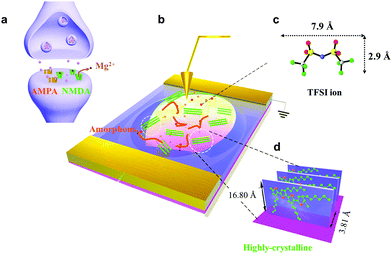
Mixed receptors of AMPA and NMDA emulated using a ‘Polka Dot’-structured two-dimensional conjugated polymer-based artificial synapse†
Hong
Han
 a,
Feng
Ge
a,
Feng
Ge
 b,
Mingxue
Ma
a,
Haiyang
Yu
a,
Huanhuan
Wei
a,
Xue
Zhao
b,
Hongbing
Yao
b,
Jiangdong
Gong
a,
Longzhen
Qiu
b,
Mingxue
Ma
a,
Haiyang
Yu
a,
Huanhuan
Wei
a,
Xue
Zhao
b,
Hongbing
Yao
b,
Jiangdong
Gong
a,
Longzhen
Qiu
 *b and
Wentao
Xu
*b and
Wentao
Xu
 *a
*a
aInstitute of Optoelectronic Thin Film Devices and Technology, Key Laboratory of Optoelectronic Thin Film Devices and Technology of Tianjin, Nankai University, Tianjin 300350, China. E-mail: wentao@nankai.edu.cn
bAcademy of Opto-Electronic Technology, State Key Lab of Advanced Display Technology, Anhui Technology Innovation Center of Special Display and Imaging Technology, Hefei University of Technology, Hefei 230009, China. E-mail: lzhqiu@hfut.edu.cn
First published on 17th July 2020
Abstract
In a biological synapse, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors mediate fast excitatory neurotransmission, whereas N-methyl-D-aspartate (NMDA) receptors trigger an enhanced memory effect; the complementary roles of AMPA and NMDA are essential in short-term plasticity (STP) to enhance memory effect (EME) transition. Herein, we report the design and fabrication of the first two-dimensional (2D) conjugated polymer (CP)-based synaptic transistor. The special design of the 2D CP with nanoscale-segregated ‘polka dot’-structured crystalline phases and adjacent amorphous phases emulate the different receptors of NMDA and AMPA on the postsynaptic membrane for the first time. The synergistic effect of mixed receptors distinguishes STP and enhanced memory effect with a critical point, which regulates the threshold level of the enhanced memory effect induction. This effect has not been reported yet. The special structure avoids easy saturation of a single receptor with consecutively increased excitatory postsynaptic current (EPSC) in response to 1200 stimuli. Furthermore, the 2D P3HT synapse successfully emulates activity-dependent synaptic plasticity, such as metaplasticity and homeostatic plasticity, which are advanced forms of plasticity, allowing the self-adaptive ability of a synapse, but have rarely been reported.
New conceptsProgress toward artificial intelligence with neuromorphic devices face significant challenges to emulate the versatile synaptic plasticity of biological synapses, and to distinguish different memory stages with a critical point. If a semiconducting thin film with nanoscale-segregated regions that interact distinctly with mobile ions could be designed, then such segregated phases could be utilized to emulate different receptors that correspond to different memory stages. Based on this new concept, we designed the first synaptic transistor based on a two-dimensional conjugated polymer film with nanoscale-segregated phases. The synergistic effect of mixed receptors distinguishes different memory stages’ mechanism. Moreover, availing this special design, advanced forms of synaptic plasticity, such as metaplasticity and homeostatic plasticity, can also be mimicked. We expect that this work will inspire further inspire neuromorphic electronics towards emulating biological intelligence with nanostructured material design. |
1. Introduction
With the rapid development of artificial intelligence, tremendous efforts have been made to understand the principles of brain operation and mimic the functions of biological neural networks.1,2 The new design of artificial synapses not only provide methodologies or platforms for better understanding of neuron's biological behaviors, but also offer possibilities to construct artificial intelligent systems.3–7In biological synapses, glutamate receptors mediate excitatory neural signal transmission, playing an important role in learning, memory acquisition and prevention of some neuropsychiatric disorders.8,9 In the prevailing view, glutamate can activate several types of receptors, typically α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and N-methyl-D-aspartate (NMDA). AMPA receptors mediate fast excitatory neurotransmission, whereas NMDA receptors trigger the induction of enhanced memory effect (EME).10–12 The complementary roles of AMPA and NMDA are essential to the transition from short-term plasticity (STP) to EME in the nervous system.
