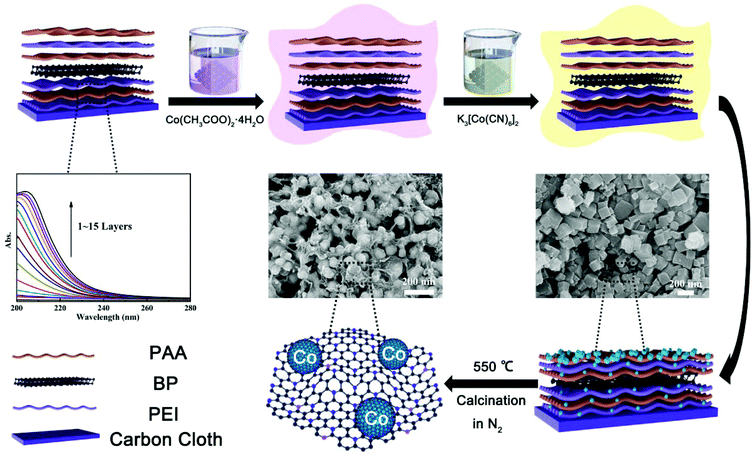
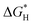A layer-by-layer strategy for the scalable preparation of uniform interfacial electrocatalysts with high structural tunability: a case study of a CoNP/N,P-graphene catalyst complex†
Limei
Lu
,
Yihe
Zhang
 *,
Xuelian
Yu
*,
Xuelian
Yu
 ,
Yi
Zhang
,
Zequn
Ma
and
Qi
An
,
Yi
Zhang
,
Zequn
Ma
and
Qi
An
 *
*
Beijing Key Laboratory of Materials Utilization of Nonmetallic Minerals and Solid Wastes, National Laboratory of Mineral Materials, School of Materials Science and Technology, China University of Geosciences, Beijing, 100083, China. E-mail: zyh@cugb.edu.cn; an@cugb.edu.cn
First published on 14th November 2019
Abstract
Electrocatalysts are important for providing clean energy and have attracted intense research attention. All electrocatalysts must function on electrode surfaces; however, interfacial engineering strategies for electrocatalytic structures remain understudied, and scalable preparation methods are especially rare. In this study, we propose a strategy that employs a layer-by-layer (LbL) assembly, subsequent in-film deposition, and calcination to prepare a complex Co nanoparticle (CoNP)/N,P-graphene catalyst on various 2D and 3D electrode surfaces. We delicately adjusted a variety of parameters and demonstrated that our LbL-based method can finely tune the total amount, the doping fraction, and the electronic structure of the complex catalysts, and provide optimal catalytic performance. When prepared on a large piece of carbon cloth, the catalysts showed a highly uniform catalytic performance, demonstrating the capability of our method for scalable fabrication. Our study emphasizes the delicate nature of the interfacial engineering of electrocatalysts, and promotes functional interface design and mechanism studies.
Introduction
Electrocatalytic hydrogen evolution is a promising technique for providing clean energy and tackling the energy and environmental crisis.1–4 Intense efforts have been directed towards developing effective electrocatalysts. However, electrocatalysts need to function on electrode surfaces.5–10 The interfacial structures of electrocatalysts on electrode surfaces significantly affect elemental coordination, electronic conductivity, and electron density around the catalytic centers.11 As a result, the overall catalytic performance is significantly influenced by the interfacial structures on the electrode surfaces.12–15 Regular surface immobilization methods that produce large-scale homogeneous interfaces, such as painting, dip-coating, casting, and doctor blading lack the capability of placing multiple components in specific sequences and with locational precision at the nanometer scale. Compared with the vast efforts directed towards developing electrocatalytic structures, strategies for mounting the electrocatalysts onto electrode surfaces remain severely understudied. A deep understanding of the methods that can be used to immobilize electrocatalysts effectively and precisely onto rigid or flexible electrode surfaces, on a large scale, is highly appealing.Most electrocatalysts are synthesized as particles from solution reactions. As the parameters of solution reactions can be finely adjusted, solution reactions can generate a versatile range of catalysts with complex components and high catalytic capabilities. However, when their catalytic performances are tested, the catalysts are usually mixed with a Nafion solution and dip-coated onto an electrode with diameters of only several millimeters.16 In practice, electrocatalytic reactions must be able to be performed on large-scale electrode surfaces in order to yield products in sufficient quantities. It is difficult to optimize homogeneously the number of active catalytic sites on the surface (the more the better) and the electron conductivity (which declines with increasing catalyst loading) on a larger scale.17 In addition, catalysts casted using polymers suffer from low long-term stabilities and the unwanted burying of active catalyst centers in the polymer films. There are thus still a number of problems that must be overcome in order to successfully immobilize these high performance composite catalysts onto large-scale electrode surfaces.
The in situ growth of electrocatalysts on substrate interfaces has many advantages. Firstly, it avoids the peeling off of the catalysts during the long-term electrocatalytic process, thus enhancing their stability.16,18 Secondly, it avoids the addition of a binder, which might bury the active sites and reduce the effective surface area.19,20 Some preparative methods are able to generate electrocatalysts from electrode surfaces, such as hydrothermal deposition, electrochemical deposition, and vapor deposition.6 However, the electrocatalysts produced by these methods are limited by the types of reactions available. Furthermore, the control of the doping amount or the doping sequence for multi-component catalyst production using these methods is problematic. In addition, it remains unclear as to whether these methods are scalable, because on a larger scale, the homogeneity of the solution reactions on substrate surfaces is sensitive to a variety of parameters, such as the distribution of reagents due to diffusion (container shape or stirring conditions), temperature gradients, and electrode conductivities.6,8,21–23 In summary, the uniform and scalable preparation of electrocatalysts, with precise componential and structural control, remains a challenge.
