCPBA = meta-chloroperoxybenzoic acid. | |||
| 1 | TBAI | TBHP | 45 |
| 2 | I2 | TBHP | 65 |
| 3 | NaI | TBHP | 32 |
| 4 | MePPh3I | TBHP | 24 |
| 5 | — | TBHP | 0 |
| 6 | I2 | — | 0 |
| 7 | I2 | CHP | 52 |
| 8 | I2 | H2O2 | 16 |
| 9 | I2 | mCPBA | 12 |
| 10 | I2 | PhI(OAc)2 | 0 |
| 11c | I2 | TBHP | 70 |
| 12d | I2 | TBHP | 67 |
| 13e | I2 | TBHP | 52 |
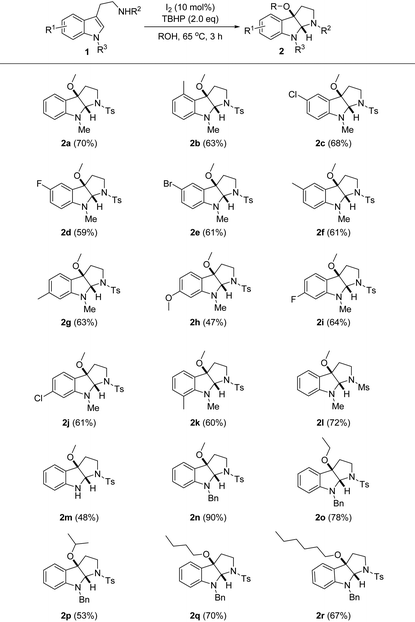
Having identified the optimal reaction conditions, we moved on to evaluate the generality of the iodine-catalyzed alkoxycyclization system (Table 2). Delightfully, various tryptamine sulfonamides 1a–k bearing sterically and electronically different substituents at the indole ring were generally well tolerated to yield products 2a–k in moderate to high yields (47–72%). Of note, the influence of the potential steric congestion between the 4-methyl and C3a-methoxy groups was not evident, as shown in the consistently good yield recorded for compound 2b. However, an electronically enriched indole ring was proven to be challenging, as shown in the diminished yield (47%) obtained for fused indoline 2h containing a methoxy group. This is likely due to the propensity of substrate 1h to undergo various other oxidative transformations indicated by the relatively complex reaction mixture. N-Mesyl tryptamine 1l exhibited marginally identical reactivity compared to 1a, and examination of other N-sulfonyl tryptamines was not carried out. Unfortunately, the respective N-Boc, N-Ac counterparts were identified as not suitable substrates herein probably because of the inadequate nucleophilicity of the attached nitrogen atom. On the other hand, the 1-methyl group of tryptamine 1a could be removed to give adduct 2m, or replaced by benzyl; the latter showed marked reactivity to deliver adduct 2n in 90% yield. Apart from these, other alcoholic reactants could also be applied uneventfully; not only was branched isopropanol applicable, but also methanol homologues with a linear alkyl chain of different lengths were nicely accommodated (2o–r, 53–78%). Practically, the use of alcoholic reactant as a solvent in superstoichiometric quantities would raise the economy issue. Taking this concern into account, upon reaction completion (in the cases of 2q and 2r), we conducted distillation after quenching the excess TBHP, and most alcohols were readily recovered (see the ESI† for details).
The system was further applied to the oxidative alkoxycyclization of other indole derivatives (Scheme 2). To our delight, the fidelity of the standard reaction conditions remained high for tryptophol 3; C3a-methoxydihydrofuroindoline 4 was obtained in 65% yield. Such a methoxylated tricyclic indoline framework is the core structure of varioloid A with significant cytotoxic activity, a natural alkaloid isolated from the marine alga-derived endophytic fungus.11 In addition, the catalytic system was also applicable for indole-3-acetic acid 5, which smoothly cyclized into the unprecedented lactone fused indoline 6 in lower yield.