To emulate the STP to EME transition, several types of transistor-structured artificial synapses have been investigated.13–19 However, the qualitative accumulation of synaptic weight differs from that of biological synapses and researchers have not been able to distinguish the STP and EME mechanism. An artificial device that emulates both the effects of AMPA and NMDA receptors as well as their correlations is desired to regulate the threshold of EME induction.
To mimic different receptors on the postsynaptic membrane, designing a semiconducting thin film with segregated regions that interact distinctly with mobile ions is desired in the synaptic transistor. A two-dimensional (2D) self-organized conjugated polymer (CP) film can be designed with separated amorphous and highly-crystalline nanoscale phases to emulate different types of receptors. Moreover, charge transport occurs mainly in the first several molecular layers near the semiconductor/gate insulator interface, and their ultralow thickness aids in studying the correlations between material structure and electrical properties.20 However, there is no study reported yet on 2D CP-based synaptic transistors.
Here, we report the design and fabrication of the first 2D CP-based synaptic transistor. The special design of the 2D CP with nanoscale-segregated ‘polka dot’-structured crystalline phases and adjacent amorphous phases of a poly(3-hexylthiophene-2,5-diyl) (P3HT) thin film emulates the different receptors of NMDA and AMPA on the postsynaptic membrane. The synergistic effect of mixed receptors distinguishes the STP and EME in mechanism, which has not yet been reported before. The device limited the saturation of postsynaptic receptors with consecutively increased excitatory postsynaptic current (EPSC) in response to 1200 stimuli. The special design also contributes to the emulation of activity-dependent synaptic plasticity, such as metaplasticity and homeostatic plasticity. Metaplasticity, an advanced form of plasticity, allows synapses to integrate plasticity-relevant signals over time and endows neural networks with the ability to self-adapt. Homeostatic plasticity, as another essential activity-dependent plasticity that acts to maintain stable activity states during perturbation, is observed in our synaptic transistor.21 The synaptic transistors with 2D CPs provide new insight into ways to build future neuromorphic electronics.
2. Experimental section
2.1 Device fabrication
P3HT (P100, Mw ≈ 41.9 kDa, Rieke Metals, Inc.) and PMMA (Mw ≈ 996 kDa, Sigma-Aldrich) were separately dissolved in chlorobenzene and mixed to achieve an ultimate concentration of 0.5 mg mL−1 P3HT and 3 wt% PMMA. Silicon substrates were cleaned using piranha solution, washed in deionized water, and dried with a nitrogen flow. The films were fabricated by spin-coating the mixed solutions on these substrates at 2000 rpm for 60 s in a glovebox, then dried in a vacuum oven overnight without heating to remove the solvent. The films were immersed in KOH solution (5 wt%) to separate them from the silicon substrate. The floated films were rinsed in deionized water three times, then transferred onto Cytop-treated SiO2/Si substrates (300 nm-thick SiO2), then washed with acetone. Thermally-evaporated gold source/drain electrodes (50 nm) were deposited on P3HT films by using shadow masks (width 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 μm; length 40 μm). Ion gel liquid composed of poly(styrene-block-methyl methacrylate-block-styrene) (PS–PMMA–PS) triblock copolymer and 1-ethyl-3-methylimidazolium bis(trifluoromethyl sulfonyl) imide ([EMIM][TFSI]) in ethyl acetate (0.7
400 μm; length 40 μm). Ion gel liquid composed of poly(styrene-block-methyl methacrylate-block-styrene) (PS–PMMA–PS) triblock copolymer and 1-ethyl-3-methylimidazolium bis(trifluoromethyl sulfonyl) imide ([EMIM][TFSI]) in ethyl acetate (0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.3
9.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, w/w/w ratio) was drop-cast to the channel region and vacuum-annealed for 2 h at room temperature to assist the removal of solvent and formation of gel-like films.
90, w/w/w ratio) was drop-cast to the channel region and vacuum-annealed for 2 h at room temperature to assist the removal of solvent and formation of gel-like films.