The complexity of catalyst structures is another challenging factor with regard to scalable production. For example, CoNPs are widely studied HER catalysts,16 but previous studies have shown that the performance of CoNP-catalyzed HER correlates closely with its coordination environment. CoNPs must be present adjacent to graphene C–N or other hybrid elements, such as S or P; it is only the interfacially coordinated atoms that show high catalytic activity.24–27 The use of a binding reagent readily disrupts the coordinative interactions between the different components.16,28 The ability to generate abundant interfacial, coordinated elements constitutes another challenge to the preparation of complex catalytic interfaces.
Layer-by-layer (LbL) self-assembly is a widely studied and mature technique that has been employed to construct complex functional interfaces with precise structural control down to the nanometer level.29–33 LbL refers to the alternative deposition of components with attractive interactions provided by electrostatic attraction, hydrogen bonding, coordination, etc.29 The alternative deposition processes allow the accommodation of ample components and strict structural control by simply adjusting the parameters of the deposition, such as the concentration of the deposition solution and the total number of cycles of the assembly process.29 However, because LbL interfaces are established upon weak inter-species interactions, polymers capable of multivalent interactions are usually necessary in the building process. These polymers are often poor conductive species which hinders electron transfer in catalytic functions. If the limited conductivities of LbL structures are overcome, LbL could serve as an effective strategy for the scalable construction of catalytic structures, allowing for the high precision and adjustability of the electrode surfaces.
In this study, we propose an LbL-based strategy for the construction of complex interfacial electrocatalysts. We took advantage of the structural control afforded by the LbL technique to prepare an organic matrix for subsequent in-film CoNP precursor deposition; the organic–metal precursor complex is then calcined to form graphene C–N structures which increases interfacial conductivity. At the same time, this generates CoNPs with a diameter of approximately 80 nm, leading to the formation of necessary coordinated Co elements. In order to further enhance the electrocatalytic performance, we added black phosphorus (BP), a two-dimensional (2D) material which possesses appealing electronic properties, as the P source during LbL assembly in order to prepare the CoNPs coordinated to N,P-codoped graphene structures. Only the doping of an appropriate amount of BP at specific locations in the interfacial structure will lead to an optimized catalytic performance; this highlights the necessity of precise control when constructing interfacial catalysts. The interfacial HER catalysts can be employed in acidic conditions for at least 14 hours with 96% performance retention. Taking advantage of the superior substrate compatibility of the LbL technique, we scaled up the preparation of the electrocatalyst on the surface of a piece of carbon cloth (10 × 10 cm), and random sampling indicated that the 30 smaller catalytic electrodes (1 × 3 cm) cut from this large piece of carbon cloth presented highly consistent electrocatalytic capabilities. This demonstrates the potential of this method for the large-scale production of interfacial catalysts. The catalysts can also be prepared on the surface of carbon nanotube papers and 3D carbon nanotube foam, demonstrating the compatibility of this method with a wide variety of electrodes. We expect this report to serve the following functions: (1) to emphasize the complicated and delicate nature of electrode surface engineering to develop effective electrocatalysts; (2) to propose the LbL-based technique as a scalable strategy for the uniform preparation of effective electrocatalysts, and provide an understanding of the process of material evolution during LbL assembly, in-film deposition, and calcination; and (3) to inspire further studies regarding electrocatalytic interfacial engineering using LbL-based technologies in order to further promote mechanism understanding and practical application. In addition, since the LbL technique is versatile and can be used to prepare functional interfaces in a wide range of research domains including catalysis, biomaterials, and sensing devices, LbL-based interfacial electrocatalysts may be further integrated with other functional units to form interfacial systems with integrated functionalities.
Results and discussion
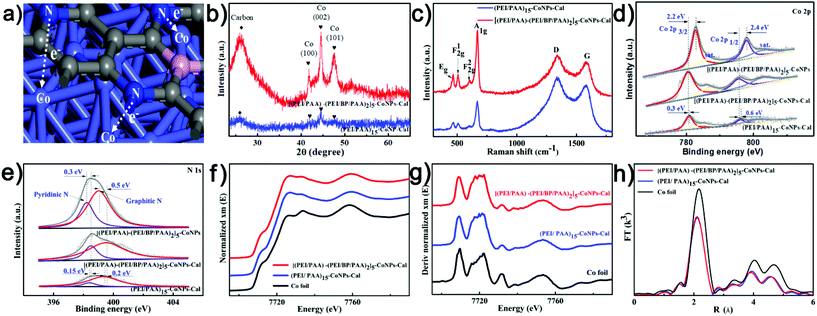
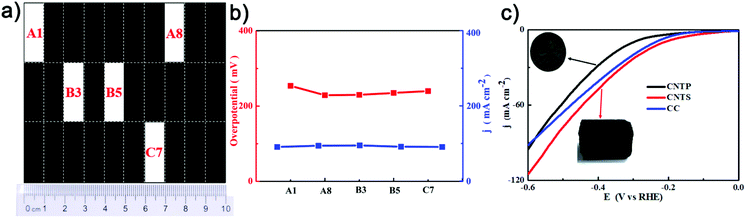
