Lead-free, stable, high-efficiency (52%) blue luminescent FA3Bi2Br9 perovskite quantum dots†
Yalong
Shen
a,
Jun
Yin
 b,
Bo
Cai
a,
Ziming
Wang
a,
Yuhang
Dong
a,
Xiaobao
Xu
a and
Haibo
Zeng
b,
Bo
Cai
a,
Ziming
Wang
a,
Yuhang
Dong
a,
Xiaobao
Xu
a and
Haibo
Zeng
 *a
*a
aMIIT Key Laboratory of Advanced Display Materials and Devices, Institute of Optoelectronics & Nanomaterials, College of Materials Science and Engineering, Nanjing University of Science and Technology, Nanjing, Jiangsu 210094, China. E-mail: zeng.haibo@njust.edu.cn
bDivision of Physical Sciences and Engineering, King Abdullah University of Science and Technology, Thuwal 23955-6900, Kingdom of Saudi Arabia
First published on 6th December 2019
Abstract
Lead halide perovskites are promising candidates as next-generation emitting materials for lighting and displays due to their superior properties. However, the toxicity of lead content severely limits their practical applications. Although lead-free Sn-based and Bi-based perovskites (Cs3Bi2Br9, MA3Bi2Br9) are reported, they all suffer from low photoluminescence quantum yield (PLQY). Here, we report the synthesis of lead-free FA3Bi2Br9 perovskite quantum dots (QDs) and their optical characterization. Through a facile ligand-assisted solution process, the as-synthesized FA3Bi2Br9 QDs exhibit a bright blue emission at 437 nm with a high PLQY of 52%. As to the origins, the observed high exciton binding energy (274.6 meV), direct bandgap nature and low defect density are proposed to guarantee the exciton generation and efficient radiative recombination. Besides, the FA3Bi2Br9 QDs show a good air stability and ethanol stability. A lead-free perovskite blue light-emitting diodes (LED) was successfully fabricated by combining FA3Bi2Br9 QDs/PS composites with a UV light chip. Our results highlight the potential of lead-free perovskites for applications in light-emitting devices.
New conceptsFor the three-primary-color displays of perovskite nanocrystals (NCs), blue emission always suffers from low photoluminescence quantum yield (PLQY), lagging behind those of green and red ones severely. Simultaneously, replacement of lead with low toxicity elements in perovskites is preferred toward potential applications. In this paper, an exceptionally simple solution process is developed to synthesize a lead-free Bi-based FA3Bi2Br9 perovskite quantum dots (QDs) with ligand-assistance. The as-prepared FA3Bi2Br9 QDs exhibit a high PLQY over 50% in the blue-emitting region, and these quantum-confined QDs were measured to possess a high exciton binding energy, which ensures the generation of excitons and their high-rate recombination. The ligand passivation of the FA3Bi2Br9 QDs helps suppress the surface defects and thus enhances the PLQY. Besides, the FA3Bi2Br9 QDs were demonstrated to have a direct bandgap and a backlit LED was constructed based on them. |
Introduction
Over the past few decades, semiconductor quantum dot (QD) materials have been successfully developed due to their unique photonic and electronic properties.1–4 In particular, lead (Pb) halide perovskite QDs exhibit ultrahigh photoluminescence quantum yield (PLQY, 70–100%), wide color gamut (∼150% NTSC) and flexible bandgap tunability, enabling them to be ideal candidates as a new generation of light-emitting diodes (LEDs) for lighting and displays.5–11 The LEDs based on perovskite QDs show excellent performance and most of them were assembled with low-cost solution processes. Despite the above outstanding properties, the intrinsic toxicity of Pb-based perovskites greatly hinders their practical applications.12–14 Therefore, developing non-toxic lead-free perovskites that retain comparable photoelectric properties with lead halide perovskites has become a practically important task.In literature reports, several non-toxic metals have been proposed to replace Pb, such as tin (Sn), manganese (Mn), and bismuth (Bi).15–17 Replacing Pb2+ with divalent Sn2+ was first considered, and the achieved Sn-based perovskites can well maintain the three-dimensional (3D) perovskite structure. Unfortunately, the ASnX3 (A = Cs, MA, FA, X = Cl, Br, and I) perovskites are very sensitive to the ambient atmosphere (oxygen, moisture, and light) since Sn2+ can be easily oxidized to Sn4+. To our best knowledge, the highest reported PLQY of CsSnX3 is below 1%.18 The vacancy-ordered double perovskites Cs2SnX6 possess a good stability, but still suffer from a low PLQY.19–22 Another divalent cation Mn2+ can be doped into the lead-based perovskites but without full substitution of Pb2+ cations, and could only achieve a half PLQY value as compared to their lead-containing analogues.23,24
Bismuth (Bi) and Pb are adjacent in the same row of the periodic table, and Bi is less toxic than Pb. Bi (6s26p3) holds the same lone-pair 6s2 state as Pb (6s26p2), exhibiting close energy levels and similar electronic properties with Pb. Different from the stoichiometry in Pb-based perovskites, the Bi-based perovskites show an A3Bi2X9 configuration, and in order to keep charge balance, Bi3+ produces a layered form of the vacancy-ordered perovskite unit cell with only two-thirds of the octahedral positions fully occupied, reducing the crystal dimensionality from 3D to two-dimensions (2D).16 More recently, Tang and coworkers reported the organic–inorganic hybrid Bi-based perovskite MA3Bi2Br9 QDs with 2D electronic structure and achieved the PLQY of 12% in the blue region (423 nm), and the MA3Bi2Br9 QDs exhibited a good ethanol stability.25 Leng et al. prepared more stable all-inorganic Bi-based Cs3Bi2Br9 nanocrystals, achieving a blue emission light at 410 nm with PLQY reaching 19.4%.26 However, the PLQYs of Bi-based perovskites are still far behind Pb-based counterparts.
Herein, we report the synthesis and optical characterization of Pb-free FA3Bi2X9 (FA+ = NH2HCNH2+) perovskite QDs. Colloidal FA3Bi2Br9 QDs were prepared via a convenient solution synthesis process with ligand-assisted. The as-synthesized FA3Bi2Br9 QDs exhibit a blue emission peak located at 437 nm with a high PLQY up to 52%. This high PLQY value is among the highest ones for Pb-free perovskites in the blue-emitting region, and comparable to those of Pb-based perovskites. Two points account for this high PLQY value. First and foremost, the FA3Bi2Br9 QDs show an extremely low defect density due to the ligand surface passivation, which suppresses the non-radiative recombination channels. Second, the big exciton binding energy (Eb) of the QDs, enables efficient radiative recombination of excitons, which, in principle, boosts the PLQY. Thanks to the Br-rich component and ligand-passivation effect, the typical FA3Bi2Br9 QDs are found to possess a good air-stability and superior ethanol stability. Furthermore, we combined the FA3Bi2Br9 QDs with commercial UV light chips to construct a LED, and demonstrated their promising applications in blue light emission.
Results and discussion
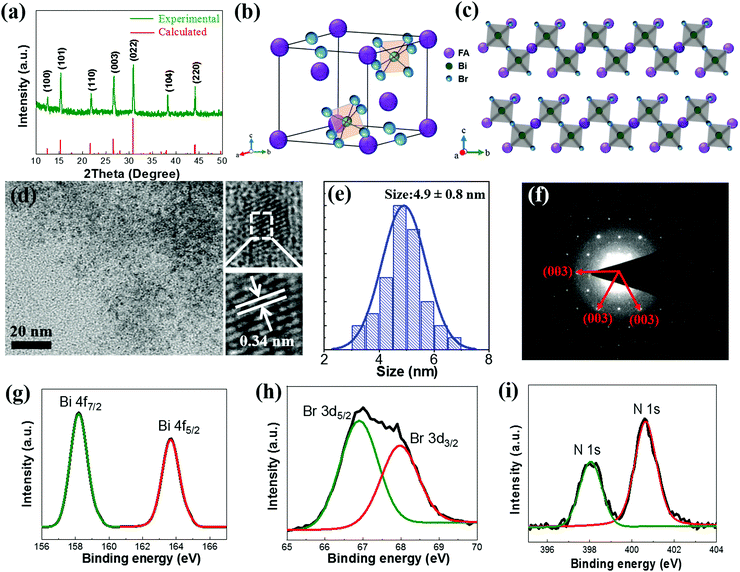
The FA3Bi2Br9 QDs were synthesized by using a facile solution process at room temperature (see the experimental details in the ESI†), and the solid FA3Bi2Br9 powder was also obtained by rotary evaporation for characterization analysis (Fig. S1, ESI†). A mixture of DMF and DMSO were used as the solvent to dissolve FABr and BiBr3 to form the precursor solution, the surface ligand oleic acids (OA) and oleylamine (OAm) was dissolved in toluene to control the crystallization and passivation of the QD surface. During the synthesis process, by adjusting the precursor concentration and the amount of ligand, the highest PL intensity was achieved by the sample, and the results are shown in Fig. S2 (ESI†). The as-prepared FA3Bi2Br9 QD solution exhibits bright blue color under UV-light irradiation, and