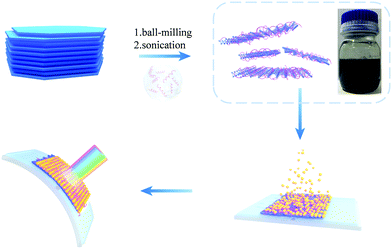
Antimonene-based flexible photodetector†
Qi
Xiao
a,
Chen-Xia
Hu
a,
Hao-Ran
Wu
a,
Yong-Yuan
Ren
 b,
Xiang-Yang
Li
a,
Qi-Qi
Yang
a,
Guan-Hua
Dun
a,
Zhi-Peng
Huang
a,
Yong
Peng
c,
Feng
Yan
b,
Xiang-Yang
Li
a,
Qi-Qi
Yang
a,
Guan-Hua
Dun
a,
Zhi-Peng
Huang
a,
Yong
Peng
c,
Feng
Yan
 b,
Qiang
Wang
b,
Qiang
Wang
 *a and
Hao-Li
Zhang
*a and
Hao-Li
Zhang
 *ad
*ad
aState Key Laboratory of Applied Organic Chemistry, Key Laboratory of Special Function Materials and Structure Design, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, 730000, P. R. China. E-mail: qiangwang@lzu.edu.cn; haoli.zhang@lzu.edu.cn
bJiangsu Key Laboratory of Advanced Functional Polymer Design and Application, Department of Polymer Science and Engineering, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123, P. R. China
cKey Laboratory of Magnetism and Magnetic Materials of MOE, Lanzhou University, Lanzhou, 730000, P. R. China
dTianjin Key Laboratory of Molecular Optoelectronic Sciences, Department of Chemistry, Collaborative Innovation Center of Chemical Science and Engineering, Tianjin University, Tianjin, 300072, P. R. China
First published on 19th August 2019
Abstract
Antimonene is an emerging semiconducting two-dimensional (2D) material with various attractive properties, but its applications in optoelectronics have rarely been explored. Herein, we report a highly efficient preparation of few-layered antimonene by a polymer ionic liquid (PIL) assisted liquid-phase exfoliation method, which gives above 20% yield of micrometer sized nanosheets. Stability studies suggested that the presence of water in the solvent significantly accelerated the decomposition of the produced antimonene, while the antimonene dispersed in dry DMF exhibited a very high stability under ambient conditions. For the first time, flexible photodetectors based on a hybrid structure of few layer antimonene nanosheets modified by PIL and CdS quantum dots (QDs) were fabricated, which showed a good responsivity of 10 μA W−1 and on/off ratio of 26.8 under the bias of 1 V. This work demonstrates that PIL assisted liquid-phase exfoliation is a very promising method to achieve highly efficient preparation, preservation, and surface modification of few-layer antimonene, and hence enable the application of antimonene-based 2D materials in optoelectronics.
New conceptsAs a new 2D material, antimonene has drawn intensive attention in recent years. However, the practical application of antimonene in the optoelectronic field has been limited by the lack of a high concentration and stable “ink” that is suitable for device fabrication. Compared with other 2D materials, antimonene has a short layer distance and strong binding energy, and hence, requires different exfoliation and surface modification methods. In this article we report the efficient fabrication and surface modification of antimonene nanosheets by polymer ionic liquid (PIL) assisted liquid exfoliation. This new method yields micron-size antimonene nanosheets over 20%, and to our best knowledge, this is the highest yield ever reported in the literature. Flexible photodetectors based on hybrid structures of surface modified few layer antimonene were successfully fabricated. Benefitting from the large-size and high stability of the PIL-modified antimonene, flexible photodetectors were fabricated and showed excellent performance. It is believed that the first antimonene-based flexible photodetector will open up the applications of antimonene materials in optoelectronics. |
Introduction
Flexible optoelectronics are attracting growing interest because of their many desirable features compared with conventional devices on rigid substrates, such as light-weight, high portability, and excellent implantability.1 A wide range of materials have been explored for the fabrication of flexible devices, including conductive polymers, nanoparticles, carbon nanotubes, graphene and quantum dots (QDs).2 Benefiting from the high carrier mobility, layer-dependent bandgap and excellent mechanical flexibility, two-dimensional (2D) materials have gained extensive attention in the field of flexible optoelectronics.3–5 Graphene is the most widely studied 2D material, but the absence of a band gap greatly limits its applications.6,7 Semiconducting transition metal dichalcogenides (TMD) exhibit preferable band gaps but it remains very challenging to achieve high carrier mobility.8–10 Black phosphorus (BP) exhibits both high carrier mobility up to 1000 cm2 V−1 s−1,11 and tunable direct band gap,12,13 but its application is still hindered by its poor stability, especially upon exposure to light, water and oxygen.14,15 To date, the development of novel 2D materials with high carrier mobility, suitable band gap and good stability that are suitable for flexible optoelectronics is still an urgent issue.Antimonene is a recently described group-VA 2D material which has a similar structure to that of BP. Theoretical studies have revealed many unique properties of antimonene for optoelectronics, including high air stability, high carrier mobility and tunable band gap with a wide range from 0–2.28 eV.16–21 Antimonene has been used for perovskite solar cells,22 electrocatalysis,23 energy storage,24–28 and cancer therapy.29 Theoretical simulation suggested that antimonene is a good candidate for photodetectors30 but this has not been experimentally verified.