[(PEI/PAA)m-(PEI/BP/PAA)n]p-CoNPs were prepared by LbL followed by in-film precipitation and calcination, as illustrated in Scheme 1. During the LbL process, the multilayers were assembled using sequential assemblies of the polyelectrolyte PEI, PAA, and BP nanosheets. BP sheets were exfoliated from bulk BP and their typical thicknesses were around 4.5–6.0 nm (Fig. S1†). The fractions of BP in the multilayers were controlled by adjusting the assembly sequences, and the total thickness of the multilayers was controlled by the number of assembly cycles. The UV-vis absorbance shown in Scheme 1 confirms that following the growth of the number of bilayers, the film absorbance gradually increases, indicating the even addition of building units in each assembly cycle. After obtaining the intended number of [(PEI/PAA)m-(PEI/BP/PAA)n]p multilayers, CoNPs were generated in the film via an in-film precipitation method using a chemical reaction between Co(CH3COO)2·4H2O and K3[Co(CN)6]2 by alternately immersing the assembled substrates in the two types of solutions. The generated CoNPs densely cover the carbon fiber surface. However, after calcination, the morphologies of the films are remarkably altered. Each CoNP is wrapped by a thin layer of amorphous species. EDS elemental mapping (Fig. S4h†) indicates that the main elements are Co (62.24%) and C (19.76%), with N being the third most abundant (17.28%). A trace amount of P is also observed (0.41%). The O content is even lower than that of P, and can be attributed to a trace amount of surface oxidation of the CoNPs and contamination with O during the analysis.The unusual morphological alterations after calcination prompted us to study the components and structures in detail. The results from multiple types of characterization indicate that the interfacial structures after calcination consist of CoNPs coordinated to N,P-codoped graphene embedded in an amorphous carbon matrix. The XRD patterns of [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs-Cal (CoNPs denote Co nanoparticles, and the last three letters ‘Cal’ denote the post-calcination samples) shown in Fig. 2b indicate diffraction peaks at 41.68°, 44.76°, and 47.56° corresponding to the Co (100), (002), and (101) planes in metal Co (JCPDS No 05-0727). There is no diffraction peak for BP, indicating that BP remains in a dispersed state after calcination or is decomposed (no aggregation). The broad peak at around 20–30 degrees corresponds to amorphous carbon.34,35 Interestingly, the XRD patterns of [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs (without calcination) correspond only to the diffraction peaks of the reagent Co(CH3COO2)·4H2O (Fig. S6†), indicating that before calcination, the CoNP precursor was amorphous. Calcination assists in further reducing this reagent and enhances the degree of crystallization of the CoNP precursor. The TGA results indicated that the [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs experienced carbonization during annealing at 550 °C (Fig. S7†).
The TEM image of [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs-Cal shown in Fig. 1f indicates that CoNPs are encapsulated in a matrix of amorphous carbon. The SAED patterns in the inset of Fig. 1g show the diffraction patterns corresponding to the Co (101, 100) planes, indicating that the particles are crystalline Co metal. Furthermore, the HRTEM images (Fig. 1g) provide interesting information regarding the detailed structures of, and around, the CoNPs. The d-spacings of the lattice fringes of the particles are 0.19 and 0.21 nm, which correspond to the (101) and (100) planes of Co, respectively, confirming that the particles are Co metal. Interestingly, the CoNPs are encapsulated by a crystalline shell with a thickness of approximately 8 nm, with a lattice spacing of 0.34 nm. The elemental survey (Fig. 1h) indicates that this crystalline structure is carbon. The formation of the layered crystalline carbon, a graphene-like structure, can be mostly attributed to the catalyzing role of the Co metals during calcination. It has been reported that the Co metal catalyzes the crystalized carbonization from a wide variety of precursors.36 In addition, the components of the multilayers, PEI and PAA, form covalent bonds upon heating,37 facilitating the formation of graphene-like structures during calcination.38 The N elements in the polymers should be present as dopants in the graphene-like structure. The P element is also considered to be a doping element in the formed graphene-structure instead of BP sheets, because: (i) the elemental mapping shows that the abundance of P is low; (ii) the shell is formed by elemental migration during calcination, and it is unlikely that the entire BP sheet would migrate; and (iii) the tight and ordered crystalline structure may contain atom defects but is unlikely to accommodate BP nanosheets as the defects. In consideration of all the evidence obtained, we speculate that the structure of the interfacial complex consists of the following: metal CoNPs with a diameter of approximately 30–80 nm are wrapped by a crystalline, N,P-codoped graphene-like shell approximately 8 nm thick. These core–shell particles are further connected by amorphous-doped carbon which is connected to the carbon cloth electrodes. The special carbonized shell prevents the CoNPs from aggregating during calcination. More importantly, according to previous reports, CoNPs, coordinated to N,P-codoped graphene, may serve as an effective catalytic center for the electrochemical HER process. We thus turned our attention to characterizing the structural features of the interfacial complex relating to its HER performance.
