Cyanostilbene-based vapo-fluorochromic supramolecular assemblies for reversible 3D code encryption†
Zhao
Gao
ab,
Ze
Chen
a,
Yifei
Han
a and
Feng
Wang
 *a
*a
aCAS Key Laboratory of Soft Matter Chemistry, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Department of Polymer Science and Engineering, University of Science and Technology of China, Hefei, Anhui 230026, P. R. China. E-mail: drfwang@ustc.edu.cn
bShaanxi Key Laboratory of Macromolecular Science and Technology, MOE Key Laboratory of Material Physics and Chemistry under Extraordinary Conditions, School of Chemistry and Chemical Engineering, Northwestern Polytechnical University, Xi’an 710072, P. R. China
First published on 11th May 2020
Abstract
Scanning codes with the capability for stimuli-triggered decryption are urgently needed to prevent information leakage and counterfeiting. Compared to conventional 1D barcodes and 2D QR codes, 3D codes show promise in this field thanks to the presence of four different colors in the icon, with great information variability. Up to now, encrypted 3D code development has primarily focused on chemical reaction-based systems, leading to information decryption in an irreversible transformation manner. Herein, a novel and intelligent 3D code encryption system has been constructed with full reversibility and a fast response, taking advantage of the luminescent vapochromism of cyanostilbene-based supramolecular assemblies. Information in the inkjet-printed 3D code is specifically decrypted through vapor fuming with chlorinated solvents, while it is reversibly encrypted upon removing the vapor. Hence, this study provides a novel and effective strategy for obtaining high-performance smart scanning codes.
New conceptsScanning codes are generally identified in an automatic manner, and can be easily mimicked by counterfeiters. To realize privacy protection, it is of interest to develop smart scanning codes, giving access to information decryption under specific conditions. 3D codes show promising prospects in this field thanks to the presence of four different colors in the icon, with greater information variability. Until now, encrypted 3D codes have been limited to chemical reaction-based systems, giving rise to information decryption in an irreversible transformation manner. To increase practical applicability, high reversibility and a fast stimulus response should be obtained during the encryption/decryption processes. To attain this objective, herein a novel type of cyanostilbene-based assembly has been developed for rapid and reversible 3D code encryption/decryption on the basis of the following two considerations. First, supramolecular assemblies with excellent processability facilitate the inkjet-printing of 3D codes on paper for daily usage. Second, vapor-triggered inter-conversion takes place between the self-assembled and monomeric states, giving rise to discernable emission signal changes. Accordingly, information encoded in the 3D code can be specifically decrypted through vapor fuming, while it is reversibly encrypted upon being placed in air. Hence, this provides an effective strategy for obtaining high-performance scanning codes with security reliability and ease of operation. |
Introduction
Scanning codes are indispensable in modern society in the fields of warehousing, manufacturing, retailing, etc.1–3 In general, the codes are visible under either ambient or UV light, enabling the automatic identification of the encoded information. Such direct read-out processes result in inevitable issues regarding information security and privacy protection, in light of the easy mimicking of scanning codes by counterfeiters. For this reason, it is useful to develop “smart” scanning codes, allowing information decryption under specific external conditions.4–11 Among the various scanning code techniques (1D barcodes, 2D QR codes, and 3D codes), 3D codes are especially suitable for achieving this goal because of the existence of four colors (red, green, blue, and black) in a 5-by-5 square cell.12 Accordingly, 3D codes possess greater information variability than their 1D and 2D counterparts, and they can be easily modulated via varying any one of the 25 subset arrays. In recent years, pioneering research by Sessler and Tang has stimulated the development of 3D code encryption materials.13–17 Despite the great progress achieved, the vast majority of encrypted 3D codes are limited to chemical reaction-based systems. Their irreversible character leads to information decryption in a single-transformation-based manner. To increase the practical applicability, good reversibility together with fast stimuli responsivity should be obtained during the encryption/decryption operation processes.To attain this objective, the use of supramolecular assemblies of π-conjugated fluorochromic molecules, such as cyanostilbenes, can be regarded as a feasible strategy.18–20 Aggregation-induced planarization occurs during the self-assembly process,21–24 accompanied by excitonic coupling between neighboring cyanostilbenes. Accordingly, discernable emission signal changes exist between the monomeric and self-assembled states, which can be efficiently modulated via external stimuli.25–28 In addition, emission colors can be further tuned via substitution variations in the π-conjugated sketelon.29,30 Benefiting from all these advantages, cyanostilbene-based supramolecular assemblies meet the color variability and reproducibility criteria for use in 3D code icons.