To further explore the scalability and synthetic potential of the present method, a gram-scale reaction was performed (Scheme 3). Consistently good yield was recorded for product 2a (1.19 g, 74%). All the isolated 2a was then taken for desulfonylation with Mg/MeOH which occurred quantitatively. Subsequent reductive methylation with HCHO/NaBH3CN in MeOH gave CPC-1 (0.59 g). Our method developed herein paves an attractive way to access this alkaloid in only three steps and with so far the best overall yield compared to known protocols.8,9a,12
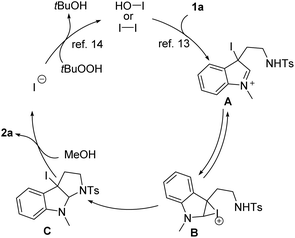
A plausible reaction mechanism is proposed in Scheme 4. The reaction is likely initiated by electrophilic iodination to form iminium A, which is likely in equilibrium with cyclic iodonium ion B.13 Intramolecular attack by sulfonamide nitrogen results in the closure of the pyrrolidine ring and generates iodopyrroloindoline C. An efficient alcoholysis event would be favored for this tertiary iodide intermediate by following a SN1 mechanism. Product 2a is accordingly formed with the concomitant release of iodide entering the next catalytic cycle via oxidation with TBHP.14 Such an ionic pathway was backed by the fact that a radical scavenger such as TEMPO virtually could not affect the transformation (see the ESI†). A notable advantage of the ionic process lies in the attainable diastereospecificity in the cyclization event, which is oftentimes difficult to synergistically control the stereochemistry of two newly formed chiral carbons in a radical process.15
In summary, we have developed the alkoxycyclization of tryptamine derivatives using molecular iodine as the catalyst. This method represents a novel, step-economic approach to efficiently assemble various functionalized C3a-alkoxypyrroloindolines under mild reaction conditions. Mechanistic studies revealed that an ionic process might be operative. In addition to the successful application of the catalytic system to the synthesis of dihydrofuran and lactone fused indoline ring systems, the value has been further highlighted by the scaled up preparation of CPC-1. Therefore, we believe that our methodology disclosed herein will find broad application for the preparation of biologically important indoline alkaloids.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Natural Science Foundation of China (21602067), the Huazhong University of Science and Technology (2016YXMS183, 2018JYCXJJ040), and the Shenzhen Science, Technology and Innovation Commission (JCYJ20180305180832515) for financial support. We are also grateful to the Analytical and Testing Centre of HUST and the Analytical and Testing Centre of School of Chemistry and Chemical Engineering (HUST) for access to their facilities.Notes and references
- (a) T. Hino and M. Nakagawa, in Chemistry and Pharmacology, ed. A. Brossi, Academic Press, New York, 1988, vol. 34, pp. 1–75 Search PubMed; (b) U. Anthoni, C. Christophersen and P. H. Nielsen, in Alkaloids: Chemical and Biological Perspectives, ed. S. W. Pelletier, Wiley, New York, 1999, vol. 13, p. 163 Search PubMed.
- (a) A. Daugan, P. Grondin, C. Ruault, A.-C. Le Monnier de Gouville, H. Coste, J. M. Linget, J. Kirilovsky, F. Hyafil and R. Labaudinière, J. Med. Chem., 2003, 46, 4533–4542 CrossRef CAS; (b) D. Crich and A. Banerjee, Acc. Chem. Res., 2007, 40, 151–161 CrossRef CAS; (c) P. Ruiz-Sanchis, S. A. Savina, P. F. Albericio and P. M. Álvarez, Chem. – Eur. J., 2011, 17, 1388–1408 CrossRef CAS.