 | ||
| Fig. 1 SEM, TEM images and TEM elemental surveys of (a–d) (PEI/PAA)15-CoNPs-Cal; (e–h) [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs-Cal. | ||
XPS and XAS indicate that the calcination step significantly alters the electronic density of CoNPs, and the addition of BP also enhances the electronic density of the Co element; both enhance the electrochemical catalytic capacities. Fig. 2d and e show the XPS spectra of [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs (without calcination), (PEI/PAA)15-CoNPs-Cal (without BP), and [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs-Cal. The high-resolution Co 2p spectrum (Fig. 2d) of [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs-Cal shows two major peaks with binding energies of 780.5 (Co 2p3/2) and 795.5 eV (Co 2p1/2), followed by two satellite peaks at 786.2 and 802.7 eV.39 The peaks corresponding to the elemental Co (780.5/795.5 eV) in the BP-hybridized calcined samples are negatively shifted by 2.2/2.4 eV (Co 2p3/2/Co 2p1/2) compared with that in [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs, and by 0.3/0.6 eV (Co 2p3/2/Co 2p1/2) compared with that in (PEI/PAA)15-CoNPs-Cal. These results indicate that calcination remarkably enhances the electron density of the Co element, supporting the notion that the Co precursor is further reduced during calcination. In addition, the introduction of BP into the structure further increases the electron density of the Co element. In the N 1s spectrum, the peaks at 398.5 and 399.5 eV can be assigned to pyridinic-N and graphitic-N, respectively.40,41 The peak corresponding to the N 1s in [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs-Cal is positively shifted by 0.3/0.5 eV (pyridinic-N/graphitic-N) compared with the samples without calcination, and by 0.15/0.2 eV compared with (PEI/PAA)15-CoNPs-Cal (Fig. 2e). These results indicate that the electron density of N decreases after calcination due to effective coordination with the CoNPs. The addition of BP decreases the electron densities of the N elements, and enhances the coordination behavior between Co and N. The increase in the electron density should be beneficial for the electrochemical catalytic capacities of the Co center.42 The C 1s peaks (Fig. S10a†) located at 284.48, 285, and 286 eV correspond to C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–P/C–O–P, and C–N/C–O, respectively, indicating chemical bonding between the C and P/N elements; this supports the structural model proposed above.43 The O 1s spectrum of [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs-Cal shown in Fig. S10b† indicates three peaks at 529.75 (Co–O),44 531.65 (oxygen vacancies),45 and 533.3 eV (adsorbed molecular water).46 The low value for the Co–O bond suggests that the surface of the catalyst is partially oxidized upon exposure to air.
C, C–P/C–O–P, and C–N/C–O, respectively, indicating chemical bonding between the C and P/N elements; this supports the structural model proposed above.43 The O 1s spectrum of [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs-Cal shown in Fig. S10b† indicates three peaks at 529.75 (Co–O),44 531.65 (oxygen vacancies),45 and 533.3 eV (adsorbed molecular water).46 The low value for the Co–O bond suggests that the surface of the catalyst is partially oxidized upon exposure to air.
XANES can be used to identify the fingerprint characteristic bonding environment.47–49 As shown in Fig. 2f, the XANES spectra of (PEI/PAA)15-CoNPs-Cal and [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs-Cal show similar oscillations to Co foil, indicating that Co is in a Co0 state, which is consistent with the XRD and TEM results. The k3-weighted extended X-ray absorption fine structure spectroscopy (EXAFS) was performed at the edge of the Co for the hybrid catalyst (Fig. S11†). The corresponding Fourier transforms (Fig. 2h) of (PEI/PAA)15-CoNPs-Cal and [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs-Cal exhibit identical peak positions (2.2 Å) to that of Co foil, indicating that the coordination environment is the same as that of Co foil, and the Co elements exist as Co metal particles. Furthermore, the peak intensities of the second and third shells are weakened, indicating that the Co coordination number is reduced, and structural defects are generated in the Co metal clusters. As indicated by the Co K-pre-edge image shown in Fig. 2g, when compared with Co foil, the (PEI/PAA)15-CoNPs-Cal and [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs-Cal energies are negatively shifted by 0.42 eV and 0.98 eV, respectively. This suggests that coordination to the N-doped graphene and N,P-codoped graphene increases the electron density of the Co element, which is consistent with the XPS results. In summary, calcination plays a significant role in generating the CoNP center which is coordinated to N,P-codoped graphene. The introduction of BP into the structure further increases the electron density of the Co element, decreases the electron densities of the N elements, and enhances the coordination between Co and N. The increase in the electron density of Co should be beneficial for the electrochemical catalytic capacities.
Raman characterization is also supportive of the structural model of the N,P-codoped graphene. The peak at 1339 cm−1 can be ascribed to the D band of the graphite structure, which is used to evaluate defects or disorders present in the structure. The peak at 1580 cm−1 can be ascribed to the G band of the graphite structure, corresponding to the in-plane stretching vibrations of the sp2 carbon atoms. The ID/IG ratio is larger for the [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs-Cal sample compared with that for (PEI/PAA)15-CoNPs-Cal, indicating the presence of abundant structural defects and disordered sites in the BP-hybridized samples, consistent with the formation of P-doping sites in the graphene-like structure. The four peaks at 467, 507, 600, and 671 cm−1 correspond to Eg, F2g1, F2g2 and A1g of CoO, respectively (Fig. 2c).44 The CoO structure showed the highest signal levels in the Raman characterization compared with the EDS and XPS measurements, possibly resulting from the signal enhancement effect of the adjacent graphene-like structure.50 All analytical data confirm the formation of a structure corresponding to CoNPs coordinated to N,P-codoped graphene, which has potential for use in electrochemical catalytic HER. We next proceeded to study the HER performance of the interfacial complex.
