Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Designing single trigger/dual-response release and degradation into amine-functional hyperbranched-polydendron nanoprecipitates†
Hannah E.
Rogers
 a,
Pierre
Chambon
ab,
Sean
Flynn
ab,
Faye Y.
Hern
ab,
Andrew
Owen
bc and
Steve P.
Rannard
a,
Pierre
Chambon
ab,
Sean
Flynn
ab,
Faye Y.
Hern
ab,
Andrew
Owen
bc and
Steve P.
Rannard
 *ab
*ab
aDepartment of Chemistry, University of Liverpool, Crown Street, L69 7ZD, UK. E-mail: srannard@liverpool.ac.uk
bMaterials Innovation Factory, University of Liverpool, Crown Street, L69 7ZD, UK
cDepartment of Molecular and Clinical Pharmacology, University of Liverpool, Block H, 70 Pembroke Place, Liverpool L69 3GF, UK
First published on 28th October 2020
Abstract
The synthesis of complex polymer architectures using relatively facile experimental protocols provides access to materials with the opportunity to control functionality and physical behaviour. The scope of hyperbranched-polydendron chemistries has been expanded here to include primary chains comprising amine-functional ‘homopolymer’, ‘statistical copolymer’ and amphiphilic ‘block copolymer’ analogues using 2-(diethyl amino)ethyl methacrylate, 2-hydroxy propyl methacrylate and t-butyl methacrylate. The different primary chain chemistry and architectures leads to a marked variation in nanoprecipitation behaviour and the response of the resulting amine-functional nanoparticles to varying pH. When acid-sensitive and acid-stable branchers, 1,4-butanediol di(methacryoyloxy)-ethyl ether and ethylene glycol dimethacrylate respectively, are utilised, nanoparticles with encapsulation properties are formed and may be triggered to either release-and-disassemble or release-disassemble-degrade to form a solution of lower molecular weight constituent primary chains.
Introduction
Stimuli-responsive materials have been of considerable interest for many applications and the formation of defined polymeric structures has been at the core of many research activities to enable fine control of material behaviour.1 Advances have led to studies of materials that are programmable,2 exhibit multiple responses in the solid state,3 respond to stimuli in solution,4 and self-assemble when triggered by a range of environmental factors.5 A number of recurring themes are present within the wealth of reports of stimuli-responsive polymeric materials; these include the use of branched6 and segmented polymer7 architectures, the manipulation of aqueous solution pH8 to drive amine-functional polymer behaviour, and the ability to encapsulate and release a payload material from a nanoscale self-assembled object.9Amine methacrylate monomers10 have been widely used to create pH-responsive polymer structures, led in part by the Armes group; their reports of shell-crosslinked micelles created from A–B diblock11 and A–B–C triblock12,13 copolymers exploited the manipulation of hydrophilicity within the different block segments using pH, temperature and electrolyte concentrations. The study of amine-methacrylate derived pH-responsive branched vinyl copolymers, synthesised by the so-called “Strathclyde” route,14 showed the reversible formation of sterically-stabilised nanoparticles during the manipulation of solution pH. The relatively high molecular weight copolymers were lightly branched with low molar concentrations of ethylene glycol dimethacrylate (EGDMA) under conventional free radical conditions and also contained oligo(ethylene glycol) methacrylate to prevent macroscale phase separation and aggregation.15
Encapsulation, release and degradation studies of assembled materials offers the potential to create delivery vehicles that may enhance the formulation capability and efficacy of small molecules in a range of applications from pharmaceutical drug delivery through to agrochemicals, antimicrobials and cosmetics.16 In each of these cases, the degradability of the building block structures to smaller subunits that may be benign, either in an in vivo context or in the wider environment, is very important and has been the aim of materials such as self-immolative polymers17,18 and triggers including reduction,19 hydrolysis,20 temperature,21 chemical,22 and light enhanced23 mechanisms.