2.2 2D CP film characterization
The surface morphologies and the thickness of the polymer thin film were investigated using tapping mode AFM (NanoScope, Veeco Instrument Inc.). The TEM observations were conducted on a JEM-2100F electron microscope, operating at an acceleration voltage of 200 kV. The samples for TEM observation were prepared by transferring the blend film onto copper mesh grids and etching PMMA in acetone. SEM images were measured on an Apreo S (Thermo Scientific). Two-dimensional (2D) GIXD was measured at the Pohang Accelerator Laboratory (PAL); 11.07 keV photons (1.12 Å) with a grazing angle of 0.12° were directed onto the sample. UV-vis transmission was measured using a Cary 5000 spectrophotometer (Agilent). XPS was measured on ESCALAB 250Xi X-ray photoelectron spectrometer (Thermo Scientific).2.3 Device electrical characterization
All electrical characteristics of the electronic devices were measured in a nitrogen-filled glove box by a Keithley 4200A-SCS semiconductor parameter analyzer.3. Results and discussion
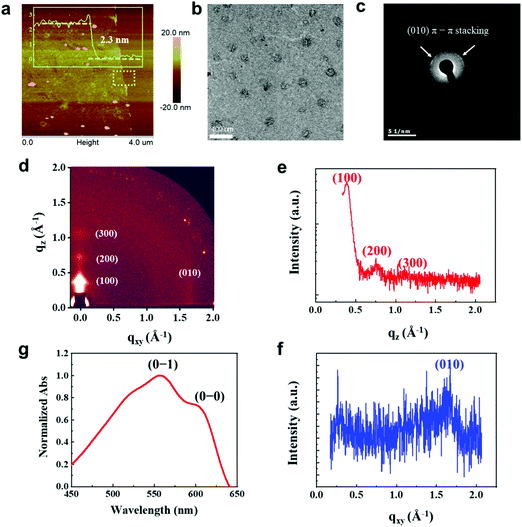
We fabricated synaptic transistors based on a 2D P3HT film to emulate the essential working principles of biological synapses (Fig. 1). The device structure is analogous to a biological synapse with mixed receptors of NMDA and AMPA (Fig. 1a and b). Electrical pulses analogous to presynaptic spikes arrive at a gate electrode that emulates the presynaptic membrane. The ion gel functions as the synaptic cleft that mediates the transmission of neurotransmitters. The electrical pulses induce ion migration in the ion gel (PS–PMMA–PS, [EMIM][TFSI]) to modulate the conductance of the conductive channel (Fig. 1c). The 2D P3HT film and source/drain electrodes are analogous to a postsynaptic membrane that receives transient signals through the synaptic junction. The conductance of P3HT can be tuned by a pulse-induced electrical double layer effect in the ion gel and by a doping effect in the P3HT layer. The 2D P3HT film with distinct amorphous and highly-crystalline phases allows ions to diffuse in and out at different speeds, and to exhibit different electrical properties. The mixed AMPA and NMDA receptors can be mimicked by amorphous and highly-crystalline phases, respectively. In the 2D P3HT film, the highly-crystalline P3HT molecular stacking orientation is perpendicular to the substrate, and increases charge carrier transport along the channel direction (Fig. 1d). The size of TFSI is shown,22 and the π–π distance of P3HT stacking is 3.81 Å, as calculated from GIXD data.Nano-structured, continuous 2D CP films have been fabricated, however the study of them is still in a preliminary stage. 2D P3HT films can be obtained by vertical phase separation from the P3HT/PMMA blend solution. This method can control the film thickness precisely down to the nanometer scale. The 2D P3HT films with apparent separated highly-crystalline and amorphous nanostructures, low free exciton bandwidth and close π–π stacking have potential for use in electronic devices. We characterized the nanostructure of 2D P3HT films by atomic force microscopy (AFM), transmission electron microscopy (TEM), scanning electron microscopy (SEM), grazing incidence X-ray diffraction (GIXD), Ultraviolet–visible spectroscopy (UV-vis), and X-ray photoelectron spectroscopy (XPS) (Fig. 2). The P3HT films were pinhole-free (Fig. S1, ESI†). The 2D P3HT thin film was ∼2.3 nm thick (Fig. 2a); white spots in the AFM image are probably P3HT aggregates, and are consistent with the globular aggregates in the TEM image (Fig. 2b). Different phases of highly-crystalline states (globular aggregates) and amorphous states formed after the film was fabricated, as seen in the TEM image (Fig. 2b). The interchain π–π stacking distance dπ–π ∼ 3.76 Å of the crystals was extracted from the arcs that represent the (0 k 0) diffractions of the electron diffraction pattern (Fig. 2c). GIXD analysis indicates that the film has highly crystalline structures with an edge-on alignment to the substrate (Fig. 2d). The (100) peaks in the out-of-plane direction (qz = 0.374 Å−1) correspond to the d-spacing (16.80 Å) of the films (Fig. 2e), and the (010) peaks at qxy = 1.649 Å-1 correspond to a π–π distance of 3.81 Å (Fig. 2f, eqn (S2), ESI†). This distance is very close to the distance (3.8 Å) between the two sulfur atoms in the thiophene backbones; this similarity indicates that the 2D P3HT films have close π–π stacking.20,23 UV-visible absorption spectra (normalized absorbance at 560 nm) show that the 2D P3HT films have low-energy features at ∼560 nm and ∼605 nm, which are associated with the vibrionic bands of (0–1) and (0–0) transitions, respectively (Fig. 2g). By calculating the free exciton bandwidth of the P3HT ultrathin film, we conclude that the 2D film has a greater extent of conjugation and higher order of molecular packing (eqn (S3), ESI†) than the P3HT bulk film.20,24 XPS confirmed the existence of sulfur in the 2D P3HT film (Fig. S4, ESI†).