Meanwhile, the practical applications are somehow hindered by the difficulty in preparing high-quality large size 2D antimonene, due to its short layer distance and strong binding energy. A few methods have been published describing successfully obtained antimonene, including mechanical exfoliation,31 liquid phase exfoliation (LPE),32 epitaxial growth,33,34 and solution-phase synthesis.35 Most of the reported methods suffer from some shortcomings, such as low yield or excessive time consumption. Recently, Wang et al. prepared antimonene with improved yield by implementing a pregrinding process before LPE, and then employed antimonene in the fabrication of perovskite solar cells.22 At present, scalable preparation of high-quality antimonene for photodetector applications remains a challenge.
In this work, we report an efficient LPE method of antimonene based on diluted polymer ionic liquid (PIL) assisted exfoliation. The stability of the antimonene in water and various organic solvents was systematically studied. Highly stable and concentrated antimonene suspensions in organic solvents were obtained, which allowed successful fabrication of antimonene-based flexible photodetectors.
Results and discussion
Scheme 1 illustrates the preparation of PIL modified antimonene nanosheets. Bulk antimony crystals were ground using a mortar to generate small sized antimony crystals, followed by ultrasonication assisted LPE. A previous study has reported that the direct liquid exfoliation of bulk antimony crystals gives a poor yield of antimonene,32 while a pregrinding process can significantly increase the yield of the following LPE process. We found that a high milling speed tended to yield very small antimonene sheets and nanoparticles in the following exfoliation step.24 A moderate grinding condition was found to be the optimal condition, which consists of 180 min milling under 500 rpm. The SEM measurements (Fig. S1, ESI†) revealed that the ground antimony materials contain uniform micro-crystals with a lateral size of about 5 μm. The antimony micro-crystals are then exfoliated in different solvents under ultrasonication with the assistance of different imidazole PILs. The PIL modified antimonene suspended in different solvents is thereafter used in the following characterization and device fabrication.PILs were used for the preparation of antimonene nanosheets, due to their unique properties, including high ionic conductivity, high thermal and chemical stability, and controllable aqueous solubility. Meanwhile, as polymers, PILs also possess multiple-charge and high molecular weight features, giving them a strong tendency to adsorb and wrap onto antimonene nanosheets.36–41 Herein, we employed DMF diluted PIL for the exfoliation of antiomnene. Two PILs were studied in this work, which were named as P([VPIm]PF6) and P([VPIm]TFSI), respectively. The synthesis of these PILs has been reported in our previous work.42 For comparison, pure DMF, 2-butanol, IPA and mixed solvent consisting of IPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (4
water (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were also studied. 2-Butanol was used as a reference as it has been previously reported to show good ability to exfoliate antimonene.22
1) were also studied. 2-Butanol was used as a reference as it has been previously reported to show good ability to exfoliate antimonene.22
For comparison, the pretreated antimony powder was exfoliated in pure DMF, IPA, IPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (4
water (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 2-butanol and the two PIL solutions with a constant concentration (1 mg mL−1) under ultrasonication. The two PILs chosen are water insoluble, and poorly soluble in 2-butanol. They were diluted with DMF to concentrations of 16.26 mg mL−1 and 19.02 mg mL−1, for P([VPIm]PF6) and P([VPIm]TFSI), respectively. For clarity, in the following discussion, the antimonene exfoliated in the DMF diluted P([VPIm]PF6), and P([VPIm]TFSI) is denoted as Sb/P([VPIm]PF6), and Sb/P([VPIm]TFSI), respectively; and the antimonene exfoliated in water and DMF is referred to as Sb/water and Sb/DMF, respectively. More experimental details, including the synthesis of the two PILs and the exfoliation process of the antimony, are available in the ESI.†
1), 2-butanol and the two PIL solutions with a constant concentration (1 mg mL−1) under ultrasonication. The two PILs chosen are water insoluble, and poorly soluble in 2-butanol. They were diluted with DMF to concentrations of 16.26 mg mL−1 and 19.02 mg mL−1, for P([VPIm]PF6) and P([VPIm]TFSI), respectively. For clarity, in the following discussion, the antimonene exfoliated in the DMF diluted P([VPIm]PF6), and P([VPIm]TFSI) is denoted as Sb/P([VPIm]PF6), and Sb/P([VPIm]TFSI), respectively; and the antimonene exfoliated in water and DMF is referred to as Sb/water and Sb/DMF, respectively. More experimental details, including the synthesis of the two PILs and the exfoliation process of the antimony, are available in the ESI.†