In recent years, we have introduced a new copolymer architecture that combines the concepts of branched vinyl copolymerisation and linear-dendritic hybrids24 (LDH; linear polymers with ideal dendron chain ends), using low concentrations of divinyl monomers25 within the controlled radical polymerisation of the LDH structures. The resulting copolymers, named hyperbranched-polydendrons (hyp-PDs), have a complex architecture of large numbers of conjoined chains with ideally-branched, highly functional dendron chain ends.26–28 We have shown that despite the broad dispersity (Ð) of hyp-PDs, self-assembly under flash nanoprecipitation conditions rapidly generates highly uniform nanoparticles that bear dendron functionality at the surface; the process is mediated by the very high molecular weight component of the molecular weight distribution.29 In all examples of hyp-PDs to date, hydrophobic monomers have comprised the primary polymer chains of the branched polymer structure, although dendrons of varying functionality and scaffold chemistry have been utilised. Here we present a fundamental study of the introduction of 2-(diethyl amino)ethyl methacrylate (DEAEMA) into the formation of hyp-PDs with the direct aim of achieving a single trigger/dual-response release and degradation of pH-responsive nanoprecipitates. A number of comparative structures have been synthesised that allow the testing of our initial hypotheses and an expansion in the understanding of the architectural variation available from the hyp-PD polymer platform.
Results and discussion
Design and preparation of pH-responsive 2-(diethyl amino)ethyl methacrylate containing hyperbranched-polydendron structures
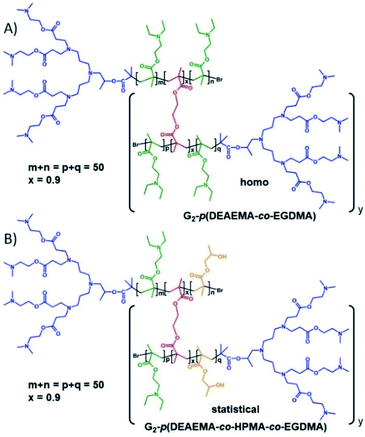
Our previous reports of hyp-PDs have largely relied upon hydrophobic monomers such as 2-hydroxy propyl methacrylate (HPMA),30n-butyl methacrylate31 and t-butyl methacrylate (tBuMA)27 to encourage nanoprecipitation of the resulting materials. Copper catalysed atom transfer radical polymerisation (ATRP) has been successfully employed to generate hyp-PDs after the formation and use of tertiary-bromide functional dendron initiators; pH-responsive behaviour has only previously been studied using hydrophobic monomers and tertiary-amine functional dendron initiators such as the second generation (G2) dendron initiator, 1, employed here (Fig. 1, see ESI Fig. S1†).32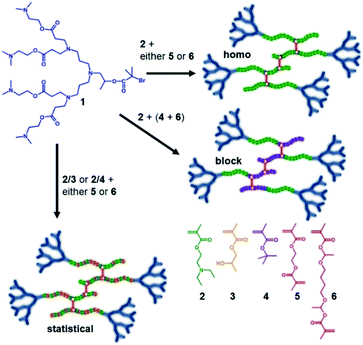 | ||
| Fig. 1 Schematic illustration of the target responsive hyperbranched-polydendron materials for this study (four conjoined primary chains shown in the simplified structures). | ||
The materials targeted for this study utilise 1 to initiate the ATRP of either (a) DEAEMA, 2, (b) monomer mixtures of DEAEMA/HPMA, 3, or DEAEMA/tBuMA, 4, or (c) blocks of DEAMEA and tBuMA. Two divinyl branching monomers were selected, namely EGDMA, 5, which would not be expected to demonstrate any pH response, and 1,4-butanediol di(methacryoyloxy)-ethyl ether (BDME, 6), expected to be cleaved under acidic pH conditions. BDME was synthesised according to a minor modification of the previously reported synthesis of ethylene glycol di(1-methacryloyloxy)ethyl ether (see ESI Fig. S2–S4†).33 The copolymerisation of DEAEMA with either EGDMA or BDME yields a hyp-PD with primary chains that are dominated by the homopolymer of DEAEMA, denoted here as a “homo hyp-PD” (Fig. 1). Similarly, DEAEMA/HPMA or DEAEMA/tBuMA mixtures create statistical copolymer primary chains, denoted as a “statistical hyp-PD”, and the sequential propagation of DEAEMA followed by a mixture of tBuMA with either EGDMA or BDME will create predominantly A–B block copolymer primary chains, denoted here as a “block hyp-PD” (Fig. 1).
Hyperbranched-polydendron synthesis comprising 2-(diethyl amino)ethyl methacrylate and ethylene glycol dimethacrylate
The ATRP synthesis of the homo hyp-PD structure using 1, DEAEMA and EGDMA (Fig. 2A) was conducted at 40 °C in a isopropanol/water mixture (IPA/H2O; 92.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5%, 39 wt% monomer) and progressed as reported for other monovinyl-methacrylate monomers using a Cu(I)Cl/bpy catalyst system (1
7.5%, 39 wt% monomer) and progressed as reported for other monovinyl-methacrylate monomers using a Cu(I)Cl/bpy catalyst system (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).