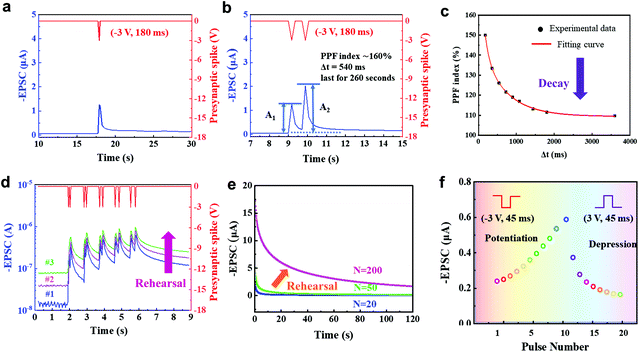
In biological synapses, EPSC occurs when a stimulus arrives at the presynaptic membrane and causes the release of neurotransmitters within the synaptic cleft, then, receptors open to permit ions to pass through the membrane. This synaptic transistor shows a similar EPSC response (Fig. 3a). A presynaptic spike (−3 V, 180 ms) was applied to the ion gel, then EPSC was recorded at a constant driving voltage of −0.1 V (VDS = −0.1 V). The EPSC reached a peak value of ∼1.26 μA, then gradually decayed back to the original current level with a relatively short relaxation time. When a negative presynaptic spike was applied, anions accumulated at the ion gel/P3HT interface or injected into the P3HT to attract additional holes in the conductive channel to form EPSC. After the spike, the distribution of anions gradually returns to random, and the EPSC decays. The energy consumption of a synaptic event is 22.68 nJ. This process is dominated by AMPA receptors in the excitatory neuron. The peak and resting currents triggered by the gate pulse increased with an increase in pulse voltage (Fig. S5, ESI†), where a favorable operating voltage of −3 V could be found. Synaptic transistors could effectively respond to further reduced stimuli by reducing their threshold voltage, which can be realized by doping the semiconducting layer.
Paired-pulse facilitation (PPF) is a typical form of STP. Short-term plasticity has unique advantages in spatiotemporal information filtering and real-time information interacting processes.25,26 PPF occurs when two presynaptic pulses arrive at a synapse in rapid succession, and the postsynaptic response generated by the second pulse is stronger when the second spike closely follows the first one.27 Similarly, in our 2D P3HT-based synaptic transistor, the second EPSC peak was higher than the first one when two consecutive input spikes (−3 V, 180 ms) with a pulse interval Δtpre = 540 ms were applied (Fig. 3b). The type of signals, such as EPSC and PPF, and their shapes in this work are similar to biological synapses.28,29 The PPF index of the 2D P3HT-based synaptic transistor decreased gradually as Δtpre increased (Fig. 3c). The trend can be fitted by a double-exponential function:
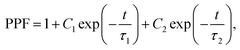 | (1) |
In biology, the formation of memory experience decay requires a repeated rehearsal process.32 The 2D P3HT-based synaptic transistor mimics this process. We performed sequential measurements with a short time interval to restart the process; the baseline, increased as the number of measurements increased, and a similar trend occurs in biological memory (Fig. 3d).
Spike-number dependent plasticity (SNDP) is a learning rule that a series of repeated external stimuli can alter the quantity of neurotransmitters released into a synapse and thereby strength the synaptic weight.33 As the number of consecutive gate pulses (−3 V) increased from 20 to 50, only a slight increase in EPSC was seen after a 20 s decay, but a significant enhancement in EPSC was observed after 200 stimuli, implying the transition from STP to EME (Fig. 3e).
Potentiation is believed to underlie learning and memory in a brain.34,35 In contrast, depression selectively weakens specific synapses in the active stage and allows encoding of new information.36,37 Potentiation triggered by ten repeated negative (−3 V, 45 ms) gate pulses and long-term depression triggered by ten repeated positive (3 V, 45 ms) gate pulses were demonstrated in our synaptic transistor (Fig. 3f). The potentiation triggered by consecutive stimuli shows good linearity (Fig. S6, ESI†).