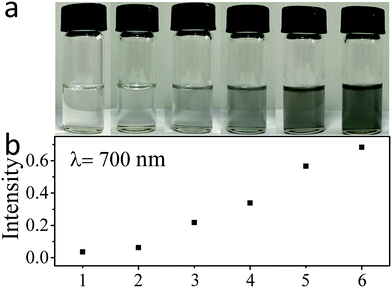
Fig. 1a shows the photographs of the antimonene suspensions formed by exfoliation in different solvents. The darker color of the exfoliated antimonene suspension indicates a more concentrated antimonene suspension. The UV-vis-NIR absorption and Raman spectra of Sb/P([VPIm]PF6) are shown in Fig. S2 (ESI†), while a comparison of their absorption intensity at 700 nm is shown in Fig. 1b. The absorbance of antimonene dispersed in pure DMF, IPA and IPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water is 0.036, 0.062 and 0.22, respectively. The phenomenon that IPA
water is 0.036, 0.062 and 0.22, respectively. The phenomenon that IPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (4
water (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixed solvent gives much higher absorbance than pure IPA is consistent with our previous reports on the liquid exfoliation of other 2D nanomaterials, which can be understood as the mixed solvent offers a better match of surface energy with the antimonene nanosheets.43 The 2-butanol gives an even higher absorbance of 0.34, consistent with previous reports that it is a good solvent to exfoliate antimonene.22 The highest absorbance values were obtained in the DMF/PILs systems, as 0.57 and 0.68 for the Sb/P([VPIm]TFSI) and Sb/P([VPIm]PF6), respectively, which are more than one order of magnitude higher than that in pure DMF and twice higher than that in 2-butanol.
1) mixed solvent gives much higher absorbance than pure IPA is consistent with our previous reports on the liquid exfoliation of other 2D nanomaterials, which can be understood as the mixed solvent offers a better match of surface energy with the antimonene nanosheets.43 The 2-butanol gives an even higher absorbance of 0.34, consistent with previous reports that it is a good solvent to exfoliate antimonene.22 The highest absorbance values were obtained in the DMF/PILs systems, as 0.57 and 0.68 for the Sb/P([VPIm]TFSI) and Sb/P([VPIm]PF6), respectively, which are more than one order of magnitude higher than that in pure DMF and twice higher than that in 2-butanol.
Quantitative measurements based on the absorbance at 700 nm (Fig. S3, ESI†) revealed a highest antimonene concentration of 0.20 mg mL−1 from the Sb/P([VPIm]PF6) suspension. The corresponding antimonene production yield with respect to the starting antimony materials can then be estimated to be above 20%. To the best of our knowledge, this is the highest yield ever reported for antimonene nanosheet production. The above results indicate that the DMF containing diluted PILs has an excellent ability to exfoliate antimonene. The excellent exfoliation ability of PILs is believed to be derived from the van der Waals force44 and strong p–π stacking45 between antimonene surfaces and the imidazole-based polymer cation. In addition, the PIL with PF6 anion shows better exfoliation ability than the PIL with TFSI anion, probably due to their different basicity, which is consistent with previous reports on ionic liquid assisted exfoliation of other 2D material.46 The Raman peaks of Sb/P([VPIm]PF6) (Fig. S2b, ESI†) display long wavenumber shifts, indicating that the bulk antimony was successfully converted into thin antimonene nanosheets32 under the PIL-assisted exfoliation conditions.
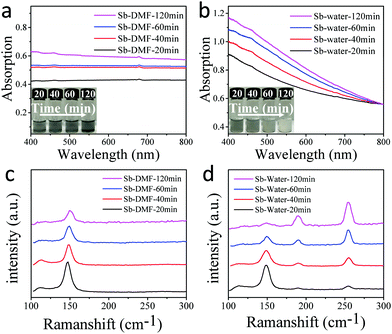
One important issue that determines the application of 2D materials is the long term stability under ambient conditions. Zamora et al. studied mechanically exfoliated antimonene and suggested that it was reasonably stable under ambient conditions or even after exposure to water containing solvents.31 However, the long term stability of antimonene upon exposure to water remains unclear. We therefore tested the stability of antimonene in DMF and water. Fig. 2a and b show the absorption spectra of the ground antimony after ultrasonication in DMF or water for 20 to 120 min. The inset is the corresponding photograph of antimonene colloids. In Fig. 2a, the absorption curves of Sb/DMF are quite smooth. The color of the colloids becomes darker upon increasing the sonication time, indicating that the antimonene colloids became more concentrated. While in Fig. 2b, as the sonication time increases, the absorption curves of Sb/water rise in the low wavelength range. As shown in the inset, the color of the colloids changed from gray to white with increase in the sonication time, which is quite different from that of the Sb/DMF. The significant absorption rise at short wavelengths suggests that a new broad band gap semiconductor has been formed during the sonication. Moreover, after being stood for 3 hours, the color of the antimonene in the DMF showed no change; in contrast, the antimonene in water completely turned white (Fig. S4, ESI†).