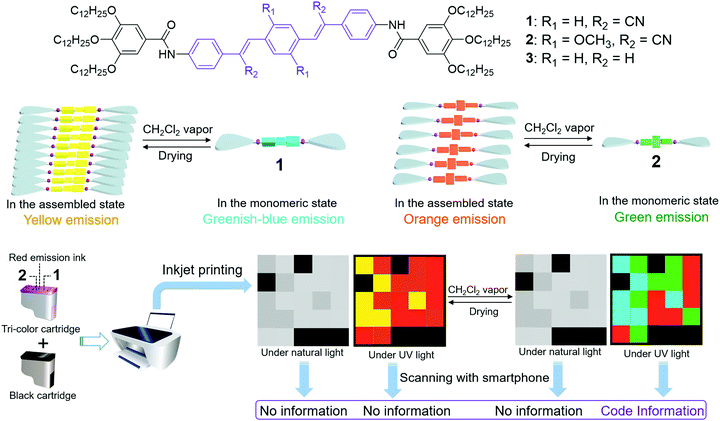
Based on this, herein we sought to develop cyanostilbene-based supramolecular assemblies for rapid and reversible 3D code encryption/decryption. The design principle of the dicyanodistyrylbenzene compounds 1–2 (Scheme 1) relies on the following two considerations. First, both compounds are prone to forming ordered supramolecular assemblies with excellent processability in apolar media, which is ascribed to the synergistic participation of hydrogen bonding, π–π stacking, and van der Waals interactions.31–35 They can be employed as ink formulators, facilitating inkjet printing on non-fluorescent paper for daily usage.36,37 Second, reversible conversion takes place between the self-assembled and monomeric states via the successive sorption and removal of chlorinated vapor.38–42 Accordingly, the emission colors can be tailored in a broad-spectrum range (1: between greenish-blue and yellow; and 2: between green and red). When 1, 2 and commercial inks (black and red) are co-deposited into the cartridge, an encrypted 3D code array is printed into a 5-by-5 square cell on paper (Scheme 1). Upon vapor exposure, four different fluorescent colors emerge with the right coordinates. This can be scanned as a ColorZip matrix for information decoding, while it is reversibly encrypted upon placing in air. Hence, the current study provides an effective strategy for the design of intelligent 3D codes with reliable security, a fast response, and full reversibility.