- (a) I. Zhalolov, V. U. Khuzhaev, B. Tashkhodzhaev, M. G. Levkovich, S. F. Aripova and N. D. Abdullaev, Chem. Nat. Compd., 1998, 34, 706–710 CrossRef CAS; (b) Z. Guo, Z. Ji, J. Zhang, J. Deng, L. Shen, W. Liu and W. Wu, J. Antibiot., 2010, 63, 231–235 CrossRef CAS; (c) U. L. Sang, Y. Asami, D. Lee, J. H. Jang, J. S. Ahn and H. Oh, J. Nat. Prod., 2011, 74, 1284–1287 CrossRef; (d) J. Xua and R. Tong, Green Chem., 2017, 19, 2952–2956 RSC; (e) Y. Li, L. Li, X. Lu, Y. Bai, Y. Wang, Y. Wu and F. Zhong, Chem. Commun., 2018, 55, 63–66 RSC; (f) K. Wu, Y. Du, Z. Wei and T. Wang, Chem. Commun., 2018, 54, 7443–7446 RSC.
- For recent studies on oxidative cyclization of tryptamine derivatives, see: (a) X. Zhang, Z.-P. Yang, C. Liu and S.-L. You, Chem. Sci., 2013, 4, 3239–3243 RSC; (b) J. Ruchti and E. M. Carreira, J. Am. Chem. Soc., 2014, 136, 16756–16759 CrossRef CAS; (c) C. Liu, J. C. Yi, Z. B. Zheng, Y. Tang, L. X. Dai and S. L. You, Angew. Chem., Int. Ed., 2016, 55, 751–754 CrossRef CAS; (d) M. E. Kieffer, K. V. Chuang and S. E. Reisman, J. Am. Chem. Soc., 2013, 135, 5557–5560 CrossRef CAS; (e) H.-F. Tu, X. Zhang, C. Zheng, M. Zhu and S.-L. You, Nat. Catal., 2018, 1, 601 CrossRef CAS; (f) X. Li, C. Golz and M. Alcarazo, Angew. Chem., Int. Ed., 2019, 58, 9496–9500 CrossRef CAS.
- For selected examples, see: (a) T. Iwaki, F. Yamada, S. Funaki and M. Somei, Heterocycles, 2005, 65, 1811–1815 CrossRef CAS; (b) I. Villanueva-Margalef, D. E. Thurstona and G. Zinzalla, Org. Biomol. Chem., 2010, 8, 5294–5303 RSC; (c) D. Tu, L. Ma, X. Tong, X. Deng and C. Xia, Org. Lett., 2012, 14, 4830–4833 CrossRef CAS; (d) X. Deng, K. Liang, X. Tong, M. Ding, D. Li and C. Xia, Org. Lett., 2014, 16, 3276–3279 CrossRef CAS PubMed; (e) W. Yang, L. Huang, Y. Yu, D. Pflästerer, F. Rominger and A. S. K. Hashmi, Chem. – Eur. J., 2014, 20, 3927–3931 CrossRef CAS; (f) T. Iwaki, Y. Fukui, M. Okigawa, F. Yamada, Y. Nagahama, S. Ogasawara, S. Tanaka, S. Funaki and M. Somei, Heterocycles, 2016, 93, 259–294 CrossRef CAS; (g) E. C. Gentry, L. J. Rono, M. E. Hale, R. Matsuura and R. R. Knowles, J. Am. Chem. Soc., 2018, 140, 3394–3402 CrossRef CAS; (h) M. Z. Wang, T. X. Si, C. F. Ku, H. J. Zhang, Z. M. Li and A. S. Chan, J. Org. Chem., 2018, 84, 831–839 CrossRef; (i) K. Liang, X. Tong, T. Li, B. Shi, H. Wang, P. Yan and C. Xia, J. Org. Chem., 2018, 83, 10948–10958 CrossRef CAS; (j) Z.-J. Liu and P.-Q. Huang, J. Org. Chem., 2019, 84, 5627–5634 CrossRef CAS.
- (a) Y. Zhu, K. Huang, J. Pan, X. Qiu, X. Luo, Q. Qin, J. Wei, X. Wen, L. Zhang and N. Jiao, Nat. Commun., 2018, 9, 2625 CrossRef; (b) X. Wu, M. Wang, L. Huan, D. Wang, J. Wang and C. Zhu, Angew. Chem., Int. Ed., 2018, 57, 1640–1644 CrossRef CAS; (c) S. Meng, L.-R. Lin, X. Luo, H.-J. Lv, J.-L. Zhao and A. S. Chan, Green Chem., 2019, 21, 6187–6193 RSC.