Fig. 2c and d are the Raman spectra of the Sb/DMF and Sb/water after sonication for different times in the air. In Fig. 2c, two peaks at approximately 110 cm−1 and 149 cm−1 are ascribed to the Eg and A1g vibration modes for antimony crystals, respectively.34 And these two peaks of Sb/DMF show a slight blue-shift and intensity reduction with the increase in the ultrasonication time, indicating that the antimonene is getting thinner.22 While in Fig. 2d, the Raman spectra of Sb/water exhibit four peaks. The peaks at 110 cm−1 and 149 cm−1 correspond to antimony, while the other two peaks at approximately 189.7 cm−1 and 255.3 cm−1 are ascribed to the F2g and Ag vibration modes of antimony trioxide,47 respectively. As the ultrasonication time increases, the peaks that are associated with antimonene decrease, and the peaks of the antimony trioxide increase gradually. After 120 minutes, the Raman peaks of antimonene nearly disappeared completely, indicating that antimonene was gradually converted to antimony trioxide. To unveil the role of oxygen in the antimonene oxidation process, a comparative ultrasonication experiment was conducted under Ar protection (Fig. S5 and S6, ESI†). Under air free conditions, the Sb/DMF was stable under ultrasonication conditions, and showed nearly no change in the spectra. While the Sb/water showed partial oxidization even under Ar protection, which may be attributed to the oxygen dissolved in water. The influence of different water content in water/DMF mixed solvent was also investigated (Fig. S7, ESI†), and the results indicate that antimonene is stable in mixed solvent as long as the water content is lower than 50%. When the water content is increased to above 60%, the suspended antimonene showed a clear trend of being gradually oxidized. The above results of the stability test provided important guidance to preserve antimonene nanosheets for optoelectronic applications.
The X-ray diffraction (XRD) patterns of bulk antimony, antimony micro-crystals after ball-milling, and antimonene are compared in Fig. 3a. The antimony powder and antimonene exhibit very similar XRD patterns, in which the peaks at 23.7° and 48.5°, corresponding to the (003) facet and the (006) facet ({001} facets) of antimony, are much weaker compared with that of the bulk antimony crystals, indicating that the size reduction of the antimony crystals is mainly along the c-axis.22,27Fig. 3b shows the high-resolution X-ray photoelectron spectroscopy (XPS) spectra of the antimony bulk. The Sb 3d5/2 and Sb 3d3/2 peaks of the antimony bulk sample can be deconvoluted into five peaks. There is a considerable percentage of oxides for bulk antimony, probably due to surface oxidization during storage and the pretreatment process. Fig. 3c is the XPS spectra of Sb/P([VPIm]PF6). Compared with that of antimony bulk, the obtained antimonene has decreased Sb0 and increased oxide (Sb2O3 and Sb2O5) content, consistent with the fact that antimonene nanosheets possess a much higher surface area.22,23,27,48 In comparison, the XPS spectra of Sb/water are displayed in Fig. 3d. The Sb0 peak is barely detected, suggesting that the surface of antimonene is almost completely oxidized in water, in agreement with the results shown by the Raman spectra in Fig. 2. All the above results indicate that we have successfully obtained antimonene nanosheets, and the use of the DMF diluted P([VPIm]PF6) assisted LPE method can prevent excessive oxidation of the antimonene flakes.
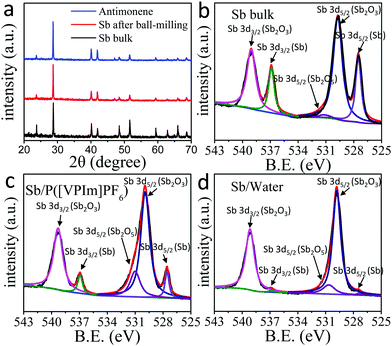 | ||
| Fig. 3 (a) X-ray diffraction (XRD) patterns of bulk antimony crystals, antimony plates after ball-milling, and Sb/P([VPIm]PF6); XPS spectra of (b) antimony bulk, (c) Sb/P([VPIm]PF6) and (d) Sb/water. | ||
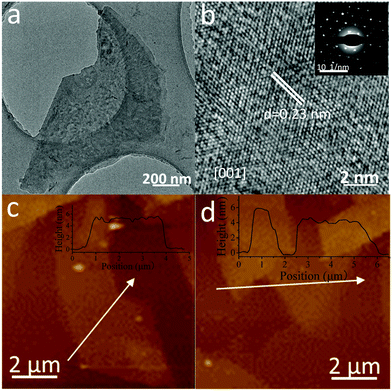
Fig. 4a is the TEM image of Sb/P([VPIm]PF6), which confirms that the obtained antimonene has a good crystal structure with a large lateral size above 1 μm. Fig. 4b shows the HRTEM image of Sb/P([VPIm]PF6) (inset shows the corresponding SAED pattern.). The derived d spacing of the (100) from the HRTEM is 0.23 nm, which is in agreement with the previous reports on antimonene.31 It can be deduced that the Sb/P([VPIm]PF6) has a rhombohedral structure (β-phase viewing along the [001] zone axis) rather than the orthorhombic structure (α-phase).34Fig. 4c and d and Fig. S8 (ESI†) show the representative AFM images of Sb/P([VPIm]PF6). To ensure all the excess PILs have been removed, the samples were washed with DMF three times prior to the AFM measurements. The lateral size of the obtained antimonene nanosheets is above the micrometer scale. The profile analysis reveals that the thickness of the Sb/P([VPIm]PF6) nanosheets varies from 2.2 to 5.1 nm, which correspond to about 6 to 14 layers, assuming that the thickness of monolayer antimonene is about 0.37 nm.31 The above results have confirmed that few layered antimonene nanosheets were successful obtained with good crystal structures and a micron-level lateral size.