 | ||
| Scheme 1 A schematic representation of the use of cyanostilbene-based supramolecular assemblies derived from 1–2 in 3D code encryption. | ||
Results and discussion
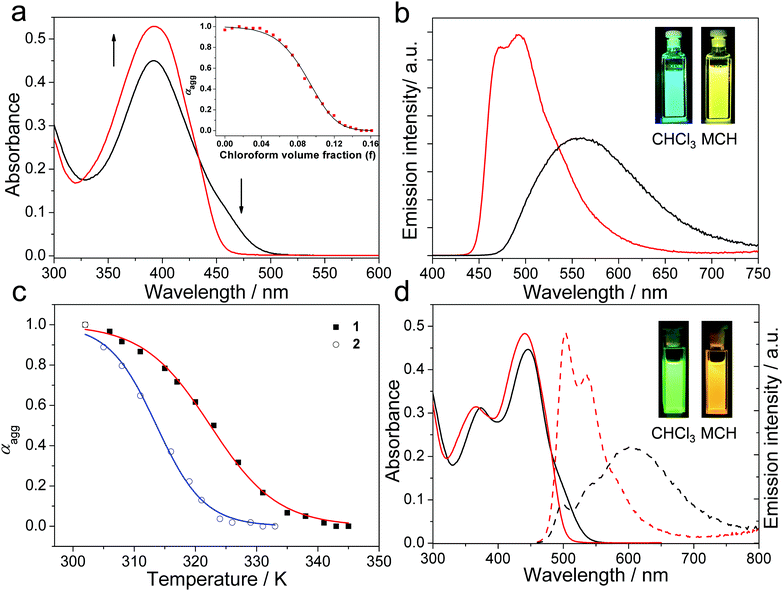
As a first step, the supramolecular assembly behavior of compound 1 is studied. In dilute CHCl3 (c = 1.00 × 10−5 M), it shows a broad absorption band centered at 392 nm (Fig. 1a). With reference to the previous literature, this band is characteristic of the π–π* transition of the dicyanodistyrylbenzene moiety in a molecularly dissolved state.43,44 Upon switching the solvent from CHCl3 to MCH (methylcyclohexane), a shoulder band emerges for 1 between 450 and 510 nm (Fig. 1a). This is characteristic of the aggregation-induced planarization of the dicyanodistyrylbenzene moiety in an apolar medium.18 The supramolecular aggregation of 1 can be further demonstrated through emission spectra experiments. In detail, the maximum emission peak of 1 is red-shifted 66 nm (λmax = 493 nm in CHCl3versus 559 nm in MCH, Fig. 1b and Table 1). Simultaneously, the fluorescence emission color of 1 exhibits a drastic change from greenish-blue to yellow (Fig. 1b, inset).| Compound | Medium | Emission λmax (nm) [τF (ns)] | Φ F | T m (K) | σ | ΔG°![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (kJ mol−1) b (kJ mol−1) |
ΔG°![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (kJ mol−1) c (kJ mol−1) |
|---|---|---|---|---|---|---|---|
| a Luminescence quantum yield measured at 293 K using rhodamine B in ethanol solution as the reference (excitation wavelength: 365 nm, ΦF = 0.69). b Obtained from the solvent-dependent mathematical fitting of UV-vis melting curves. c Calculated from modified van’t Hoff plots at 293 K. c = 1.00 × 10−5 M for both 1 and 2. | |||||||
| 1 | CHCl3 | 472, 493 (1.36) | 0.59 | 322.4 | 1 | −44.0 | −40.6 |
| MCH | 559 (5.29) | 0.33 | |||||
| 2 | CHCl3 | 504, 536 (1.58) | 0.39 | 313.6 | 1 | −36.1 | −35.8 |
| MCH | 499, 545, 603 (19.8) | 0.17 | |||||
Upon varying the CHCl3/MCH volume ratio, the absorption spectra of 1 display two isosbestic points at 327 and 434 nm (Fig. S2, ESI†), suggesting a reversible transition between self-assembled and monomeric states. A sigmoidal curve is obtained via plotting the fraction of aggregated species (αagg) against the CHCl3 volume fraction (f) at 470 nm (Fig. 1a, inset, and Fig. S2, ESI†). The curve can be nicely fitted to a previously reported solvent-dependent mathematical model (eqn (S1)–(S4), ESI†).45 Accordingly, the cooperativity parameter (σ) is determined to be 1, demonstrating the involvement of an isodesmic mechanism46,47 in the self-assembly process of 1. Moreover, the self-assembly thermodynamics of 1 can be also elucidated via temperature-dependent UV-vis measurements. A sigmoidal melting curve is acquired via plotting αagg against temperature in pure MCH (Fig. 1c and Fig. S3, ESI†). Non-linear fitting of the melting curve provides a ΔH value of −174.1 kJ mol−1, together with a Tm (melting temperature at which αagg is 0.5) value of 322.4 K (Table S2, ESI†). According to the modified Van’t Hoff plot, the ΔG° (Gibbs free energy) value of the self-assembly process of 1 is calculated to be −40.6 kJ mol−1 at 293 K (Fig. S6, ESI†), which is consistent with the value obtained via solvent-dependent experiments (−44.0 kJ mol−1, Table S1, ESI†).