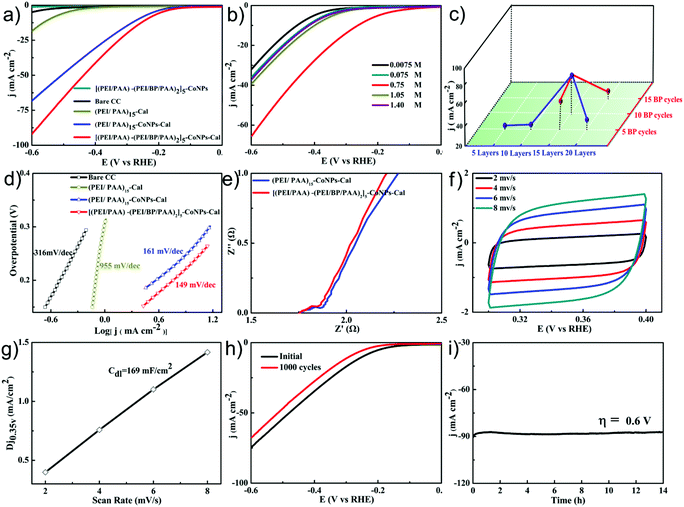
The HER performance of the [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs-Cal interfacial complex was evaluated in 0.5 M H2SO4 solution in a conventional three-electrode system. Bare CC, (PEI/PAA)15-Cal, (PEI/PAA)15-CoNPs-Cal, and [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs (without calcination) were also fabricated for comparison. The LSV curves are shown in Fig. 3a (the reported potentials are all versus a reversible hydrogen electrode (RHE)). Bare CC, (PEI/PAA)15-Cal, or [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs exhibits barely any electrocatalytic activity for HER. (PEI/PAA)15-CoNPs-Cal exhibits considerable HER electrocatalytic activity, indicating that the CoNPs are the main catalytic centers. After adding a BP nanosheet into the complex, the electrocatalytic activity further increases. In order to reach a catalytic current density of 10 mA cm−2, (PEI/PAA)15-CoNPs-Cal needs an overpotential of −266 mV, while [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs-Cal requires −238 mV.
The electrochemical activities are influenced by a range of preparative parameters, demonstrating the high potential of the LbL process for adjusting and optimizing the electrode performance. Firstly, the number of bilayers (Fig. 3c) significantly alters the performance. Due to the competing effects of the increased activity of the catalyst center and reduced conductivities, a structure with 15 bilayers provided the best electrocatalytic activity. Secondly, the concentrations of the reaction solutions to generate CoNPs remarkably influence the electrochemical catalytic performance. When the concentrations of Co(CH3COO)2 and K3[Co(CN)6]2 were increased from 0.0075 to 1.4 mol L−1, the catalytic performance first increased and then declined (Fig. 3b). A concentration of 0.75 mol L−1 provided the optimal catalytic performance. A possible explanation for this volcano-shaped trend is that an increase in the number of active centers influences the change in performance following the gradual increase in concentration. After the optimal performance is reached, the dominating factor is the enhanced charge transfer resistance and decreased active surface areas due to the increase in the size of CoNPs, leading to decreased catalytic performance. Finally, the dosage of BP was optimized (Fig. 3c) by inserting various BP cycles into the LbL process. It is noteworthy that the doping amount of BP peaks at 10 BP layers, which also corresponds to the best performance. Further increasing the number of BP layers to 15 hinders the electrocatalytic performance. These results indicate that the assembly and doping of the electrochemical catalyst, and its subsequent performance, are influenced by several competing parameters. Being able to systematically tune each parameter is important to defining the optimal parameters. The capability to delicately tune the interfacial properties of the catalyst in the LbL assembly process was highlighted during these parameter optimization studies, demonstrating the unique merits of the LbL process in preparing interfacial electrochemical catalyst structures.
The Tafel slope of [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs-Cal shown in Fig. 3d is 149 mV dec−1 within an overpotential range between 0.13 and 0.33 V, and is smaller than those of (PEI/PAA)15-CoNPs-Cal (161 mV dec−1), (PEI/PAA)15-Cal (955 mV dec−1), and Bare CC (316 mV dec−1). This indicates that the [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs-Cal structure exhibits a faster kinetic process for HER and that the Volmer step is the rate-controlling mechanism. The electrochemical impedance spectroscopy (EIS) was performed in the frequency range of 0.1 Hz–100 kHz and at an overpotential of 0.2 V. The Nyquist plots of various catalysts shown in Fig. 3e indicate that [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs-Cal possesses a charge transfer resistance comparable to that of (PEI/PAA)15-CoNPs-Cal. This suggests that the dominating factor for the enhanced electrocatalytic performance resulting from BP doping is the alteration of the electronic structure of the complex, and not the interfacial conductivities.
In order to assess the number of catalytic active centers in the interfacial complex, we measured the double-layer capacitances (Cdl) to obtain the electrochemically active surface areas (ECSA) in 0.5 M H2SO4. The cyclic voltammogram (CV) curves of [(PEI/PAA)-(PEI/BP/PAA)2]5-CoNPs-Cal are shown in Fig. 3f at various scan rates. The Cdl was calculated to be 169 mF cm−2 by plotting the 1/2(ja − jc) at 0.35 V (where jc and ja are the cathodic and anodic current densities, respectively) against the scan rate (Fig. 3f and g, check Notes S2† for details). The ECSA was found to be 0.84 m2 according to the equation (see Notes S3† for details).
As an HER catalyst, long-term stability is important for its broader application. We evaluated the long-term stabilities of the catalysts using CV and chronoamperometry methods. As shown in Fig. 3h, the polarization curves before and after 1000 CV cycles changed only slightly, and the current density dropped to 91% at an overpotential of 0.6 V. Furthermore, chronoamperometric measurements were carried out at a fixed overpotential of 0.6 V, as shown in Fig. 3i. The electrolysis current density remained at 96% after 14 h of continuous operation, implying that the interfacial complex possesses high HER stability in 0.5 M H2SO4.