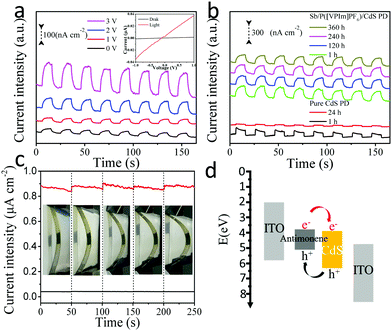
It is known that PIL can be used as a polyelectrolyte for the fabrication of various kinds of all-solid state devices.49 Herein for the first time, a flexible photodetector based on PIL modified few layer antimonene was fabricated. The structure of the device is shown in Fig. S9 (ESI†); the width of the flexible indium tin oxide/polyethylene-terephthalate (ITO/PET) substrate is about 0.4 cm, the length of which is about 2 cm. The concentrated suspension of Sb/P([VPIm]PF6) nanosheets (0.20 mg mL−1) was directly used for centrifugal casting on the ITO/PET substrate to give a uniform thin film. The Sb/P([VPIm]PF6) film is on the top of the ITO electrodes with a gap of 100 μm. To enhance the response to visible light, CdS QDs were hybridized on the antimonene/PIL surface by a S-CBD50 method. The subsequent S-CBD exposed the antimonene layer above the solution surface of about 0.05 cm. The Raman spectra (Fig. S9a, ESI†) indicate successful deposition of CdS QDs onto the antimonene layer. Morphology characterization of the device can be seen in Fig. S9c and d (ESI†). The thickness of the active layer is about 5 μm on average from the cross-sectional SEM images (Fig. S9b, ESI†).
Fig. 5a shows the photocurrent response of the Sb/P([VPIm]PF6)/CdS-based flexible photodetector device under different bias voltages, with the light source power intensity maintained around 20 mW cm−2. The photocurrent density increased with the raised bias voltage, which was about 20 nA cm−2 under 0 V bias, and increased to 160 nA cm−2 under 3 V. The on/off ratio of the flexible photodetector device was 26.8 under the bias of 1 V, as shown in the inset. The responsivity of the Sb/P([VPIm]PF6)/CdS-based flexible photodetector device is 10 μA W−1 under the bias potential of 3 V. For comparison, the performance of pure CdS QDs and a pure antimonene based flexible photodetector was also measured (Fig. S14 and S15, ESI†). Due to the pyroelectric effect of CdS,51–53 the shape of the photocurrent-time curve is different from the hybrid film based photodetector, which also confirmed that CdS QDs have been successfully hybridized with Sb/P([VPIm]PF6). When comparing their I–V curves, the on/off ratio of the pure CdS QD based flexible photodetector device was 9.4 under the bias of 1 V, only one third of the hybrid device. Moreover, the on/off ratio of the Sb/P([VPIm]PF6) based flexible photodetector device is smaller, illustrating that CdS QDs mainly play a role in light absorption in the hybrid devices.
Fig. 5b shows the stability test of the Sb/P([VPIm]PF6)/CdS-based flexible photodetector, with the CdS QD-based device as a reference. The photocurrent density of the pure CdS QD based flexible photodetector exhibited a big drop of more than 80% in 24 hours. And the photocurrent intensity of the pure CdS QD based flexible photodetector dropped by nearly 40% during a single test, indicating the poor stability of the pure CdS QD based flexible photodetector. In contrast, the photocurrent density of the detector only decreased by less than 10% after being exposed to the ambient environment for 15 days. Moreover, during the experiment time of over 160 s, the photocurrent density kept nearly unchanged. Above all, the hybridization of Sb/P([VPIm]PF6) and CdS QDs significantly improved the photocurrent density and stability of the device.