Interestingly, the steric cyano groups on 1 have a crucial impact on the self-assembly mechanism. This conclusion can be manifested via performing self-assembly experiments using the counterpart compound 3 (Scheme 1). This results in a non-sigmoidal melting curve, denoting the adoption of a nucleation–elongation cooperative mechanism (Fig. S7, ESI†).48–52 The discrepancy between the self-assembly mechanisms of 1 and 3 gives rise to different morphologies. Specifically, 1 is prone to self-assemble into short nanofibers that are approximately 500 nm in length. No gelation can be visualized in concentrated MCH solution (Fig. S8, ESI†). The phenomenon is in stark contrast to the behavior of 3, which tends to form long nanofibers (several tens of micrometers in length) with strong gelation capabilities (critical gelation concentration: ∼8 mM in MCH, Fig. S10, ESI†).
On this basis, the self-assembly behaviors of 1 and 2 are compared. As expected, 2 is dominated by the monomeric state in dilute CHCl3, while self-assembly takes place in apolar MCH. The conclusion can be evidenced via the emergence of a UV-vis shoulder band (λ = 490–551 nm), as well as the red-shifting of the emission signal (λmax: 504 nm in CHCl3versus 603 nm in MCH) in the self-assembled state (Fig. 1d). Notably, both the π–π* absorption and emission transitions of 2 show bathochromic shifts compared to 1 (λmax in MCH: 441 nm versus 392 nm for the absorption signals; 603 nm versus 559 nm for the emission signals). This is due to the presence of two OCH3 groups in the structure of 2.29,30 Accordingly, the highest occupied molecular orbital (HOMO)–lowest unoccupied molecular orbital (LUMO) energy gap of 2 is narrower than that of 1 (2.72 eV for 2versus 2.92 eV for 1, Fig. S11, ESI†).
By performing solvent- and temperature-dependent UV-vis experiments, it is apparent that 2 also adopts an isodesmic assembly mechanism in MCH (Fig. S4 and S5, ESI†). Nevertheless, the thermodynamic parameters for the supramolecular assembly processes are different between 1 and 2 (Table 1). Briefly, 2 shows a lower Tm value than 1 under the same conditions (313.6 K versus 322.4 K). In addition, the ΔG° value of 2 is lower than that of 1 based on modified van’t Hoff plots at 293 K (−35.8 kJ mol−1 for 2versus −40.6 kJ mol−1 for 1, Fig. S6, ESI†). Based on density functional theory (DFT) calculations at the B3LYP/6-31G(d) level (Fig. S12, ESI†), it is rationalized that the two OCH3 moieties on 2 give the dicyanodistyrylbenzene unit a distorted conformation, thereby restricting close packing in the self-assembled structure.