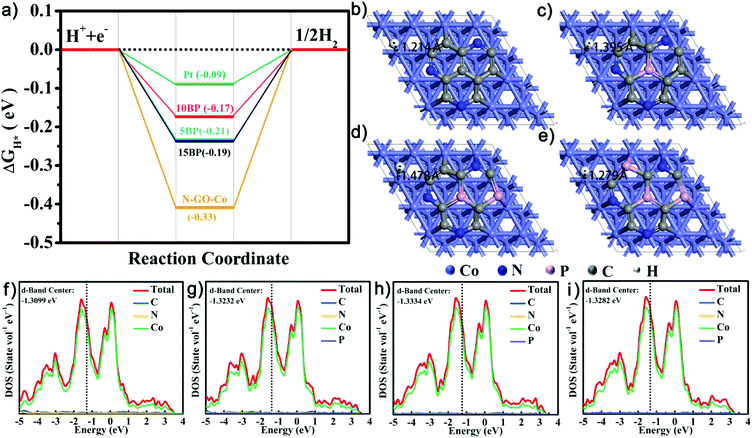
In order to confirm the underlying mechanism of P doping, we further conducted density functional theory (DFT) simulations, as shown in Fig. 4. Based on discussions regarding the relative carbon-shelled HER catalysts, the carbon shells are permeative to H atom, and H should adsorb onto the surface of the Co particles. Using such models, the simulation results indicate that the amount of P doping influences the adsorption geometries of H+ and the H+ adsorption energy. In Fig. 4b–e, following the increase of P doping, the distances between the adsorbed H atom and the Co atom first increase and then decrease. As indicated by previous studies, with regard to HER, the energy change upon H+ sorption (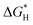 ) is generally used as an indicator of catalytic activity. The thermo-neutral adsorption of H atoms (the energy change upon H+ adsorption close to zero, which has a balance between the transfer of proton and the removal of adsorbed hydrogen51) has been recognized as the ideal type of H adsorption for HER catalysis. The calculated
) is generally used as an indicator of catalytic activity. The thermo-neutral adsorption of H atoms (the energy change upon H+ adsorption close to zero, which has a balance between the transfer of proton and the removal of adsorbed hydrogen51) has been recognized as the ideal type of H adsorption for HER catalysis. The calculated 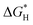 values for CoNPs are coordinated with 0.19 eV (high P doping) and −0.17 eV (medium P doping). These results indicate that only the appropriate level of P doping will benefit thermo-neutral H adsorption, which is consistent with the experimental results. We calculated DOS to explore the effect of P doping on HER and the change in the electronic structure of CoNPs. After DOS normalization, the Fermi level generally needs to be adjusted to be 0 eV. The farther the D band is from the Fermi level, the weaker the adsorption of small molecules is and the smaller the
values for CoNPs are coordinated with 0.19 eV (high P doping) and −0.17 eV (medium P doping). These results indicate that only the appropriate level of P doping will benefit thermo-neutral H adsorption, which is consistent with the experimental results. We calculated DOS to explore the effect of P doping on HER and the change in the electronic structure of CoNPs. After DOS normalization, the Fermi level generally needs to be adjusted to be 0 eV. The farther the D band is from the Fermi level, the weaker the adsorption of small molecules is and the smaller the 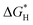 value, which are more beneficial for HER. After P doping, the atomic spacing is stretched, which causes the d-band overlap to decrease, the d-band to narrow, and the d-band center to move toward higher energy (near the Fermi level). As Fig. 4f–i shows, the d-band center is downshifted from the Fermi level due to the P doping. There is an optimal value at medium P doping for the d-band center, which results in the decrease of the binding strength of H.52 All the results indicate that the adsorption energy of H is sensitive to the level of P doping in the catalysts, and only an appropriate P doping fraction will benefit the thermos-neutral H adsorption.53
value, which are more beneficial for HER. After P doping, the atomic spacing is stretched, which causes the d-band overlap to decrease, the d-band to narrow, and the d-band center to move toward higher energy (near the Fermi level). As Fig. 4f–i shows, the d-band center is downshifted from the Fermi level due to the P doping. There is an optimal value at medium P doping for the d-band center, which results in the decrease of the binding strength of H.52 All the results indicate that the adsorption energy of H is sensitive to the level of P doping in the catalysts, and only an appropriate P doping fraction will benefit the thermos-neutral H adsorption.53
The LbL technique possesses the unique advantage of being able to prepare scalable interfacial structures homogeneously with 3D features. We therefore proceeded to test whether our method was capable of preparing electrochemical catalysts on a large piece of carbon cloth (10 × 10 cm, Fig. 5a) with uniform performance over the entire area. Once prepared, the large piece of CC was divided into 30 parts with identical dimensions of 1 × 3 cm. We selected 5 different pieces across the entire area for electrochemical tests (A1, A8, B3, B5 and C7, as shown in Fig. 5a). The overpotentials at a current density of 10 mA cm−2 are highly consistent, being approximately 250 mV across these samples. Similarly, the current densities at an overpotential of 0.6 V are also consistent, being approximately −97 mA cm−2 (Fig. 5b).
In addition, in order to demonstrate the generality of our preparative method towards different substrates, we also used carbon nanotube paper (CNTP) and carbon nanotube sponges (CNTS) as the substrates. The LSV curves shown in Fig. 5c indicate that both substrates show clear electrochemical catalytic performances. The catalytic performance of the modified CNTS is even higher than that of the modified CC, showing a 30% increase in current density at an over potential of −0.6 V, due to its larger surface area. These results demonstrate that our reported LbL method for the preparation of electrochemically catalytic interfaces not only achieves scalable uniform preparation, but is also compatible with a variety of conductive substrates.