We have also tested the mechanical stabilities of the Sb/P([VPIm]PF6)/CdS-based flexible photodetector at various bending states, as shown in Fig. 5c. The inset photographs indicate the corresponding bending curvatures of the same flexible photodetector device. The photocurrent exhibited a very slight decline at different curvatures, indicating that the photodetector is nearly immune to the external bending stress, which can be attributed to the following factors: the excellent mechanical flexibility of the 2D Sb/P([VPIm]PF6); the PIL modified antimonene nanosheets provide good material–electrode contacts; and the antimonene nanosheets offer a flat substrate for the proper distribution of CdS QDs.
A schematic mechanism for the Sb/P([VPIm]PF6)/CdS based flexible photodetector is provided in Fig. 5d. The valence band and conduction band of the antimonene layer are −5.3 and −3.7 eV, versus vacuum, respectively.22 Upon visible light illumination, excitons are generated in the CdS QD layer according to the absorption spectra of the CdS QDs film and Sb/P([VPIm]PF6)/CdS hybrid film as shown in Fig. S11 (ESI†). The Sb/P([VPIm]PF6) can act as a hole transport layer22 to efficiently transport the holes from CdS upon photoexcitation to the electrode. The energy band alignment of the device ensures that electrons are injected from the photoexcited layer of the CdS QDs and collected by one of the ITO electrodes, while the valence band holes reach the other ITO electrode through the hole transport layer of antimonene, under the bias voltage. The above hypothesis is supported by the steady-state fluorescence spectra of the Sb/P([VPIm]PF6)/CdS hybrid film (Fig. S12a, ESI†). The emission intensity of the CdS QDs was greatly quenched due to the hybridization of the antimonene layer, implying a possible charge transfer process from the CdS QDs to the antimonene layer. This result was also confirmed by the time-resolved fluorescence spectra (Fig. S12b, ESI†). The average fluorescence lifetime decreased from 1.24 ns for CdS QDs to 0.66 ns for the hybrid (Table S1, ESI†), further verifying the charge transfer process between them. Meanwhile, the photocorrosion of CdS QDs may be suppressed and the stability of the Sb/P([VPIm]PF6)/CdS layer is enhanced.
Conclusions
Highly concentrated antimonene suspensions containing large nanosheets were successfully prepared by a PIL assisted liquid-phase exfoliation method. The over 20% yield of antimonene, to our best knowledge, is the highest value reported in the literature. The pretreatment procedure including a grinding process matched with a low rotation speed ball-milling to avoid long time sonication, greatly improved the efficiency and quality of the produced antimonene. In addition, we report that antimonene will be oxidized in solutions when the water content exceeds 60% under ultrasonication, providing important guidance to preserve stable antimonene for optoelectronic applications. Benefitting from the successful exfoliation of antimonene and the PIL on the surface, flexible photodetectors based on the hybrid structure of few layer antimonene were fabricated, which showed a good responsivity of 10 μA W−1 and on/off ratio of 26.8 under the bias of 1 V. Given the strong anisotropy of the β-phase antimonene between the armchair and zigzag directions, future efforts to improve the performance and realize the polarization detection30 of an antimonene photodetector may include an ordered arrangement of antimonene nanosheets in the active layer or fabrication of a single layer antimonene based photodetector. This work provides a promising approach for scalable fabrication of stable large size antimonene and paves the way for its application in optoelectronics.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the Ministry of Science and Technology of China (2017YFA0204903), National Natural Science Foundation of China (NSFC. 51733004, 51525303, 21673106, 21702085, 21602093, 21572086), 111 Project and the Fundamental Research Funds for the Central Universities (lzujbky-2017-109). We wish to thank the Electron Microscopy Center of Lanzhou University for the microscopy and microanalysis of our specimens.References
- J. A. Rogers, T. Someya and Y. Huang, Science, 2010, 327, 1603–1607 CrossRef CAS.
- C. Xie and F. Yan, Small, 2017, 13, 1701822 CrossRef.
- B. Chitara, L. S. Panchakarla, S. B. Krupanidhi and C. N. Rao, Adv. Mater., 2011, 23, 5419–5424 CrossRef CAS.
- L. Wang, J. Jie, Z. Shao, Q. Zhang, X. Zhang, Y. Wang, Z. Sun and S.-T. Lee, Adv. Funct. Mater., 2015, 25, 2910–2919 CrossRef CAS.
- Y. Zhang, J. Liu, Z. Wang, Y. Xue, Q. Ou, L. Polavarapu, J. Zheng, X. Qi and Q. Bao, Chem. Commun., 2016, 52, 13637–13655 RSC.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- Y. Zhang, Y. W. Tan, H. L. Stormer and P. Kim, Nature, 2005, 438, 201–204 CrossRef CAS.
- F. H. Koppens, T. Mueller, P. Avouris, A. C. Ferrari, M. S. Vitiello and M. Polini, Nat. Nanotechnol., 2014, 9, 780–793 CrossRef CAS.
- H. S. Lee, S. W. Min, Y. G. Chang, M. K. Park, T. Nam, H. Kim, J. H. Kim, S. Ryu and S. Im, Nano Lett., 2012, 12, 3695–3700 CrossRef CAS.