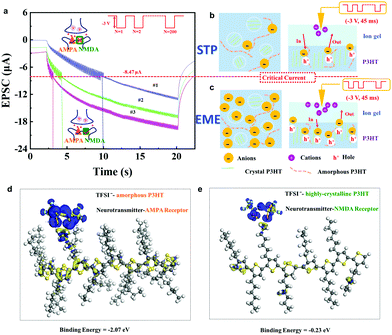
We successfully mimicked the synergistic effects of mixed receptors in a 2D P3HT synaptic transistor. When we applied 200 consecutive gate pulses (−3 V, 45 ms), the amplitude in current vibration altered when the current level was increased to 8.47 μA (Fig. 4a). These vibrations were much stronger below 8.47 μA than at above 8.47 μA. We then took repeated measurements consecutively using the same stimuli, and the same feature synaptic current value (8.47 μA) was observed. This type of critical current has not yet been reported in previous reports with electrical-double-layer (EDL) formation and electrochemical doping processes.38–40 The vibration amplitude of synaptic current correlates to the interaction between TFSI− anions and different phases in the 2D P3HT thin film (Fig. 2b and 4b, c).
The distinctively separated amorphous and highly-crystalline phases can be regarded as AMPA receptors and NMDA receptors, respectively. Both are ligand-gated ion channels and have distinctive properties in different phases of synaptic plasticity.41 According to first principles calculations, the binding energy between TFSI− and P3HT chains in the highly-crystalline region (−0.23 eV) is much lower than that between TFSI− and amorphous P3HT (−2.07 eV) (Fig. 4d, e and Fig. S10, ESI.† Simulation Methods). Therefore, TFSI− anions preferentially bind to amorphous P3HT chains (AMPA receptor), and consequently cause STP (Fig. 4a and b). This could be further confirmed by the electron density difference (Fig. 4d and e).
When the amorphous P3HT region is saturated with anions, TFSI− starts to inject into the highly-crystalline phase (NMDA receptor) with compact molecular stacking. Once injected and trapped in the compactly stacked P3HT chains, TFSI− anions do not easily move in and out. This results in an enhanced memory effect with a reduced vibration amplitude of synaptic current (Fig. 4a and c). This is similar to the biological process. When glutamates are released into the synaptic cleft, the AMPA receptor channel opens at first, while the NMDA receptor is closed due to a Mg2+ block. When the synaptic current is increased to meet the depolarization threshold, the NMDA channel is opened to trigger subsequent events, leading to the enhanced memory effect.42 In previous work, the critical current of transition from STP to EME was unclear, and the distinction between STP and EME was also unclear. However, when the 2D CP film was used in our synaptic transistor, the critical value of transition from STP to EME is shown. The design of 2D CPs with separated phases helps to distinguish STP and EME in principle and enables the precise regulation of synaptic behavior.
When we applied 400 consecutive gate pulses (−3 V, 45 ms), the postsynaptic current kept increasing without saturation (Fig. S7, ESI†). This phenomenon can be attributed to the synergistic effect of amorphous and highly-crystalline regions in the 2D P3HT film. When one receptor (amorphous state) saturates, the other receptor (highly-crystalline phase) opens to avoid saturation. Therefore, the synergistic effect of mixed receptors not only clearly distinguishes STP and EME, but also limits the saturation of postsynaptic receptors.43 Tuning the postsynaptic currents in a broad range is very important.44–49 We applied even more stimuli to find that EPSC was consecutively tuned in response to 1200 spikes (Fig. S8, ESI†).