the corresponding PLQY was measured to be as high as 52%, which is not the highest but better than most other Pb-free perovskite QDs (Table S1, ESI†).27X-ray diffraction (XRD) measurements of the FA3Bi2Br9 powder sample were conducted to study the crystal structural information, and the results are presented in Fig. 1a (a = b = 7.96 Å, c = 9.78 Å, α = β = 90°, γ = 120°), confirming that the perovskite is crystalline with the hexagonal structure and holds the trigonal P3m1 space group (No. 164), which is consistent with our calculated patterns and the previous reports on similar compounds.28 As illustrated in Fig. 1b, the crystal structure of FA3Bi2Br9 perovskite shows that one FA+ group is surrounded by six metal halide octahedral layers, the BiBr6 octahedra is fused into Bi2Br93− dimers through sharing their triangular faces, and it is derived from the 3D ABX3 perovskite by moving every third Bi layer along (111) to achieve correct charge balance, forming a quasi-2D structure (Fig. 1c). The characterization was taken by TEM and high-resolution TEM (HRTEM) measurements, as shown in Fig. 1d. According to the typical TEM images, the as-synthesized FA3Bi2Br9 QDs are polydisperse with a partially quasi-spherical shape, which exhibits an average size of 4.9 ± 0.8 nm (Fig. 1e). The HRTEM image of FA3Bi2Br9 QDs reveals a high crystallinity with the typical interplanar distance of 0.34 nm, matching well with that of the (003) plane in an FA3Bi2Br9 structure (d(003) = 0.341 nm). The selected-area electron-diffraction (SAED) pattern of an individual QD in Fig. 1f confirms the highly crystalline nature of the FA3Bi2Br9 QDs with a hexagonal diffraction pattern, again in agreement with the XRD patterns.
We further carried out X-ray photoelectron spectroscopy (XPS) measurements to evaluate the surface chemistry of the FA3Bi2Br9 perovskite. As given in Fig. S3 (ESI†), the presence of Bi and Br is clearly presented in survey XPS spectra, and the Br/Bi ratio of the QD sample is 4.9, which is higher than the ideal atomic ratio (4.5) according to the chemical formula. The achieved Br-rich FA3Bi2Br9 QDs may increase the air-stability of the perovskite under ambient conditions. The high-resolution XPS spectra of N (1s), Bi (4f), and Br (3d) are shown in Fig. 1g–i. We find that the two XPS peaks of Bi-4f at binding energy of 158.2 and 163.7 eV, respectively, are attributed to the 4f7/2 and 4f5/2 bonding of Bi3+. The Br-3d peaks can be fitted into two peaks at binding energies of 66.9 and 68.0 eV, which correspond to the 3d5/2 and 3d3/2 bonding of Br−, respectively. Two peaks at 398.1 and 400.6 eV can be found in the N 1s XPS spectrum, indicating that there are two existing chemical states of N element, one was attributed to FA+ and the other to the surface OAm+ of FA3Bi2Br9 QDs.
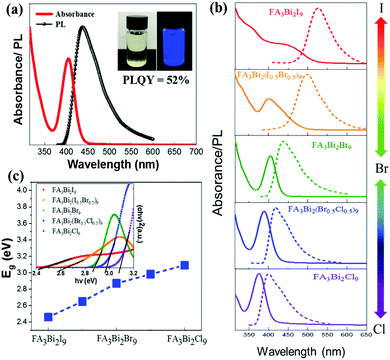
Fig. 2a shows the absorption and photoluminescence (PL) spectra of the colloidal FA3Bi2Br9 QDs solution (the insets show the photographs of the QD solution under light and UV-irradiation). The FA3Bi2Br9 QD solution shows a clear pale-yellow color under sunlight, which is a good indication of the FA3Bi2Br9 QD nucleation without any aggregation. While under UV-irradiation, a bright blue color can be clearly observed, implying outstanding PL properties of the FA3Bi2Br9 QDs. The absorption spectra of FA3Bi2Br9 QDs show a pronounced band edge exciton peak at 404 nm, and PL spectra centered at 437 nm with a broad full width at half maxima (FWHM) of 65 nm. The Stokes shift of the sample was determined to be 231 meV, which indicates almost no overlap between absorption and PL spectra.29 Such large Stokes shift renders the QDs a weak self-absorption, making them appropriate for usage as a phosphor in lighting applications. The excitation peak of the FA3Bi2Br9 QDs is located at 350 nm, as demonstrated by the excitation wavelength (Fig. S4, ESI†).