- A. A. Adhikari and J. D. Chisholm, Org. Lett., 2016, 18, 4100–4103 CrossRef CAS.
- (a) T. Itoh, H. Ishikawa and Y. Hayashi, Org. Lett., 2009, 11, 3854–3857 CrossRef CAS; (b) G.-W. Wang, A.-X. Zhou, J.-J. Wang, R.-B. Hu and S.-D. Yang, Org. Lett., 2013, 15, 5270–5273 CrossRef CAS; (c) Q. Ren, J. Huang, L. Wang, W. Li, H. Liu, X. Jiang and J. Wang, ACS Catal., 2012, 2, 2622–2625 CrossRef CAS.
- (a) X. Lu, Y. Bai, Y. Li, Y. Shi, L. Li, Y. Wu and F. Zhong, Org. Lett., 2018, 20, 7937–7941 CrossRef CAS; (b) Y. Ni, Q. Yu, Q. Liu, H. Zuo, H.-B. Yu, W.-J. Wei, R.-Z. Liao and F. Zhong, Org. Lett., 2018, 20, 1404–1408 CrossRef CAS; (c) Y. Ni, H. Zuo, Y. Li, Y. Wu and F. Zhong, Org. Lett., 2018, 20, 4350–4353 CrossRef CAS; (d) D. Wang, X. Lu, S. Sun, H. Yu, H. Su, Y. Wu and F. Zhong, Eur. J. Org. Chem., 2019, 6028–6033 CrossRef CAS; Q. Yu, Y. Fu, J. Huang, J. Qin, H. Zuo, Y. Wu and F. Zhong, ACS Catal., 2019, 9, 7285–7291 Search PubMed.
- (a) T. Abe, T. Suzuki, M. Anada, S. Matsunaga and K. Yamada, Org. Lett., 2017, 19, 4275–4278 CrossRef CAS; (b) X. W. Liang, Y. Cai and S. L. You, Chin. J. Chem., 2018, 36, 925–928 CrossRef CAS; (c) Q. Cheng, F. Zhang, Y. Cai, Y. L. Guo and S. L. You, Angew. Chem., Int. Ed., 2018, 57, 2134–2138 CrossRef CAS; (d) K. Liang, X. Tong, T. Li, B. Shi, H. Wang, P. Yan and C. Xia, J. Org. Chem., 2018, 83, 10948–10958 CrossRef CAS; (e) T. Abe, S. Aoyama, M. Ohmura, M. Taniguchi and K. Yamada, Org. Lett., 2019, 21, 3367–3371 CrossRef CAS.
- P. Zhang, X.-M. Li, X.-X. Mao, A. Mándi, T. Kurtán and B.-G. Wang, Beilstein J. Org. Chem., 2016, 12, 2012–2018 CrossRef CAS.
- (a) A. Singh and G. P. Roth, Org. Lett., 2011, 13, 2118–2121 CrossRef CAS; (b) W.-H. Chiou, C.-L. Kao, J.-C. Tsai and Y.-M. Chang, Chem. Commun., 2013, 49, 8232–8234 RSC.
- (a) Y.-X. Li, H.-X. Wang, S. Ali, X.-F. Xia and Y.-M. Liang, Chem. Commun., 2012, 48, 2343–2345 RSC; (b) S. Badigenchala, V. Rajeshkumar and G. Sekar, Org. Biomol. Chem., 2016, 14, 2297–2305 RSC.
- M. Uyanik and K. Ishihara, Org. Biomol. Chem., 2014, 12, 5807–5817 RSC.
- H. Ren, J.-R. Song, Z.-Y. Li and W.-D. Pan, Org. Lett., 2019, 21, 6774–6778 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1889795. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ob02287b |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2020 |