2).
Each primary chain targeted a number average degree of polymerisation (DPn) of 50 monomer units and at this relatively low solids content, a degree of intramolecular cyclisation would be expected as previously reported;34 the reaction concentration was somewhat dictated by the balance of solubilities of the different reaction components. Polymerisations in the absence of EGDMA, to form the LDH structures (DPn = 50 monomer units), were also conducted to establish the appropriate conditions and enable estimations of the structure of the final complex architectures (see ESI Fig. S2–S4†)
The introduction of HPMA or tBuMA into the DEAEMA monomer feedstock led to no noticeable complications within the formation of the statistical LDH copolymer, or for the statistical hyp-PD when EGDMA was also incorporated (see ESI Fig. S5–S11†). The ratio of DEAEMA/HPMA or DEAEMA/tBuMA was varied from 33/17 to 25/25 and 17/33, each targeting a DPn = 50 monomer units, in both the statistical LDH and statistical hyp-PD structures. The triple detection size exclusion chromatography (TD-SEC) analysis of all homo- and statistical LDH and hyp-PDs is shown in Table 1. All hyp-PDs utilised a [1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [EGDMA] ratio of 1.0
[EGDMA] ratio of 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 to avoid gelation and maintain an average of less than one brancher per chain.
0.9 to avoid gelation and maintain an average of less than one brancher per chain.
| Polymer | M n theory (g mol−1) | TD-SECa | Ð | |
|---|---|---|---|---|
| M n (g mol−1) | M w (gmol−1) | |||
| a Triple detection size exclusion chromatography using THF/2% TEA eluent; all polymerisation attained >99% monomer conversion as determined by 1H NMR. | ||||
| LDH | ||||
| G2-p(DEAEMA50) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090 090 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 650 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.45 |
| G2-p(DEAEMA33-co-HPMA17) | 9400 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.24 |
| G2-p(DEAEMA25-co-HPMA25) | 9070 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550 550 |
1.31 |
| G2-p(DEAEMA17-co-HPMA33) | 8740 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.31 |
| G2-p(DEAEMA33-co- tBuMA17) | 9360 | 9000 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 350 |
1.15 |
| G2-p(DEAEMA25-co- tBuMA25) | 9020 | 9800 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 350 |
1.16 |
| G2-p(DEAEMA17-co- tBuMA33) | 8670 | 9850 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 050 |
1.12 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| hyp-PDs | ||||
| G2-p(DEAEMA50-co-EGDMA0.9) | — | 125![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
341![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
2.72 |
| G2-p(DEAEMA33-co-HPMA17-co-EGDMA0.9) | — | 247![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
398![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.61 |
| G2-p(DEAEMA25-co-HPMA25-co-EGDMA0.9) | — | 268![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 971 971![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
7.33 |
| G2-p(DEAEMA17-co-HPMA33-co-EGDMA0.9) | — | 682![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 510 510![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
6.61 |
| G2-p(DEAEMA33-co-tBuMA17 -co-EGDMA0.9) | — | 209![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
392![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.88 |
| G2-p(DEAEMA25-co-tBuMA25-co-EGDMA0.9) | — | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
2.10 |
| G2-p(DEAEMA17-co-tBuMA33-co-EGDMA0.9) | — | 149![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
531![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
3.56 |
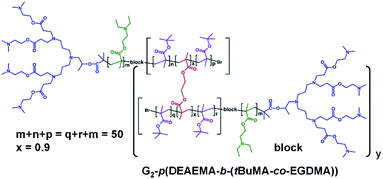
The synthesis of block hyp-PDs was undertaken after a series of block LDH structures containing various molar ratios of DEAEMA and tBuMA had been successfully demonstrated (Table 2, see ESI Fig. S12 and S13†); this represents the first report for the formation of such structures (Fig. 3).