To obtain the mixed halide perovskite QDs, we have synthesized FA3Bi2X9 (X = Cl, Br, and I) QDs by controlling anion exchange reactions. Diffuse reflectance UV-Vis spectroscopy was performed and the corresponding Tauc plots are depicted in the inset of Fig. 2c. The bandgaps increase once Br atoms were partially replaced by Cl or decreased by I, leading to composition-dependent photoluminescence (the emission PL peak shifts from 399 to 526 nm, Fig. 2b). Therefore, the bandgap of FA3Bi2X9 varied from 2.46 to 3.09 eV with a change in X from Cl to I (Fig. 2c). Bandgap values were obtained by assuming that these mixed FA3Bi2X9 perovskites are direct bandgap materials, which could be verified via subsequent DFT calculations. The change in the color of samples was found to be in agreement with the trend in bandgap shift, as shown in Fig. S5 (ESI†). Furthermore, the comparison of PLQY, emission peak and FWHM between FA3Bi2Br9 QDs and the as-prepared FA3Bi2Cl9 and FA3Bi2I9 is summarized in Table S2 (ESI†).
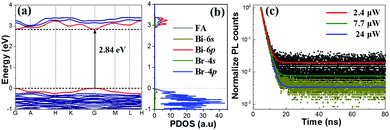
Among these QDs, FA3Bi2Br9 shows an experimental bandgap of 2.87 eV, which is consistent with the calculated value (a direct bandgap of 2.84 eV at the Γ-point, Fig. 3a). For the projected density of states (PDOS) as presented in Fig. 3b, the valence band maximum (VBM) of FA3Bi2Br9 is composed of both Br-4p and Bi-6s, and the conduction band minimum (CBM) is mostly contributed by the Bi-6p (Fig. S6, ESI†), in accordance with the properties of the direct bandgap Pb-based perovskites (the VBM is primarily composed of halide-p orbital, while the CBM has mostly Pb-p orbital character).30,31 This indicates that the absorption continuum is due to the interband electronic transitions with the main contributions coming from Bi3+(6s)Br−(4p) → Bi3+(6p) around the Γ point. Our DFT results also suggest that the lone pair s electrons (Bi-6s) are one of the prerequisites for achieving the excellent optical properties of the FA3Bi2X9 system.32 (Note that the Bi-6s shows a low occupation in the PDOS results because of the low electron ratio of Bi/Br, but it contributes greatly to the optical properties of the perovskite.)
We further conducted the time-resolved PL measurements to reveal the exciton recombination dynamics of the FA3Bi2Br9 QDs. Fig. 3c shows the PL decay of the FA3Bi2Br9 QDs under different light intensities (2.4, 7.7, 24 μW). All the PL decay curves show monoexponential decay behavior, and by fitting the PL decay curves with the monoexponential model, the corresponding PL lifetimes of 3.54, 3.51 and 3.45 ns are obtained, respectively. Upon the unchanged monoexponential PL lifetime behavior, the FA3Bi2Br9 QDs are believed to have an extremely low defect density due to the surface-passivation, which favors radiative recombination and leads to a high PLQY. Generally, the obtained short-lived lifetime can be attributed to exciton recombination, and the monoexponential fitting results give reasonably good agreement with the high PLQY of 52% of the FA3Bi2Br9 QDs.