Moreover, synaptic NMDA receptors play a pivotal role in activity-dependent synaptic plasticity. Comparing the three curves in Fig. 4a, the critical value occurs at around 10 s (blue), 4 s (green), and 3 s (pink) on the first, second and third measurements, respectively. Later measurements were affected by the previous one and this long-term facilitation condition stimulated a neuron into a state of relative excitement and activity ahead of time, and thereby increased its learning ability, i.e., a previous training process reduced the time needed to recall information. This facilitation phenomenon of EME is ‘plasticity of plasticity’, so called metaplasticity, which means that synaptic plasticity is influenced by previous experience; this phenomenon is essential in learning and memory.50
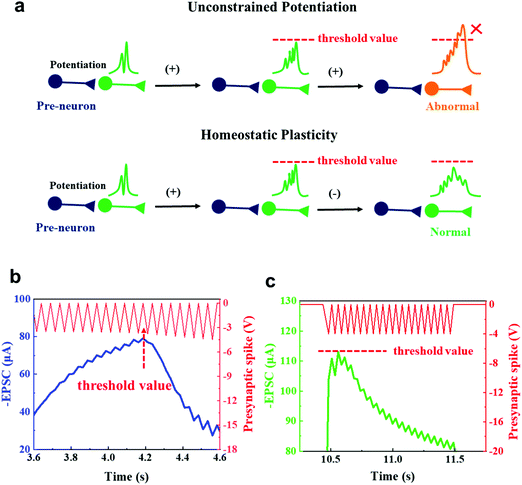
Activity-dependent neural networks are able to adapt to a fluctuant environment, and maintain a reasonable range of dynamic activity.51 Unconstrained consecutive potentiation of synaptic plasticity could result in a loss of synaptic specificity and a state away from equilibrium. Homeostatic plasticity prevents this runaway potentiation (Fig. 5a).52 Homeostatic plasticity protects neural cells by reducing the strengths of synapses once EPSC reaches an unsafe level until the firing rate returns to normal.53
This activity-dependent plasticity function was mimicked using our device. We applied gradually increasing presynaptic spikes from −3.5 V to −4.5 V to the presynaptic terminal (Fig. 5b). At first, the postsynaptic currents increased as the amplitude of the presynaptic spike increased, but after the amplitude exceeded −3.9 V, the postsynaptic currents decreased. This threshold value of −3.9 V was observed in several measurements in which the presynaptic spikes with incremental amplitudes were applied (Fig. S9a–d, ESI†). No obvious degradation was observed, and the process was reproducible. Too many external stimuli can cause similar synaptic behavior. When we applied 20 spikes at −4 V, the postsynaptic currents first showed potentiation, then showed depression (Fig. 5c). When the applied voltage exceeds a certain threshold value, a controllable gate current leakage occurs to reduce source-drain current (ISD), avoiding damage of the conductive channel. This finding could be an important addition to previous work, which reported homeostatic plasticity with an emphasis on global control of the overall neural network behavior.54 In this work, we pay special attention to the precise regulation of the synaptic plasticity of individual synapses. The negative feedback sets an appropriate range of synaptic plasticity in response to excitation and inhibition, and allows synaptic activity to propagate through a network without either dying out or increasing uncontrollably into an epilepsy-like state.52
4. Conclusion
In conclusion, we have demonstrated a synaptic transistor based on a ‘polka dot’-structured 2D CP film with separated crystalline and amorphous phases. The two phases mimicked the mixed receptors of AMPA and NMDA on the postsynaptic membrane. Besides the basic neuronal functions, such as EPSC, PPF, potentiation and depression and SNDP, the synergistic effect of different receptors was also mimicked. The effect can distinguish STP and EME with a critical point to regulate synaptic behavior precisely, and limits the saturation of postsynaptic current. The special design also successfully emulated activity-dependent synaptic plasticity, such as metaplasticity and homeostatic plasticity, both of which are typical and important advanced forms of plasticity. Metaplasticity can train a synapse to attain an excited state more easily. Homeostatic plasticity maintains normal activity states in response to perturbation. These special synaptic behaviors contribute to the well-designed nanostructures of a 2D CP ultrathin film, and thereby provide a new path to the implementation of neuromorphic devices and systems.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported in part by Key Area R&D Program of Guangdong Province with grant No. 2018B030338001, Tianjin Science Foundation for Distinguished Young Scholars (Grant No. 19JCJQJC61000), Natural Science Foundation of Tianjin (18JCYBJC16000), Hundred Young Academic Leaders Program of Nankai University (2122018218), the Fundamental Research Funds for the Central Universities (075-63191740, 075-63191745), the National Natural Science Foundation of China (NSFC, 51573036), the Distinguished Youth Foundation of Anhui Province (1808085J03), the 111 Project (B16027), the International Cooperation Base (2016D01025), Tianjin International Joint Research and Development Center, The authors thank 9A beamlines (the Pohang Accelerator Laboratory in Korea) for providing the beam time. The authors would like to thank Dr B. Chen and Prof. Q. Zhou (Nanjing Tech University) for performing the DFT simulations of studied material properties with CASTEP.References
- M. Wang, Z. Yan, T. Wang, P. Cai, S. Gao, Y. Zeng, C. Wan, H. Wang, L. Pan, J. Yu, S. Pan, K. He, J. Lu and X. Chen, Nat. Electron., 2020 DOI:10.1038/s41928-020-0422-z.
- J. Tang, F. Yuan, X. Shen, Z. Wang, M. Rao, Y. He, Y. Sun, X. Li, W. Zhang, Y. Li, B. Gao, H. Qian, G. Bi, S. Song, J. J. Yang and H. Wu, Adv. Mater., 2019, 31, 1902761 CrossRef CAS PubMed.
- C. Wan, P. Cai, M. Wang, Y. Qian, W. Huang and X. Chen, Adv. Mater., 2020, 32, 1902434 CrossRef CAS PubMed.