- F. Xia, H. Wang, D. Xiao, M. Dubey and A. Ramasubramaniam, Nat. Photonics, 2014, 8, 899–907 CrossRef CAS.
- H. Liu, A. T. Neal, Z. Zhu, Z. Luo, X. Xu, D. Tomanek and P. D. Ye, ACS Nano, 2014, 8, 4033–4041 CrossRef CAS.
- J. Qiao, X. Kong, Z. X. Hu, F. Yang and W. Ji, Nat. Commun., 2014, 5, 4475 CrossRef CAS.
- S. Das, W. Zhang, M. Demarteau, A. Hoffmann, M. Dubey and A. Roelofs, Nano Lett., 2014, 14, 5733–5739 CrossRef CAS.
- J. O. Island, G. A. Steele, H. S. J. v. d. Zant and A. Castellanos-Gomez, 2D Mater., 2015, 2, 011002 CrossRef.
- J. S. Kim, Y. Liu, W. Zhu, S. Kim, D. Wu, L. Tao, A. Dodabalapur, K. Lai and D. Akinwande, Sci. Rep., 2015, 5, 8989 CrossRef CAS.
- M. Pumera and Z. Sofer, Adv. Mater., 2017, 29, 1605299 CrossRef.
- S. Zhang, W. Zhou, Y. Ma, J. Ji, B. Cai, S. A. Yang, Z. Zhu, Z. Chen and H. Zeng, Nano Lett., 2017, 17, 3434–3440 CrossRef.
- S. Zhang, Z. Yan, Y. Li, Z. Chen and H. Zeng, Angew. Chem., Int. Ed., 2015, 54, 3112–3115 CrossRef CAS.
- Y. Song, Z. Liang, X. Jiang, Y. Chen, Z. Li, L. Lu, Y. Ge, K. Wang, J. Zheng, S. Lu, J. Ji and H. Zhang, 2D Mater., 2017, 4, 045010 CrossRef.
- G. Abellan, P. Ares, S. Wild, E. Nuin, C. Neiss, D. R. Miguel, P. Segovia, C. Gibaja, E. G. Michel, A. Gorling, F. Hauke, J. Gomez-Herrero, A. Hirsch and F. Zamora, Angew. Chem., Int. Ed., 2017, 56, 14389–14394 CrossRef CAS.
- G. Pizzi, M. Gibertini, E. Dib, N. Marzari, G. Iannaccone and G. Fiori, Nat. Commun., 2016, 7, 12585 CrossRef CAS.
- X. Wang, J. He, B. Zhou, Y. Zhang, J. Wu, R. Hu, L. Liu, J. Song and J. Qu, Angew. Chem., Int. Ed., 2018, 57, 8668–8673 CrossRef CAS.
- F. Li, M. Xue, J. Li, X. Ma, L. Chen, X. Zhang, D. R. MacFarlane and J. Zhang, Angew. Chem., Int. Ed., 2017, 56, 14718–14722 CrossRef CAS.
- E. Martínez-Periñán, M. P. Down, C. Gibaja, E. Lorenzo, F. Zamora and C. E. Banks, Adv. Energy Mater., 2018, 8, 1702606 CrossRef.
- Z. Li, X. Tan, P. Li, P. Kalisvaart, M. T. Janish, W. M. Mook, E. J. Luber, K. L. Jungjohann, C. B. Carter and D. Mitlin, Nano Lett., 2015, 15, 6339–6348 CrossRef CAS.
- W. Tian, S. Zhang, C. Huo, D. Zhu, Q. Li, L. Wang, X. Ren, L. Xie, S. Guo, P. K. Chu, H. Zeng and K. Huo, ACS Nano, 2018, 12, 1887–1893 CrossRef CAS.
- J. Gu, Z. Du, C. Zhang, J. Ma, B. Li and S. Yang, Adv. Energy Mater., 2017, 7, 1700447 CrossRef.
- J. Liu, Z. Yang, J. Wang, L. Gu, J. Maier and Y. Yu, Nano Energy, 2015, 16, 389–398 CrossRef CAS.
- W. Tao, X. Ji, X. Zhu, L. Li, J. Wang, Y. Zhang, P. E. Saw, W. Li, N. Kong, M. A. Islam, T. Gan, X. Zeng, H. Zhang, M. Mahmoudi, G. J. Tearney and O. C. Farokhzad, Adv. Mater., 2018, 30, 1802061 CrossRef PubMed.
- F. Chu, M. Chen, Y. Wang, Y. Xie, B. Liu, Y. Yang, X. An and Y. Zhang, J. Mater. Chem. C, 2018, 6, 2509–2514 RSC.
- P. Ares, F. Aguilar-Galindo, D. Rodriguez-San-Miguel, D. A. Aldave, S. Diaz-Tendero, M. Alcami, F. Martin, J. Gomez-Herrero and F. Zamora, Adv. Mater., 2016, 28, 6332–6336 CrossRef CAS PubMed.