To further explore the mechanism of photoluminescence decay in FA3Bi2Br9 QDs, the temperature-dependent PL measurements were performed. Taking into account the activation of nonradiative recombination centers in FA3Bi2Br9 perovskite, we chose the temperature range of 208–298 K to analyze the temperature-dependent PL spectra.26 As presented in Fig. S7a (ESI†), the PL intensity exhibited obvious increase with decreasing temperature. Fig. S7b (ESI†) shows the integrated PL intensity versus temperature, and the curve can be fitted using the eqn (1)
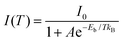 | (1) |
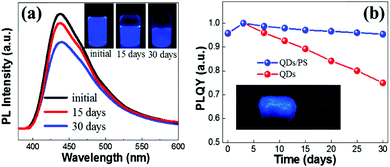
The air stability of the FA3Bi2Br9 QDs was investigated by measuring the PL intensity evolution under ambient conditions. As illustrated in Fig. 4a, after storage in the air for 30 days, we can still achieve 75% PL intensity of the QDs without any encapsulation as compared to that of the fresh sample (the insets in Fig. 4a show the photographs of QDs under UV irradiation). Based on the good air stability performance, we built an encapsulation of QDs by simply mixing the QDs with a common polymer polystyrene (PS). This one-pot encapsulation is well suitable for our FA3Bi2Br9 QDs because of their superior flexibility. The FA3Bi2Br9 QDs/PS composite was exactly demonstrated to have an excellent flexibility, as presented in the inset of Fig. 4b. Next, PLQY evolution measurements were conducted for the QDs and QDs/PS composites (Fig. 4b). Unexpectedly, in the first few days of storage, the PLQY undergoes a little degree of improvement. Such PLQY enhancement is probably due to the “photoactivation” phenomenon, benefitting from the smoothing of FA3Bi2Br9 QDs and the removal of dangling bonds or some surface defects.26 During the further storage, the PLQY of the FA3Bi2Br9 QDs/PS showed a gradual decrement due to the surface defects induced by oxygen exposure, this phenomenon was similar to that of Pb-based perovskites. After 30 days of storage, the QDs/PS composite can still keep 95% of the PLQY as compared to the 73% of the PLQY of QDs without encapsulation, implying the efficient encapsulated protection from the polymer PS. Furthermore, we investigated the stability of FA3Bi2Br9 QDs in ethanol. Interestingly, by adding 1 mL of ethanol into 1 mL of the as-prepared FA3Bi2Br9 QD solution, the PL intensity only shows a slight decrease (Fig. S8, ESI†), which demonstrates the much more stable performance than FAPbBr3 perovskite in terms of the endurance for ethanol.
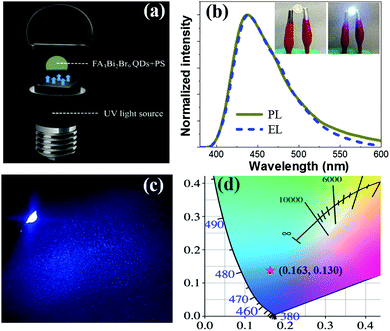
To date, there are few research studies about light-emitting diodes (LEDs) based on Pb-free halide perovskite QDs. The capability of fine emission tuning in the highly desirable wavelength of 437 nm, along with high PLQY, and good air stability, make the FA3Bi2Br9 QDs suitable for light-emitting device applications. Blue LEDs were assembled through combining UV light chips (rongcheng mic-electronics Co. Ltd, ShenZhen) and FA3Bi2Br9 QDs/PS composite (Fig. 5a). Separated high-energy UV light sources for photoexcitation were applied to emit blue emission from the FA3Bi2Br9 QDs. As a result, the as-fabricated blue LED well maintained the electroluminescence (EL) emissions at 437 nm (Fig. 5b), which barely shifts in comparison to the PL spectrum. This means that the FA3Bi2Br9 QDs keep their original electronic structure and properties under ambient voltage. Furthermore, the device well maintained the EL emissions in a wide range of operating currents from 20 to 100 mA (Fig. S9, ESI†). A maximum EQE of 3.05% was obtained, as shown in Fig. S10, ESI.† The insets of Fig. 5b present the photographs of the devices in closed and open states. Notably, bright blue color emitted from the underlying UV chips through photoluminescence by the FA3Bi2Br9 QDs are shown in Fig. 5c, indicating superior lighting effects for the FA3Bi2Br9 QDs (Table S3, ESI†). The CIE 1931 color coordinate of (0.163, 0.130) corresponding to the blue LED is shown in Fig. 5d. The satisfactory EL performance of FA3Bi2Br9 QDs and their good air-stability were expected to hold great potential in wide applications including lighting, lasing and so on.