| Polymer | M n theory (g mol−1) | TD-SECa | Ð | |
|---|---|---|---|---|
| M n (g mol−1) | M w (gmol−1) | |||
| a Triple detection size exclusion chromatography using THF/2% TEA eluent; all polymerisation attained >98% monomer conversion as determined by 1H NMR. b Polymerisation attained 94% conversion (Mn theory @ 94% = 7460 g mol−1) | ||||
| LDH | ||||
| G2-p(DEAEMA33-b-tBuMA17) | 9360 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 850 |
1.15 |
| G2-p(DEAEMA25-b-tBuMA25) | 9020 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 450 |
1.40 |
| G2-p(DEAEMA17-b-tBuMA33) | 8670 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 350 |
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.12 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| hyp-PDs | ||||
| G2-p(DEAEMA33-b-(tBuMA17-co-EGDMA0.9)) | — | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
192![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
2.21 |
| G2-p(DEAEMA25-b-(tBuMA25-co-EGDMA0.9)) | — | 265![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
528![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.99 |
| G2-p(DEAEMA17-b-(tBuMA33-co-EGDMA0.9)) | — | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
251![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
2.73 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Comparison | ||||
| G2-p(tBuMA50) | 7940 | 6900b | 7650 | 1.11 |
| G2-p(tBuMA50-co-EGDMA0.95) | — | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
273![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
3.10 |
Again, ATRP polymerisation in IPA/H2O was conducted at 40 °C initially synthesising the DEAEMA block segment before addition of tBuMA or the tBuMA/EGDMA mixture at approximately 80% conversion to form a block LDH or block hyp-PD respectively; in this way, branching was restricted to the second hydrophobic block segment with limited mixing of the block chemistries.35 For consistency, block copolymers with a total (A + B) block length of DPn = 50 monomer units were targeted and the DEAEMA/tBuMA ratio was varied from 33/17 to 25/25 and 17/33 to match the ratios within the statistical hyp-PD structures.
It is clear from the SEC data (Tables 1 and 2) that the initiator efficiency (IE) of 1 is relatively low when used with this amine-containing methacrylate monomer, as we also reported for HPMA homopolymerisation previously.32 When DEAEMA is polymerised solely, this appears to be approximately 50% (as indicated by a comparison of targeted and experimentally determined number average molecular weight (Mn)), but the inclusion of comonomers within the synthesis of statistical copolymerisation appears to lead to better targeting of DPn (IE > 70%) and Ð values are consistently below 1.32 (<1.17 when tBuMA present). Low initiator efficiency will lead to experimental [1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [EGDMA] ratios > 1 per chain, however this has been compensated by intramolecular cyclisation that would be expected at these concentrations, as discussed above.
[EGDMA] ratios > 1 per chain, however this has been compensated by intramolecular cyclisation that would be expected at these concentrations, as discussed above.
The purified homo and statistical hyp-PD samples (Table 1) have a considerable weight average number of conjoined primary chains, ranging from 14–370 chains, calculated as the ratio of weight average molecular weight (Mw) of the branched polymer and the Mn of the corresponding LDH synthesised without brancher, i.e. Mw(hyp-PD)/Mn(LDH). This increases with decreasing DEAEMA, as does Mw generally, and may indicate a negative impact of DEAEMA on the ATRP reaction under these conditions. Given that each primary chain-end bears 7 tertiary amine functional groups these materials comprise, on a weight average basis, between 98 and >2500 dendron-derived tertiary amines at the periphery of the complex architecture.
The block hyp-PDs (Table 2) rely upon the homopolymerisation of DEAEMA within the first linear block segment to form an external amine-functional block segment, and are subject to the low initiator efficiency of the homopolymerisation as described above; however, relatively low dispersity LDH block copolymers were synthesised in the absence of EGDMA and high molecular weight block hyp-PDs were synthesised, as expected, when EGDMA was included in the formation of the second block. As described above, the weight average number of chains for the block hyp-PD samples varied from 5–16 conjoined primary chains, and the structures therefore bear between 35 and 112 peripheral dendron-derived tertiary amine groups. In order to investigate the impact of DEAEMA on the initiator efficiency of 1, the LDH of tBuMA was synthesised (Table 2; see ESI Fig. S14 and S15†) and close targeting of Mn with low Ð was observed; the corresponding homo hyp-PD G2-p(tBuMA50-co-EGDMA0.95) was synthesised using a higher EGDMA content, with a weight average number of 40 conjoined primary chains (see ESI Fig. S16†).