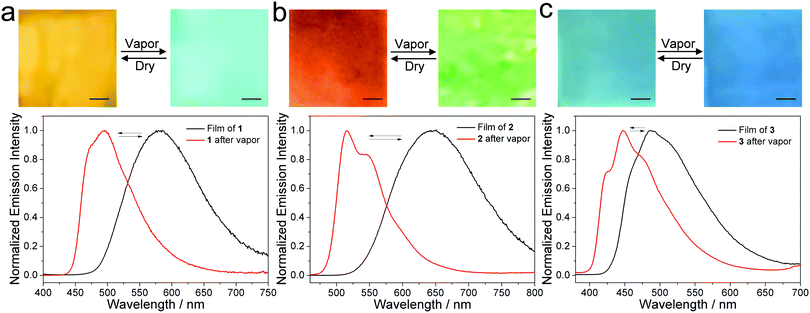
As discussed above, supramolecular assembly/disassembly induces dramatic emission color changes in both 1 and 2. Benefiting from this, these two compounds can be exploited as vapo-fluorochromic materials.38–42 In detail, films are prepared via drop-casting an MCH solution of 1 or 2 onto a glass slide. These are exposed to a series of solvent vapors, including hexane, diethylether, dichloromethane, chloroform, acetic ether, 2-propanol, acetone, ethanol, methanol, acetonitrile, and water. Films of 1 only respond to dichloromethane and chloroform vapor, leading to a prompt emission color change from yellow to greenish-blue (Fig. S13, ESI†). Simultaneously, the maximum emission peak of 1 is blue-shifted from 580 to 494 nm after exposure to dichloromethane vapor. This result confirms the conversion from a self-assembled to a molecularly dissolved state (Fig. 2a, and Video 1, ESI†). After placing the film in air, the original yellow color is quickly restored. In terms of 2, luminescent vapochromic behavior exists upon exposure to and the removal of chlorinated vapor, between red (λmax = 647 nm) and green (λmax = 515 nm) colors (Fig. 2b, and Video 2, ESI†). In stark contrast, no discernable emission color change occurs for films of 3 under the same conditions (Fig. 2c). Such results emphasize the importance of cyano groups for vapo-fluorochromic behavior.
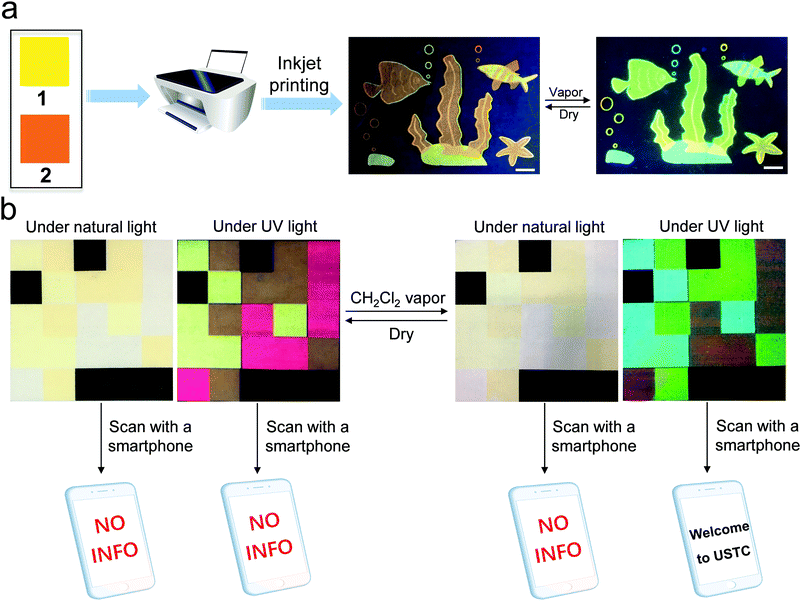
Considering that 1 and 2 provide four emission colors (red, yellow, green, and greenish-blue) between the assembled and disassembled states, a multicolor vapo-fluorochromic pattern can be fabricated via combining the two compounds. For example, MCH solutions of 1 and 2 (1.00 × 10−3 M) are separately used to fill ink cartridges. A colorful “sea world” pattern is inkjet-printed (Fig. 3a) onto non-fluorescent paper, which is invisible under natural light yet discernable under UV light. When the sea world pattern is fumed with dichloromethane vapor, the fluorescent color quickly changes. Hence, it is evident that the vapo-fluorochromism behavior of 1 and 2 is maintained on an inkjet-printed substrate, without mutual interference.