Conclusions
In summary, we have reported the use of an LbL method for the uniform and scalable preparation of interfacial electrochemical catalysts with high componential and structural tunability. The LbL building units, the polyelectrolyte matrix, serve dual roles as: (i) the matrix for in-film deposition of the electrocatalytically active metal CoNPs; and (ii) the carbon source during the catalytic calcination process for the generation of doped crystalline carbon. The doping of the crystalline carbon is further enhanced by introducing BP as the P source during LbL assembly. As a result of the assembly, in-film deposition, and catalytic calcination, an effective electrocatalytically active center, CoNPs coordinated to N,P-codoped graphene, was prepared. A variety of parameters associated with the assembly, reaction, and calcination process remarkably influence the catalytic performance. These factors include the addition of BP as a co-assembly unit, the number of bilayers, the fraction of BP in the multilayers, and the concentration of the deposition reaction reagents. The remarkable capabilities of the LbL technique to delicately tune all of these parameters to achieve optimal catalytic performance were highlighted during the preparative process. DFT simulations indicated that only the appropriate amount of P doping provides the optimal H+ adsorption energy for HER. The prepared interfacial catalysts possess high long-term stabilities in 0.5 M H2SO4 after 1000 CV cycles or after 14 h of continuous operation at −0.6 V. The catalysts prepared on a large piece of carbon cloth (10 × 10 cm) show uniform performance across the entire electrode area. In addition, the preparative method is compatible with various 2D and 3D substrates, such as carbon nanotube paper and carbon nanotube sponge. In particular, the catalysts on carbon nanotube sponges show a clearly superior performance compared with those on carbon cloth, indicating that increasing the electrode surface area using 3D structures can further enhance the catalytic performance. The versatility of the LbL technique to generate various interfacial structural features provides a suitable method for interfacial catalyst immobilization. Further optimization of the performance of interfacial catalytic structures using the LbL method is ongoing in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the NSFC (51772279, 21673209), the Fundamental Research Funds for the Central Universities (2652018289), and Beijing Nova Programme Interdisciplinary Cooperation Project (Z181100006218122). The authors would like to thank Conghua Qi from Shiyanjia Lab (http://www.shiyanjia.com) for the XAS analysis.Notes and references
- J. Shan, T. Ling, K. Davey, Y. Zheng and S. Z. Qiao, Adv. Mater., 2019, 1900510 CrossRef PubMed.
- Y. Xu, M. Kraft and R. Xu, Chem. Soc. Rev., 2016, 45, 3039–3052 RSC.
- W. S. Zhi, J. Kibsgaard, C. F. Dickens, I. Chorkendorff, J. K. Nørskov and T. F. Jaramillo, Science, 2017, 355, eaad4998 CrossRef PubMed.
- C. G. Morales-Guio, L.-A. Stern and X. Hu, Chem. Soc. Rev., 2014, 43, 6555–6569 RSC.
- V. R. Stamenkovic, B. S. Mun, M. Arenz, K. J. J. Mayrhofer, C. A. Lucas, G. Wang, P. N. Ross and N. M. Markovic, Nat. Mater., 2007, 6, 241–247 CrossRef CAS PubMed.
- J. Zhang, K. Sasaki, E. Sutter and R. R. Adzic, Science, 2007, 315, 220–222 CrossRef CAS PubMed.
- H. You, S. Yang, B. Ding and H. Yang, Chem. Soc. Rev., 2013, 42, 2880–2904 RSC.
- N. Du, C. Wang, X. Wang, L. Yue, J. Jiang and Y. Xiong, Adv. Mater., 2016, 28, 2077–2084 CrossRef CAS.
- V. R. Stamenkovic, B. S. Mun, K. J. Mayrhofer, P. N. Ross and N. M. Markovic, J. Am. Chem. Soc., 2006, 128, 8813–8819 CrossRef CAS PubMed.
- Z. Chen, M. Waje, W. Li and Y. Yan, Angew. Chem., 2010, 119, 4138–4141 CrossRef.
- R. X. Guang, J. Bai, X. J. Jia, M. L. Jong and Yu Chen, Chem. Sci., 2017, 8, 8411–8418 RSC.
- H. L. Liu, F. Nosheen and X. Wang, Chem. Soc. Rev., 2015, 44, 3056–3078 RSC.
- F. Zaera, Chem. Soc. Rev., 2013, 42, 2746–2762 RSC.
- A. Oh, H. Baik, D. S. Choi, J. Y. Cheon, B. Kim, H. Kim, S. J. Kwon, S. H. Joo, Y. Jung and K. Lee, ACS Nano, 2015, 9, 2856–2867 CrossRef CAS PubMed.
- Z. Niu, D. Wang, Y. Rong, Q. Peng and Y. Li, Chem. Sci., 2012, 3, 1925–1929 RSC.
- H. Sun, Z. Yan, F. Liu, W. Xu, F. Cheng and J. Chen, Adv. Mater., 2019, 1806326–1806343 CrossRef PubMed.
- Y. Li, J. H.-C. Liu, C. A. Witham, W. Huang, M. A. Marcus, S. C. Fakra, P. Alayoglu, Z. Zhu, C. M. Thompson and A. Arjun, J. Am. Chem. Soc., 2011, 133, 13527–13533 CrossRef CAS PubMed.
- C. Zhang, Z. Pu, I. S. Amiinu, Y. Zhao, J. Zhu, Y. Tang and S. Mu, Nanoscale, 2018, 10, 2902–2907 RSC.
- Z. Zou, J. Zhao, J. Xue, R. Huang and C. Jiang, J. Electroanal. Chem., 2017, 799, 187–193 CrossRef CAS.
- D. Yan, Y. Li, J. Huo, R. Chen and S. Wang, Adv. Mater., 2017, 29, 1606459 CrossRef.
- Z. Peng and Y. Hong, J. Am. Chem. Soc., 2009, 131, 7542–7543 CrossRef CAS PubMed.
- Y. Lu and W. Chen, Chem. Commun., 2011, 47, 2541–2543 RSC.
- F. Ye, H. Liu, W. Hu, J. Zhong, Y. Chen, H. Cao and J. Yang, Dalton Trans., 2012, 41, 2898–2903 RSC.