Synthesis and acid degradation of hyperbranched-polydendrons comprising 2-(diethyl amino)ethyl methacrylate and BDME, 6
The synthesis of BDME-containing hyp-PDs was conducted using a primary chain DPn = 50 monomer units, for consistency; however, the incorporation of BDME was not as efficient as EGDMA and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BDME molar ratio of 1
BDME molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was required to generate high molecular weight materials. This may again be due to significant intramolecular cyclisation and a reduction in the intermolecular branching by BDME as described above. At a 1
2 was required to generate high molecular weight materials. This may again be due to significant intramolecular cyclisation and a reduction in the intermolecular branching by BDME as described above. At a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio, three polymer structures were targeted as comparisons with EGDMA-containing hyp-PDs described above with all syntheses reaching >99% monomer conversion (Table 3, see Fig. S17 and S18†); the selection of these structures is explained later.
2 molar ratio, three polymer structures were targeted as comparisons with EGDMA-containing hyp-PDs described above with all syntheses reaching >99% monomer conversion (Table 3, see Fig. S17 and S18†); the selection of these structures is explained later.
| Polymer | SECa | Ð | |
|---|---|---|---|
| M n (g mol−1) | M w (gmol−1) | ||
| a Triple detection size exclusion chromatography using THF/2% TEA eluent; all polymerisation attained >99% monomer conversion as determined by 1H NMR. | |||
| hyp-PDs | |||
| G2-p(DEAEMA50-co-BDME2.0) | 157![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
321![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
2.04 |
| G2-p(DEAEMA33-co-HPMA17-co-BDME2.0) | 305![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
733![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
2.40 |
| G2-p(DEAEMA17-b-(tBuMA33-co-BDME2.0)) | 444![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
594![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.34 |
The introduction of an acid-sensitive brancher allows the triggering of branched copolymer degradation under acid conditions. The susceptibility of the BDME linking chemistry to HCl was confirmed by dissolving the three hyp-PD architectures containing BDME in acetone prior to dropwise addition of 1 M HCl at ambient temperature.
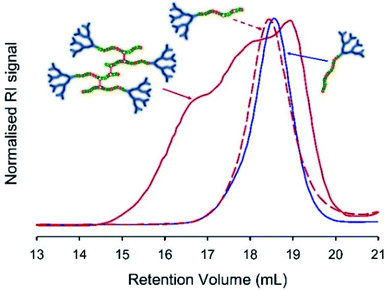
Freeze-drying was used to remove all solvents, and the resulting polymer samples were dissolved in THF/TEA (2% v/v) and compared with the corresponding LDH samples of near identical composition (Table 1), and the starting hyp-PD structure, using SEC. In principle, the cleavage of the BDME link would lead to the formation of a polymer distribution comprising LDH structures of very similar structure and molecular weight to the LDH polymers formed in the absence of brancher (Fig. 4, see ESI Fig. S19 andS20†); this was indeed observed for each of the homo, statistical and block structures and further supports the conjoined primary chain nature of the hyp-PD structure and is analogous to studies conducted on non-dendron bearing branched polymers.36
Nanoprecipitation of amine-functional EGDMA-containing hyperbranched-polydendrons of varying primary chain structure
As mentioned above, nanoprecipitation of hyp-PDs has been shown to benefit from a low concentration of structures containing large numbers of conjoined primary chains. In our earlier reports, a simple rapid nanoprecipitation approach was remarkably successful in forming stable nanoparticles with narrow distributions; we have utilised a similar process here, however, the presence of amine functionality within the dendron chain-end and primary chains does provide the opportunity to study nanoprecipitation within aqueous anti-solvent environments of different pH. To standardise the rapid nanoprecipitation process for the materials containing EGDMA, each polymer was dissolved in acetone (good solvent) at a concentration of 5 mg mL−1 and 2 mL of the solution was rapidly added to 10 mL of stirred water (anti-solvent); after overnight solvent removal via evaporation, the resulting aqueous nanoparticle dispersion was at a concentration of 1 mg mL−1, as reported previously. Samples were unfiltered and all studies were undertaken on unpurified aqueous dispersions.The nanoprecipitation of each hyp-PD was studied by following this approach using deionised water as the bad solvent phase at a pH = 7.8 (Table 4). The G2-p(DEAEMA50-co-EGDMA0.9) hyp-PD generated a narrow dispersity sample (PDI = 0.065) with a z-average diameter (Dz) of 60 nm and a zeta potential (ζ) of +22 mV as determined by dynamic light scattering (DLS) and electrophoretic mobility studies. The positive zeta potential was expected due to protonation of the amine functional groups within this hyp-PD. The statistical hyp-PDs based on G2-p(DEAEMAx-co-HPMAy-co-EGDMA0.9) were also nanoprecipitated under these conditions leading to particle dispersions of larger Dz, ranging between 115–145 nm (see ESI Fig. S21†), but maintaining relatively narrow distributions (PDI = 0.099–0.166) with the most monodisperse dispersion being formed from polymers with the highest HPMA content; this also correlates to the highest Mn and Mw values and again indicates the importance of having large numbers of conjoined primary chains as we have reported previously.29 Within these dispersions a low zeta potential was observed, correlating to the reduction in DEAEMA content as may be expected (ζ = 13–16 mV) with the lowest value being obtained for the highest HPMA content.