On this basis, we turned to exploring their potential application in 3D code encryption materials. Briefly, 1, 2 and commercial red emissive ink are loaded separately into a tri-color inkjet cartridge. In combination with a black ink cartridge, a 3D code icon is inkjet-printed into a 5-by-5 square cell on paper. It is in the encrypted state, as the icon fails to be recognized by the application software COLORCODE® downloaded onto a smartphone under both ambient and UV light conditions (Fig. 3b). This phenomenon is highly plausible according to 3D code theory,12 since the encoded information can only be read when the given colors appear at the right coordinates. When the paper is exposed to dichloromethane vapor for 20 s, the emission colors of 1 and 2 vary from yellow to greenish-blue and form red to green, respectively (Fig. 3b). Simultaneously, the information can be decoded by a smartphone (the words “Welcome to USTC”) under UV light. The 3D code pattern can be reversibly decrypted and encrypted over multiple cycles upon fuming and drying the paper in a successive manner. Notably, the inkjet-printed 3D code shows suitable stability under ambient conditions, as evidenced by the unchanging emission color over a period of at least two months (Fig. S14, ESI†).
Conclusions
In summary, herein it is demonstrated that the supramolecular assembly of the cyanostilbenes 1 and 2 proceeds via an isodesmic mechanism, giving rise to high contrast between the emission colors in the monomeric and assembled states. As a result of the broad-spectrum emission color variations of 1 and 2, an intelligent 3D code has been successfully fabricated via an inkjet printing technique. The information encoded in the 3D code is decrypted through vapor fuming with chlorinated solvents, while it is reversibly encrypted upon removing the vapor. Hence, cyanostilbene-based vapo-fluorochromic assemblies open up a new avenue for obtaining materials with reversible information protection properties, security reliability, and ease of operation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21922110, 21871245, 21674106), the Fundamental Research Funds for the Central Universities (WK3450000004, WK3450000005, G2019KY05111), and the Natural Science Basic Research Plan in Shaanxi Province of China (2020JQ-134).References
- O. Graydon, Nat. Photonics, 2013, 7, 343 Search PubMed.
- J. M. Meruga, C. Fountain, J. Kellar, G. Crawford, A. Baride, P. S. May, W. Cross and R. Hoover, Int. J. Comput. Appl., 2015, 37, 17 Search PubMed.
- O. Lustgarten, R. Carmieli, L. Motiei and D. Margulies, Angew. Chem., Int. Ed., 2019, 58, 184 CrossRef CAS PubMed.
- R. Klajn, P. J. Wesson, K. J. M. Bishop and B. A. Grzybowski, Angew. Chem., Int. Ed., 2009, 48, 7035 CrossRef CAS PubMed.
- X. Hou, C. Ke, C. J. Bruns, P. R. McGonigal, R. B. Pettman and J. F. Stoddart, Nat. Commun., 2015, 6, 6884 CrossRef PubMed.
- D. Samanta, D. Galaktionova, J. Gemen, L. J. W. Shimon, Y. Diskin-Posner, L. Avram, P. Král and R. Klajn, Nat. Commun., 2018, 9, 641 CrossRef PubMed.
- Y. Zhang, H. Yang, H. Ma, G. Bian, Q. Zang, J. Sun, C. Zhang, Z. An and W.-Y. Wong, Angew. Chem., Int. Ed., 2019, 58, 8773 CrossRef CAS PubMed.
- B. Yoon, J. Lee, I. S. Park, S. Jeon, J. Lee and J.-M. Kim, J. Mater. Chem. C, 2013, 1, 2388 RSC.
- V. K. Praveen, B. Vedhanarayanan, A. Mal, R. K. Mishra and A. Ajayaghosh, Acc. Chem. Res., 2020, 53, 496 CrossRef CAS PubMed.
- Y. Zhang, X. Le, Y. Jian, W. Lu, J. Zhang and T. Chen, Adv. Funct. Mater., 2019, 29, 1905514 CrossRef CAS.
- P. Li, D. Zhang, Y. Zhang, W. Lu, J. Zhang, W. Wang, Q. He, P. Theato and T. Chen, ACS Macro Lett., 2019, 8, 937 CrossRef CAS.
