Supramolecular secondary helical structures in solid-state N-protected amino acids†
Abstract
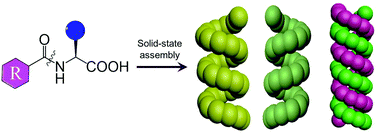
Helix is an important secondary structure in proteins and polypeptides, which, however, has rarely been recognized in amino acids or their simple derivatives. In this work, we firstly unveil the generic existence of supramolecular helical secondary structures in solid-state N-protected amino acids. Throughout searching in Cambridge Structural Database followed by screening, ∼10% N-protected amino acids were evidenced to form H-bonded helical structures, thus covering 15 coded amino acids and diverse types of protecting groups. Helical structures were typically classified as 21 and 31 symmetry, and specific double-strand helical structures were discovered. Computational studies on the calculated electronic circular dichroism spectra show well-defined correlation to experimental results, indicative of the supramolecular secondary structures that possess feature Cotton effects similar to naturally occurring α-helices in proteins. Such feature Cotton effects could be transferred to protecting groups, which is of vital significance to the emerging chiroptical materials. This work highlighting the neglected structural analysis would offer a better understanding and deep insight into the structure relationship on the supramolecular chiral materials, crystal engineering and chiroptical materials based on amino acids and their derivatives.


 Please wait while we load your content...
Please wait while we load your content...