| Polymer | Dynamic light scattering data | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial pH 4.0 (final pH = 5.9–7.4)a | Initial pH 7.8 (final pH = 6.9–7.9)b | Acid addition (final pH = 2.6–3.1)c | ||||||||
| D z | PDI | D n | D z | PDI | D n | D z | PDI | D n | ||
| a Data from nanoprecipitating into aqueous HCl at pH = 4.0. b Data from nanoprecipitating into deionised water at pH = 7.8. c Data obtained after treating nanoprecipitates with aqueous HCl and forming a final pH = 2.6–3.1. is = insufficient scattering (suggests solvation); — = experiment not conducted; u = unsuccessful experiment. | ||||||||||
| G2-p(DEAEMA50-co-EGDMA0.9) | is | is | is | 60 | 0.065 | 45 | → | is | is | is |
| G2-p(DEAEMA33-co-HPMA17-co-EGDMA0.9) | — | — | — | 145 | 0.113 | 105 | → | is | is | is |
| G2-p(DEAEMA25-co-HPMA25-co-EGDMA0.9) | — | — | — | 185 | 0.166 | 140 | is | is | is | |
| G2-p(DEAEMA17-co-HPMA33-co-EGDMA0.9) | — | — | — | 115 | 0.099 | 80 | is | is | is | |
| G2-p(DEAEMA33-co-tBuMA17-co-EGDMA0.9) | 50 | 0.222 | 20 | u | u | u | → | is | is | is |
| G2-p(DEAEMA25-co-tBuMA25-co-EGDMA0.9) | 135 | 0.284 | 35 | u | u | u | is | is | is | |
| G2-p(DEAEMA17-co-tBuMA33-co-EGDMA0.9) | 130 | 0.222 | 60 | u | u | u | 141 | 0.293 | 51 | |
| G2-p(DEAEMA33-b-(tBuMA17-co-EGDMA0.9)) | is | is | is | u | u | u | → | is | is | is |
| G2-p(DEAEMA25-b-(tBuMA25-co-EGDMA0.9)) | is | is | is | u | u | u | is | is | is | |
| G2-p(DEAEMA17-b-(tBuMA33-co-EGDMA0.9)) | 85 | 0.197 | 45 | u | u | u | 155 | 0.354 | 77 | |
| G2-p(tBuMA50-co-EGDMA0.95) | 235 | 0.295 | 80 | u | u | u | → | — | — | — |
| G2-p(DEAEMA50-co-BDME2.0) | — | — | — | 60 | 0.241 | 30 | → | is | is | is |
| G2-p(DEAEMA33-co-HPMA17-co-BDME2.0) | — | — | — | 410 | 0.204 | 310 | is | is | is | |
| G2-p(DEAEMA17-b-(tBuMA33-co-BDME2.0)) | — | — | — | 110 | 0.086 | 75 | 135 | 0.073 | 105 | |
The statistical hyp-PDs containing tBuMA did not nanoprecipitate into water at pH = 7.8 and neither did the block hyp-PDs (Table 4). It is interesting that our previous nanoprecipitation reports of HPMA-derived materials (without amine functionality) led to stable nanoparticles with highly negative ζ values when nanoprecipitated in this way, and here we see very different behaviour when tBuMA is employed. For comparison, we synthesised the homo hyp-PD G2-p(tBuMA50-co-EGDMA0.95) (Table 2), and this also failed to nanoprecipitate under these conditions. In order to study this in greater detail, the range of statistical, block and homopolymer hyp-PDs containing tBuMA were nanoprecipitated into water containing HCl at pH = 4; the hypothesis underpinning this approach suggested the protonation of the amine functionality during nanoprecipitation may encourage colloidal stability and the unbuffered water would potentially lead to nearly neutral conditions after polymer addition and removal of solvent. As expected, the neutralisation during nanoprecipitation led to final pH values ranging from 5.9–7.4, depending on the branched copolymer composition, but the success of this approach varied quite considerably across the range of materials used.