Conclusions
In summary, an appealing solution-processed method was developed for the synthesis of well-defined Pb-free FA3Bi2Br9 QDs, and bright blue emission (437 nm), and high absolute PLQY (52%) were achieved at room temperature. The high PLQY value represents the top level among Pb-free perovskite QDs in the same wavelength region. By varying different precursor solutions, the fine-tuned FA3Bi2X9 QDs composition was obtained with the emission peak ranging from 399 to 526 nm. The FA3Bi2Br9 was proposed to have a direct bandgap of 2.84 eV by DFT, showing good agreement with the experimental data. The extremely low defect density and high Eb of the QDs, were supported to achieve ultrahigh PLQY. Furthermore, the FA3Bi2Br9 QDs exhibit a good air stability and excellent ethanol stability. To fulfill the practical applications, an LED with bright blue color was fabricated based on these QDs. This work will open a new avenue to obtain high-quality blue color perovskite QDs, and provide a good candidate for Pb-free perovskite LED applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Natural Science Foundation of Jiangsu Province (BK20180489), NSFC (61874054), Fundamental Research Funds for the Central Universities (30918011208). Most of the experiments were performed at the Materials Characterization Facility of Nanjing University of Science and Technology.References
- C. Liu, H. Peng, K. Wang, C. Wei, Z. Wang and X. Gong, Nano Energy, 2016, 30, 27–35 CrossRef CAS.
- H. Wei, Y. Fang, Y. Yuan, L. Shen and J. Huang, Adv. Mater., 2015, 27, 4975–4981 CrossRef CAS PubMed.
- Y. Wang, K. Lu, L. Han, Z. Liu, G. Shi, H. Fang, S. Chen, T. Wu, F. Yang, M. Gu, S. Zhou, X. Ling, X. Tang, J. Zheng, M. A. Loi and W. Ma, Adv. Mater., 2018, 30, 1704871 CrossRef PubMed.
- H. Sun, Z. Yang, M. Wei, W. Sun, X. Li, S. Ye, Y. Zhao, H. Tan, E. L. Kynaston, T. B. Schon, H. Yan, Z. H. Lu, G. A. Ozin, E. H. Sargent and D. S. Seferos, Adv. Mater., 2017, 29, 1701153 CrossRef PubMed.
- C. Xie, P. You, Z. Liu, L. Li and F. Yan, Light: Sci. Appl., 2017, 6, 17023 CrossRef PubMed.
- Y. Shen, C. Wei, L. Ma, S. Wang, X. Wang, X. Xu and H. Zeng, J. Mater. Chem. C, 2018, 6, 12164–12169 RSC.
- X. Li, F. Cao, D. Yu, J. Chen, Z. Sun, Y. Shen, Y. Zhu, L. Wang, Y. Wei, Y. Wu and H. Zeng, Small, 2017, 13, 1603996 CrossRef PubMed.
- Y. Wang and H. Sun, Small Methods, 2018, 2, 1700252 CrossRef.
- M. I. Saidaminov, A. L. Abdelhady, B. Murali, E. Alarousu, V. M. Burlakov, W. Peng, I. Dursun, L. Wang, Y. He, G. Maculan, A. Goriely, T. Wu, O. F. Mohammed and O. M. Bakr, Nat. Commun., 2015, 6, 7586 CrossRef PubMed.
- L. Lv, Y. Xu, H. Fang, W. Luo, F. Xu, L. Liu, B. Wang, X. Zhang, D. Yang, W. Hu and A. Dong, Nanoscale, 2016, 8, 13589–13596 RSC.
- Q. Shan, J. Song, Y. Zou, J. Li, L. Xu, J. Xue, Y. Dong, B. Han, J. Chen and H. Zeng, Small, 2017, 13, 1701770 CrossRef PubMed.
- J. Liang, C. Wang, P. Zhao, Z. Lu, Y. Ma, Z. Xu, Y. Wang, H. Zhu, Y. Hu, G. Zhu, L. Ma, T. Chen, Z. Tie, J. Liu and Z. Jin, Nanoscale, 2017, 9, 11841–11845 RSC.
- N. Yantara, S. Bhaumik, F. Yan, D. Sabba, H. A. Dewi, N. Mathews, P. P. Boix, H. V. Demir and S. Mhaisalkar, J. Phys. Chem. Lett., 2015, 6, 4360–4364 CrossRef CAS PubMed.
- F. Jiang, D. Yang, Y. Jiang, T. Liu, X. Zhao, Y. Ming, B. Luo, F. Qin, J. Fan, H. Han, L. Zhang and Y. Zhou, J. Am. Chem. Soc., 2018, 3, 1019–1027 CrossRef PubMed.