- N.-A. M. Cabã, M. L. Stone, J. R. Schmidt, J. G. Thomas, Q. Ding, H. C. Chang, M. L. Tsai, J. H. He and S. Jin, Nat. Mater., 2015, 14, 1245–1251 CrossRef PubMed.
- H. Guo, Q. Feng, K. Xu, J. Xu, J. Zhu, C. Zhang and T. Liu, Adv. Funct. Mater., 2019, 29, 1903660–1903670 CrossRef.
- C. Zhang, M. Antonietti and T. P. Fellinger, Adv. Funct. Mater., 2014, 24, 7655–7665 CrossRef CAS.
- H. Guo, Q. Feng, J. Zhu, J. Xu, Q. Li, S. Liu, K. Xu, C. Zhang and T. Liu, J. Mater. Chem. A, 2019, 7, 3664–3672 RSC.
- G. F. Chen, T. Y. Ma, Z. Q. Liu, N. Li, Y. Z. Su, K. Davey and S. Z. Qiao, Adv. Funct. Mater., 2016, 26, 3314–3323 CrossRef CAS.
- Q. An, T. Huang and F. Shi, Chem. Soc. Rev., 2018, 47, 5061–5098 RSC.
- J. J. Richardson, M. Björnmalm and F. Caruso, Science, 2015, 348, aaa2491 CrossRef PubMed.
- B. Joao and J. O. F. Mano, Chem. Rev., 2014, 114, 8883–8942 CrossRef.
- Y. Zhang, Q. An, W. Tong, H. Li, Z. Ma, Y. Zhou, T. Huang and Y. Zhang, Small, 2018, 14, 1802136–1802146 CrossRef PubMed.
- X. Luan, T. Huang, Y. Zhou, Q. An, Y. Wang, Y. Wu, X. Li, H. Li, F. Shi and Y. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 34080–34088 CrossRef CAS PubMed.
- Q. Zhou, Z. Shen, C. Zhu, J. Li, Z. Ding, P. Wang, F. Pan, Z. Zhang, H. Ma and S. Wang, Adv. Mater., 2018, 30, 1800140–1800147 CrossRef PubMed.
- Z. Zhang, Y. Liu, L. Ren, H. Zhang, Z. Huang, X. Qi, X. Wei and J. Zhong, Electrochim. Acta, 2016, 200, 142–151 CrossRef CAS.
- X. Zou, X. Huang, A. Goswami, R. Silva, B. R. Sathe and T. Asefa, Angew. Chem., Int. Ed., 2014, 53, 4372–4376 CrossRef CAS PubMed.
- X. Zhang, H. Chen and H. Zhang, Chem. Commun., 2007, 14, 1395–1405 RSC.
- R. Longfang and W. Xuechuan, J. Chem. Soc. Pak., 2009, 31, 447–452 Search PubMed.
- E. Gottlieb, K. Matyjaszewski and T. Kowalewski, Adv. Mater., 2019, 31, 1804626–1804641 CrossRef.
- H. Fei, J. Dong, M. J. Arellanojiménez, G. Ye, N. D. Kim, E. L. G. Samuel, Z. Peng, Z. Zhu, F. Qin and J. Bao, Nat. Commun., 2015, 6, 8668–8675 CrossRef CAS.
- Y. Zhu, G. Chen, Y. Zhong, W. Zhou and Z. Shao, Adv. Sci., 2018, 5, 1700603–1170610 CrossRef PubMed.
- Y. Tong, X. Yu, H. Wang, B. Yao, C. Li and G. Shi, ACS Catal., 2018, 8, 4637–4644 CrossRef CAS.
- Y. Peng, B. Lu and S. Chen, Adv. Mater., 2018, 30, 1801995–1802019 CrossRef.
- J. Tian, J. Chen, J. Liu, Q. Tian and P. Chen, Nano Energy, 2018, 48, 284–291 CrossRef CAS.
- Y. Chen, R. Ren, Z. Wen, S. Ci, J. Chang, S. Mao and J. Chen, Nano Energy, 2018, 47, 66–73 CrossRef CAS.
- B. Liu, L. Zhang, W. Xiong and M. Ma, Angew. Chem., Int. Ed., 2016, 55, 6564–6564 CrossRef.
- N. Kornienko, J. Resasco, N. Becknell, C.-M. Jiang, Y.-S. Liu, K. Nie, X. Sun, J. Guo, S. R. Leone and P. Yang, J. Am. Chem. Soc., 2015, 137, 7448–7455 CrossRef CAS.
- Z. Sun, W. Yan, T. Yao, Q. Liu, Y. Xie and S. Wei, Dalton Trans., 2013, 42, 13779–13801 RSC.
- B. Ravel and M. Newville, J. Synchrotron Radiat., 2005, 12, 537–541 CrossRef CAS PubMed.
- T. Schmid, L. Opilik, C. Blum and R. Zenobi, Angew. Chem., Int. Ed., 2013, 52, 5940–5954 CrossRef CAS.
- J. Greeley and J. K. Nørskov, Surf. Sci., 2007, 601, 1590–1598 CrossRef CAS.
- B. Hammer and J. K. Norskov, Nature, 1995, 376, 238–240 CrossRef CAS.
- Y. Pan, K. Sun, Y. Lin, X. Cao, Y. Cheng, S. Liu, L. Zeng, W.-C. Cheong, D. Zhao and K. Wu, Nano Energy, 2019, 56, 411–419 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, electrochemical data and additional characterization, including Fig. S1–S13 and Table S1. See DOI: 10.1039/c9nr09018e |
| This journal is © The Royal Society of Chemistry 2020 |