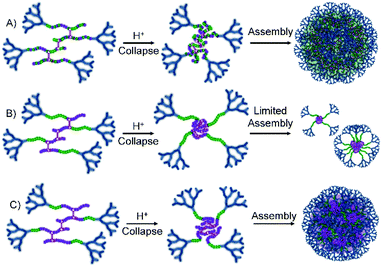
G2-p(DEAEMA50-co-EGDMA0.9) was unable to nanoprecipitate under these conditions, leading to a clear and homogeneous solution; however, the statistical G2-p(DEAEMAx-co-tBuMAy-co-EGDMA0.9) polymer series generated small nanoparticles (Dz = 50 nm) at low tBuMA content and larger particles (Dz approximately 130 nm) at higher tBuMA ratios (Fig. 5A, see ESI Fig. S22†). Similar behaviour was seen for block hyp-PDs G2-p(DEAEMAx-b-(tBuMAy-co-EGDMA0.9)) but at the lowest block lengths of tBuMA (DPn = 17 or 25 units), insufficient light scattering was seen, suggesting no precipitation and high levels of solvation. At the highest hydrophobic block length (DPn = 33 monomer units), nanoprecipitation was observed (see ESI Fig. S23†) with nanoparticles of relatively small size formed (Dz = 85 nm).
This variation in behaviour between the chemically similar but architecturally different materials is notable and suggests a difference in hydrophobicity between the statistically mixed and segmented chemistries. Although we have not confirmed this rationale, it seems plausible that the block hyp-PDs assemble into stabilised unimolecular structures, or small aggregates (Fig. 5B), when the block length is short and the protonated dendron-terminated DEAEMA block dominates the behaviour during addition to the low pH aqueous environment; solubility behaviour within amphiphilic block structures is known to be composition and block length dependent.37 As the hydrophobic block length increases to 66% of the total primary chain, a switch to tBuMA block dominance is seen and a more conventional nanoprecipitation appears to occur (Fig. 5C); this observation determined the selection of the block hyp-PD structure G2-p(DEAEMA17-b-(tBuMA33-co-BDME2.0)) for studies using acid sensitive branchers.
Within the statistical copolymers, the mixing of DEAEMA and tBuMA leads to a clear impact on the solution behaviour of the protonated DEAEMA chain, even at 34 mole % tBuMA, and larger nanoparticles are formed as the tBuMA content increases, leading to an additional decrease in protonated amine functional groups and subsequent surface charge density of the protonated macromolecules; the tBuMA-containing nanoprecipitates from this statistical copolymer series exhibited ζ values between +35–52 mV.
Nanoprecipitation of amine-functional BDME-containing hyperbranched-polydendrons with varying primary chain structures
The hyp-PD structures comprising BDME as the branching agent were also subjected to nanoprecipitation at pH 7.8 and successfully formed nanoparticle dispersions (Table 4). Interestingly, the block copolymer G2-p(DEAEMA17-b-(tBuMA33-co-BDME2.0)) was able to form a stable nanoparticle dispersion despite the analogous EGDMA-containing G2-p(DEAEMA17-b-(tBuMA33-co-EGDMA0.9)) failing to do so at this pH; it is unclear why this is the case but the different chemical nature of BDME compared to EGDMA may impact the hydrophobicity of the branched copolymers, suggesting a fine balance of properties that is also seen in the EGDMA-derived block hyp-PDs of different tBuMA segment length (Table 4).The nanoprecipitates formed from BDME-containing hyp-PDs have two direct comparisons to EGDMA-containing nanoparticles formed at pH 7.8, namely G2-p(DEAEMA50-co-EGDMA0.9) and G2-p(DEAEMA33-co-HPMA17-co-EGDMA0.9). The homo hyp-PD synthesised with BDME formed nanoparticles of very similar hydrodynamic diameter but broader PDI at near neutral pH (see ESI Fig. S24†); however, the statistical hyp-PD comprising HPMA and BDME formed larger nanoparticles with a relatively broad PDI (Table 4). Again, it is unclear why this is the case and a detailed study of this behaviour is required. Conversely, G2-p(DEAEMA17-b-(tBuMA33-co-BDME2.0)) formed nanoparticle dispersions of narrow PDI.