- D. H. Park, C. J. Han, Y. G. Shul and J. H. Choy, Sci. Rep., 2014, 4, 4879 CrossRef CAS PubMed.
- X. Ji, R.-T. Wu, L. Long, X.-S. Ke, C. Guo, Y.-J. Ghang, V. M. Lynch, F. Huang and J. L. Sessler, Adv. Mater., 2018, 1705480 CrossRef PubMed.
- X. Ji, W. Chen, L. Long, F. Huang and J. L. Sessler, Chem. Sci., 2018, 9, 7746 RSC.
- X. Ji, Z. Li, X. Liu, H.-Q. Peng, F. Song, J. Qi, J. W. Y. Lam, L. Long, J. L. Sessler and B. Z. Tang, Adv. Mater., 2019, 1902365 CrossRef CAS PubMed.
- Z. Li, H. Chen, B. Li, Y. Xie, X. Gong, X. Liu, H. Li and Y. Zhao, Adv. Sci., 2019, 6, 1901529 CrossRef CAS PubMed.
- Y.-D. Yang, X. Ji, Z.-H. Lu, J. Yang, C. Gao, H. Zhang, B. Z. Tang, J. L. Sessler and H.-Y. Gong, Nat. Commun., 2020, 11, 77 CrossRef CAS PubMed.
- B.-K. An, J. Gierschner and S. Y. Park, Acc. Chem. Res., 2012, 45, 544 CrossRef CAS PubMed.
- Y. Sagara, S. Yamane, M. Mitani, C. Weder and T. Kato, Adv. Mater., 2016, 28, 1073 CrossRef CAS PubMed.
- M. Martínez-Abadía, R. Giménez and M. B. Ros, Adv. Mater., 2018, 30, 1704161 CrossRef PubMed.
- J. Gierschner and S. Y. Park, J. Mater. Chem. C, 2013, 1, 5818 RSC.
- J. Shi, L. E. A. Suarez, S.-J. Yoon, S. Varghese, C. Serpa, S. Y. Park, L. Lüer, D. Roca-Sanjuán, B. Milián-Medina and J. Gierschner, J. Phys. Chem. C, 2017, 121, 23166 CrossRef CAS.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718 CrossRef CAS PubMed.
- J. Li, J. Wang, H. Li, N. Song, D. Wang and B. Z. Tang, Chem. Soc. Rev., 2020, 49, 1144 RSC.
- H.-J. Kim, D. R. Whang, J. Gierschner, C. H. Lee and S. Y. Park, Angew. Chem., Int. Ed., 2015, 54, 4330 CrossRef CAS PubMed.
- S. J. Wezenberg, C. M. Croisetu, M. C. A. Stuart and B. L. Feringa, Chem. Sci., 2016, 7, 4341 RSC.
- X. Wang, Z. Gao, J. Zhu, Z. Gao and F. Wang, Polym. Chem., 2016, 7, 5217 RSC.
- A. Lavrenova, D. W. R. Balkenende, Y. Sagara, S. Schrettl, Y. C. Simon and C. Weder, J. Am. Chem. Soc., 2017, 139, 4302 CrossRef CAS PubMed.
- B. K. An, S. H. Gihm, J. W. Chung, C. R. Park, S. K. Kwon and S. Y. Park, J. Am. Chem. Soc., 2009, 131, 3950 CrossRef CAS PubMed.
- J. Han, J. You, X. Li, P. Duan and M. Liu, Adv. Mater., 2017, 29, 1606503 CrossRef PubMed.
- X. Wang, Y. Han, Y. Liu, G. Zou, Z. Gao and F. Wang, Angew. Chem., Int. Ed., 2017, 56, 12466 CrossRef CAS PubMed.
- J. Matern, Y. Dorca, L. Sánchez and G. Fernández, Angew. Chem., Int. Ed., 2019, 58, 16730 CrossRef CAS PubMed.