- C. Wan, K. Xiao, A. Angelin, M. Antonietti and X. Chen, Adv. Intell. Syst., 2019, 1, 1900073 CrossRef.
- K. Xiao, C. Wan, L. Jiang, X. Chen and M. Antonietti, Adv. Mater., 2020, 2000218, DOI:10.1002/adma.202000218.
- Z. Lv, Y. Wang, J. Chen, J. Wang, Y. Zhou and S.-T. Han, Chem. Rev., 2020, 120, 3941–4006 CrossRef CAS PubMed.
- J. Zhang, S. Dai, Y. Zhao, J. Zhang and J. Huang, Adv. Intell. Syst., 2020, 2, 1900136 CrossRef.
- S. Nakanishi, Science, 1992, 258, 597–603 CrossRef CAS PubMed.
- M. L. Mayer, Nature, 2006, 440, 456–462 CrossRef CAS PubMed.
- D. H. Ebert and M. E. Greenberg, Nature, 2013, 493, 327 CrossRef CAS PubMed.
- V. R. Rao and S. Finkbeiner, Trends Neurosci., 2007, 30, 284–291 CrossRef CAS PubMed.
- B. Herguedas, J. García-Nafría, O. Cais, R. Fernández-Leiro, J. Krieger, H. Ho and I. H. Greger, Science, 2016, 352, aad3873 CrossRef PubMed.
- Y. Park, M. J. Park and J. S. Lee, Adv. Funct. Mater., 2018, 28, 1804123 CrossRef.
- J. Zhu, Y. Yang, R. Jia, Z. Liang, W. Zhu, Z. U. Rehman, L. Bao, X. Zhang, Y. Cai and L. Song, Adv. Mater., 2018, 30, 1800195 CrossRef PubMed.
- H. Wang, M. Yang, Q. Tang, X. Zhao, Y. Tong and Y. Liu, Adv. Funct. Mater., 2019, 29, 1901107 CrossRef.
- Y. Ren, L. Hu, J.-Y. Mao, J. Yuan, Y.-J. Zeng, S. Ruan, J.-Q. Yang, L. Zhou, Y. Zhou and S.-T. Han, J. Mater. Chem. C, 2018, 6, 9383–9393 RSC.
- S. Zhang, L. Zhou, J. Mao, Y. Ren, J. Yang, G. Yang, X. Zhu, S. Han, V. A. L. Roy and Y. Zhou, Adv. Mater. Technol., 2019, 4, 1800342 CrossRef.
- Z. Lv, Y. Zhou, S. Han and V. A. L. Roy, Mater. Today, 2018, 21, 537–552 CrossRef CAS.
- C. S. Yang, D. S. Shang, N. Liu, G. Shi, X. Shen, R. C. Yu, Y. Q. Li and Y. Sun, Adv. Mater., 2017, 29, 1700906 CrossRef PubMed.
- F. Ge, S. Wei, Z. Liu, G. Wang, X. Wang, G. Zhang, H. Lu, K. Cho and L. Qiu, ACS Appl. Mater. Interfaces, 2018, 10, 9602–9611 CrossRef CAS PubMed.
- G. G. Turrigiano and S. B. Nelson, Nat. Rev. Neurosci., 2004, 5, 97 CrossRef CAS PubMed.
- H.-C. Huang, C.-W. Huang, C.-T. Hsieh and H. Teng, J. Mater. Chem. A, 2014, 2, 14963–14972 RSC.
- P. Müller-Buschbaum, Adv. Mater., 2014, 26, 7692–7709 CrossRef PubMed.
- K. Zhao, H. U. Khan, R. Li, Y. Su and A. Amassian, Adv. Funct. Mater., 2013, 23, 6024–6035 CrossRef CAS.
- X. Li, J. Tang, Q. Zhang, B. Gao, J. J. Yang, S. Song, W. Wu, W. Zhang, P. Yao, N. Deng, L. Deng, Y. Xie, H. Qian and H. Wu, Nat. Nanotechnol., 2020 DOI:10.1038/s41565-020-0722-5.
- C. Wang, Z. Yang, S. Wang, P. Wang, C.-Y. Wang, C. Pan, B. Cheng, S.-J. Liang and F. Miao, Adv. Intell. Syst., 2020, 2, 1900103 CrossRef.
- P. P. Atluri and W. G. Regehr, J. Neurosci., 1996, 16, 5661–5671 CrossRef CAS PubMed.
- L. Groc and D. Choquet, Science, 2020, 368(6496), eaay4631, DOI:10.1126/science.aay4631.