- C. Gibaja, D. Rodriguez-San-Miguel, P. Ares, J. Gomez-Herrero, M. Varela, R. Gillen, J. Maultzsch, F. Hauke, A. Hirsch, G. Abellan and F. Zamora, Angew. Chem., Int. Ed., 2016, 55, 14345–14349 CrossRef CAS PubMed.
- Y. Shao, Z. L. Liu, C. Cheng, X. Wu, H. Liu, C. Liu, J. O. Wang, S. Y. Zhu, Y. Q. Wang, D. X. Shi, K. Ibrahim, J. T. Sun, Y. L. Wang and H. J. Gao, Nano Lett., 2018, 18, 2133–2139 CrossRef CAS PubMed.
- J. Ji, X. Song, J. Liu, Z. Yan, C. Huo, S. Zhang, M. Su, L. Liao, W. Wang, Z. Ni, Y. Hao and H. Zeng, Nat. Commun., 2016, 7, 13352 CrossRef CAS PubMed.
- L. Peng, S. Ye, J. Song and J. Qu, Angew. Chem., Int. Ed., 2019, 58, 9891–9896 CrossRef CAS PubMed.
- G. Wang, L. Wang, S. Zhuo, S. Fang and Y. Lin, Chem. Commun., 2011, 47, 2700–2702 RSC.
- M. Hamedi, L. Herlogsson, X. Crispin, R. Marcilla, M. Berggren and O. Inganas, Adv. Mater., 2009, 21, 573–577 CrossRef CAS PubMed.
- R. Marcilla, D. Mecerreyes, G. Winroth, S. Brovelli, M. del Mar Rodriguez Yebra and F. Cacialli, Appl. Phys. Lett., 2010, 96, 043308 CrossRef.
- R. Marcilla, F. Alcaide, H. Sardon, J. A. Pomposo, C. Pozo-Gonzalo and D. Mecerreyes, Electrochem. Commun., 2006, 8, 482–488 CrossRef CAS.
- T. Fukushima, A. Kosaka, Y. Yamamoto, T. Aimiya, S. Notazawa, T. Takigawa, T. Inabe and T. Aida, Small, 2006, 2, 554–560 CrossRef CAS PubMed.
- C. M. Tollan, R. Marcilla, J. A. Pomposo, J. Rodriguez, J. Aizpurua, J. Molina and D. Mecerreyes, ACS Appl. Mater. Interfaces, 2009, 1, 348–352 CrossRef CAS PubMed.
- J. Lu, F. Yan and J. Texter, Prog. Polym. Sci., 2009, 34, 431–448 CrossRef CAS.
- K. G. Zhou, N. N. Mao, H. X. Wang, Y. Peng and H. L. Zhang, Angew. Chem., Int. Ed., 2011, 50, 10839–10842 CrossRef CAS PubMed.
- C.-X. Hu, Q. Xiao, Y.-Y. Ren, M. Zhao, G.-H. Dun, H.-R. Wu, X.-Y. Li, Q.-Q. Yang, B. Sun, Y. Peng, F. Yan, Q. Wang and H.-L. Zhang, Adv. Funct. Mater., 2018, 28, 1805311 CrossRef.
- W. Zhao, Z. Xue, J. Wang, J. Jiang, X. Zhao and T. Mu, ACS Appl. Mater. Interfaces, 2015, 7, 27608–27612 CrossRef CAS.
- T. Morishita, H. Okamoto, Y. Katagiri, M. Matsushita and K. Fukumori, Chem. Commun., 2015, 51, 12068–12071 RSC.
- G. Mestl, P. Ruiz, B. Delmon and H. Knozinger, J. Phys. Chem., 1994, 98, 11276–11282 CrossRef CAS.
- R. Gusmao, Z. Sofer, D. Bousa and M. Pumera, Angew. Chem., Int. Ed., 2017, 56, 14417–14422 CrossRef CAS.
- W. Qian, J. Texter and F. Yan, Chem. Soc. Rev., 2017, 46, 1124–1159 RSC.
- C. X. Guo, H. B. Yang, Z. M. Sheng, Z. S. Lu, Q. L. Song and C. M. Li, Angew. Chem., Int. Ed., 2010, 49, 3014–3017 CrossRef CAS.
- A. K. Katiyar, S. Mukherjee, M. Zeeshan, S. K. Ray and A. K. Raychaudhuri, ACS Appl. Mater. Interfaces, 2015, 7, 23445–23453 CrossRef CAS.
- Y. V. Shaldin and S. Matyjasik, Semiconductors, 2014, 48, 562–569 CrossRef CAS.
- Y. Dai, X. Wang, W. Peng, C. Xu, C. Wu, K. Dong, R. Liu and Z. L. Wang, Adv. Mater., 2018, 30, 1705893 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nh00445a |
| This journal is © The Royal Society of Chemistry 2020 |