- F. Giustino and H. J. Snaith, ACS Energy Lett., 2016, 1, 1233–1240 CrossRef CAS.
- J. Sun, J. Yang, J. I. Lee, J. H. Cho and M. S. Kang, J. Phys. Chem. Lett., 2018, 9, 1573–1583 CrossRef CAS PubMed.
- A. Abate, Joule, 2017, 1, 659–664 CrossRef CAS.
- T. C. Jellicoe, J. M. Richter, H. F. J. Glass, M. Tabachnyk, R. Brady, S. E. Dutton, A. Rao, R. H. Friend, D. Credgington, N. C. Greenham and M. L. Boehm, J. Am. Chem. Soc., 2016, 138, 2941–2944 CrossRef CAS PubMed.
- X. Qiu, Y. Jiang, H. Zhang, Z. Qiu, S. Yuan, P. Wang and B. Cao, Phys. Status Solidi RRL, 2016, 10, 587–591 CrossRef CAS.
- A. Wang, X. Yan, M. Zhang, S. Sun, M. Yang, W. Shen, X. Pan, P. Wang and Z. Deng, Chem. Mater., 2016, 28, 8132–8140 CrossRef CAS.
- X. F. Qiu, B. Q. Cao, S. Yuan, X. F. Chen, Z. W. Qiu, Y. A. Jiang, Q. Ye, H. Q. Wang, H. B. Zeng, J. Liu and M. G. Kanatzidis, Sol. Energy Mater. Sol. Cells, 2017, 159, 227–234 CrossRef CAS.
- S. Gupta, T. Bendikov, G. Hodes and D. Cahen, ACS Energy Lett., 2016, 1, 1028–1033 CrossRef CAS.
- D. Parobek, B. J. Roman, Y. Dong, H. Jin, E. Lee, M. Sheldon and D. H. Son, Nano Lett., 2016, 16, 7376–7380 CrossRef CAS PubMed.
- W. J. Mir, M. Jagadeeswararao, S. Das and A. Nag, ACS Energy Lett., 2017, 2, 537–543 CrossRef CAS.
- M. Y. Leng, Z. W. Chen, Y. Yang, Z. Li, K. Zeng, K. H. Li, G. D. Niu, Y. S. He, Q. C. Zhou and J. Tang, Angew. Chem., Int. Ed., 2016, 55, 15012–15016 CrossRef CAS PubMed.
- M. Leng, Y. Yang, K. Zeng, Z. Chen, Z. Tan, S. Li, J. Li, B. Xu, D. Li, M. P. Hautzinger, Y. Fu, T. Zhai, L. Xu, G. Niu, S. Jin and J. Tang, Adv. Funct. Mater., 2018, 28, 1704446 CrossRef.
- J. Zhang, Y. Yang, H. Deng, U. Farooq, X. K. Yang, J. Khan, J. Tang and H. S. Song, ACS Nano, 2017, 11, 9294–9302 CrossRef CAS PubMed.
- B. Yang, J. Chen, F. Hong, X. Mao, K. Zheng, S. Yang, Y. Li, T. Pullerits, W. Deng and K. Han, Angew. Chem., Int. Ed., 2017, 56, 12471–12475 CrossRef CAS PubMed.
- D. Zhang, S. W. Eaton, Y. Yu, L. Dou and P. Yang, J. Am. Chem. Soc., 2015, 137, 9230–9233 CrossRef CAS PubMed.
- Q. Lin, A. Armin, R. C. R. Nagiri, P. L. Burn and P. Meredith, Nat. Photonics, 2014, 9, 106–112 CrossRef.
- M. D. Smith and H. I. Karunadasa, Acc. Chem. Res., 2018, 51, 619–627 CrossRef CAS PubMed.
- A. Walsh, D. J. Payne, R. G. Egdell and G. W. Watson, Chem. Soc. Rev., 2011, 40, 4455–4463 RSC.
- S. Kumar, J. Jagielski, N. Kallikounis, Y. H. Kim, C. Wolf, F. Jenny, T. Tian, C. J. Hofer, Y. C. Chiu, W. J. Stark, T. W. Lee and C. J. Shih, Nano Lett., 2017, 17, 5277–5284 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nh00685k |
| This journal is © The Royal Society of Chemistry 2020 |