- H. S. Venkatesh, W. Morishita, A. C. Geraghty, D. Silverbush, S. M. Gillespie, M. Arzt, L. T. Tam, C. Espenel, A. Ponnuswami, L. Ni, P. J. Woo, K. R. Taylor, A. Agarwal, A. Regev, D. Brang, H. Vogel, S. Hervey-Jumper, D. E. Bergles, M. L. Suvà, R. C. Malenka and M. Monje, Nature, 2019, 573, 539–545 CrossRef CAS PubMed.
- R. S. Zucker and W. G. Regehr, Annu. Rev. Physiol., 2002, 64, 355–405 CrossRef CAS PubMed.
- Y. H. Liu, L. Q. Zhu, P. Feng, Y. Shi and Q. Wan, Adv. Mater., 2015, 27, 5599–5604 CrossRef CAS PubMed.
- R. Atkinson and R. Shiffrin, Psychol. Learn. Motiv., 1968, 2, 89 Search PubMed.
- H. Yu, J. Gong, H. Wei, W. Huang and W. Xu, Mater. Chem. Front., 2019, 3, 941–947 RSC.
- Y. Ben-Ari, Nat. Rev. Neurosci., 2002, 3, 728 CrossRef CAS PubMed.
- Y. Feng, X. Gao, Y.-N. Zhong, J.-L. Wu, J.-L. Xu and S.-D. Wang, Adv. Intell. Syst., 2019, 1, 1900022 CrossRef.
- W. Xu, S.-Y. Min, H. Hwang and T.-W. Lee, Sci. Adv., 2016, 2, e1501326 CrossRef PubMed.
- D. V. Buonomano and W. Maass, Nat. Rev. Neurosci., 2009, 10, 113 CrossRef CAS PubMed.
- C. Qian, J. Sun, L. A. Kong, G. Gou, J. Yang, J. He, Y. Gao and Q. Wan, ACS Appl. Mater. Interfaces, 2016, 8, 26169–26175 CrossRef CAS PubMed.
- L. A. Kong, S. Jia, C. Qian, F. Ying, J. Wang, J. Yang and Y. Gao, Org. Electron., 2017, 47, 126–132 CrossRef CAS.
- H. Han, H. Yu, H. Wei, J. Gong and W. Xu, Small, 2019, 15, 1900695 CrossRef PubMed.
- V. M. Ho, J.-A. Lee and K. C. J. S. Martin, Science, 2011, 334, 623–628 CrossRef CAS PubMed.
- R. A. Nicoll, J. A. Kauer and R. C. Malenka, Neuron, 1988, 1, 97–103 CrossRef CAS PubMed.
- W. C. Abraham and W. P. Tate, Prog. Neurobiol., 1997, 52, 303–323 CrossRef CAS PubMed.
- Y. V. D. Burgt, A. Melianas, S. T. Keene, G. Malliaras and A. Salleo, Nat. Electron., 2018, 1, 386–397 CrossRef.
- Y. van de Burgt, E. Lubberman, E. J. Fuller, S. T. Keene, G. C. Faria, S. Agarwal, M. J. Marinella, A. Alec Talin and A. Salleo, Nat. Mater., 2017, 16, 414–418 CrossRef CAS PubMed.
- C. Du, W. Ma, T. Chang, P. Sheridan and W. D. Lu, Adv. Funct. Mater., 2015, 25, 4290–4299 CrossRef CAS.
- W. Tian-Yu, H. Zhen-Yu, L. Hao, C. Lin and Q.-Q. Zhu, ACS Appl. Mater. Interfaces, 2018, 10, 37345–37352 CrossRef PubMed.
- N. Gong, T. Idé, S. Kim, I. Boybat, A. Sebastian, V. Narayanan and T. Ando, Nat. Commun., 2018, 9, 2102 CrossRef CAS.
- Y. Ren, C.-L. Chang, L.-Y. Ting, L. Zhou, J.-Y. Mao, S.-R. Zhang, H.-H. Chou, J.-Q. Yang, Y. Zhou and S.-T. Han, Adv. Intell. Syst., 2019, 1, 1900008 CrossRef.
- W. C. Abraham and M. F. Bear, Trends Neurosci., 1996, 19, 126–130 CrossRef CAS.
- Y. Humeau and D. Choquet, Nat. Neurosci., 2019, 22, 1536–1543 CrossRef CAS PubMed.
- G. G. Turrigiano, Cell, 2008, 135, 422–435 CrossRef CAS.
- L. F. Abbott and S. B. Nelson, Nat. Neurosci., 2000, 3, 1178–1183 CrossRef CAS PubMed.
- D. A. Koutsouras, G. G. Malliaras and P. Gkoupidenis, MRS Commun., 2018, 8, 493–497 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0nh00348d |
| This journal is © The Royal Society of Chemistry 2020 |