- A. Jain, S. Dhiman, A. Dhayani, P. K. Vemula and S. J. George, Nat. Commun., 2019, 10, 450 CrossRef CAS PubMed.
- Z.-C. Gao, C.-P. Wei, Y.-F. Han, M. Yuan, X.-Z. Yan and F. Wang, Chinese J. Polym. Sci., 2018, 36, 399 CrossRef CAS.
- Z.-S. Yang, Y.-K. Tian, Z.-J. Li, L. Ao, Z.-C. Gao and F. Wang, Acta Polym. Sin., 2017, 1, 121 Search PubMed.
- L. R. Hart, J. L. Harries, B. W. Greenland, H. M. Colquhoun and W. Hayes, ACS Appl. Mater. Interfaces, 2015, 7, 8906 CrossRef CAS PubMed.
- Z. Gao, Y. Han and F. Wang, Nat. Commun., 2018, 9, 3977 CrossRef PubMed.
- O. S. Wenger, Chem. Rev., 2013, 113, 3686 CrossRef CAS PubMed.
- B. Jiang, J. Zhang, J.-Q. Ma, W. Zheng, L.-J. Chen, B. Sun, C. Li, B.-W. Hu, H. Tan, X. Li and H.-B. Yang, J. Am. Chem. Soc., 2016, 138, 738 CrossRef CAS PubMed.
- Y. Li, L. Chen, Y. Ai, E. Y.-H. Hong, A. K.-W. Chan and V. W.-W. Yam, J. Am. Chem. Soc., 2017, 139, 13858 CrossRef CAS PubMed.
- Q. Li, H. Zhu and F. Huang, J. Am. Chem. Soc., 2019, 141, 13290 CrossRef CAS PubMed.
- E. Li, K. Jie, M. Liu, X. Sheng, W. Zhu and F. Huang, Chem. Soc. Rev., 2020, 49, 1517 RSC.
- J. Kunzelman, M. Kinami, B. R. Crenshaw, J. D. Protasiewicz and C. Weder, Adv. Mater., 2008, 20, 119 CrossRef CAS.
- S. Varghese, S. J. Yoon, E. M. Calzado, S. Casado, P. G. Boj, M. A. Díaz-García, R. Resel, R. Fischer, B. Milián-Medina, R. Wannemacher, S. Y. Park and J. Gierschner, Adv. Mater., 2012, 24, 6473 CrossRef CAS PubMed.
- P. A. Korevaar, C. Schaefer, T. F. A. De Greef and E. W. Meijer, J. Am. Chem. Soc., 2012, 134, 13482 CrossRef CAS PubMed.
- T. F. A. De Greef, M. M. J. Smulders, M. Wolffs, A. P. H. J. Schenning, R. P. Sijbesma and E. W. Meijer, Chem. Rev., 2009, 109, 5687 CrossRef CAS PubMed.
- Z. Li, Y. Han and F. Wang, Nat. Commun., 2019, 10, 3735 CrossRef PubMed.
- C. Kulkarni, R. Munirathinam and S. J. George, Chem. – Eur. J., 2013, 19, 11270 CrossRef CAS PubMed.
- C. Kulkarni, K. K. Bejagam, S. P. Senanayak, K. S. Narayan, S. Balasubramanian and S. J. George, J. Am. Chem. Soc., 2015, 137, 3924 CrossRef CAS PubMed.
- Y. Han, M. Liu, R. Zhong, Z. Gao, Z. Chen, M. Zhang and F. Wang, Inorg. Chem., 2019, 58, 12407 CrossRef CAS PubMed.
- C. Wang, Z. Chen, M. Liu, H. Zhong and F. Wang, Polym. Chem., 2019, 10, 3210 RSC.
- J. Chen, Z. Zhang, C. Wang, Z. Gao, Z. Gao and F. Wang, Chem. Commun., 2017, 53, 11552 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0nh00186d |
| This journal is © The Royal Society of Chemistry 2020 |