Studying the pH response of nanoprecipitated hyp-PD particles
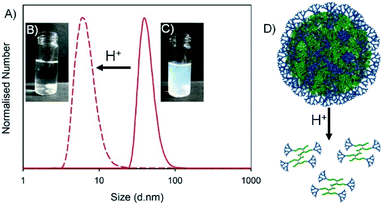
The successful nanoprecipitates formed from this library of hyp-PDs were all generated in aqueous conditions that varied between pH 5.9–8.0 after solvent evaporation. Each of these nanoparticle dispersions were treated dropwise with aqueous HCl to form a final pH between 2.6–3.1 (Table 4). The visual turbidity from most samples was lost after treatment with HCl, indicating a swelling and disassembly of the nanoprecipitate dispersions (Fig. 6). DLS analysis of each sample showed a considerable loss of measured scattering intensity; for example, the nanoparticles formed from G2-p(DEAEMA50-co-EGDMA0.9) and the statistical hyp-PDs G2-p(DEAEMAx-co-HPMAy-co-EGDMA0.9) exhibited a decrease in derived count rate (DCR) to <5% of their initial value. Where sufficient but limited light scattering was present, the number distribution was observed to decrease to number average diameters (Dn) of <10 nm (Fig. 6A, see ESI Fig. S25†). This behaviour was also seen for statistical hyp-PDs containing ≤50 mole % tBuMA within the primary chains of the branched macromolecules.Interestingly, the hyp-PD derived nanoprecipitates formed from the statistical and block architectures containing 66 mole % tBuMA, namely G2-p(DEAEMA17-co-tBuMA33-co-EGDMA0.9), G2-p(DEAEMA17-b-(tBuMA33-co-EGDMA0.9)) and G2-p(DEAEMA17-b-(tBuMA33-co-BDME2.0)) showed an increase in Dz and Dn values after addition of HCl, suggesting the assemblies remain intact and swell during the protonation of the DEAEMA monomer residues (see ESI Fig. S26†).
These samples also represent the highest content of tBuMA in both block and statistical primary chain architectures and suggests a lack of penetration of aqueous acid into the hydrophobic core of the nanoprecipitate as this behaviour is clearly not evident when HPMA is present at high concentrations within the primary chains. Additionally, the hydrophobic environment within the block hyp-PD G2-p(DEAEMA17-b-(tBuMA33-co-BDME2.0))-derived nanoparticle acts to protect the acid sensitive brancher and avoid cleavage of the hyp-PD structure. The large variation in behaviour allows the judicious selection of monomers and copolymer compositions within the design of stimuli-responsive nanoprecipitates derived from hyp-PDs to target different responses under varying acidic conditions.
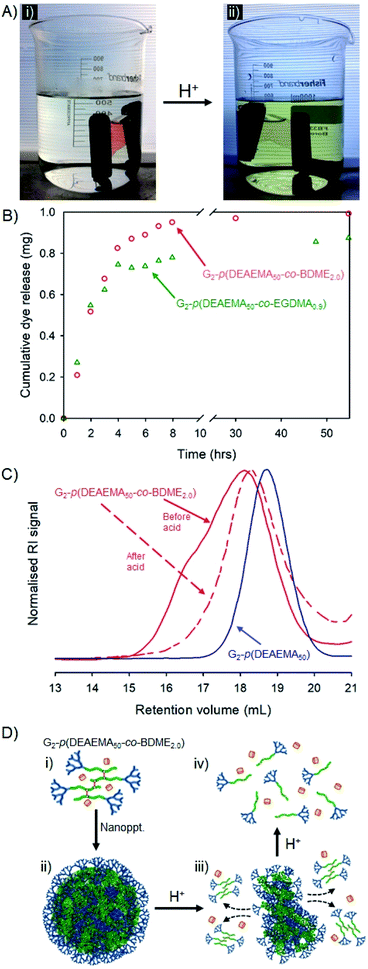
Encapsulation, pH-triggered release, nanoparticle disassembly and hyp-polydendron degradation: dual response from a pH stimulus
The hyp-PD studies above provide a conceptual and experimental framework for the design of a guest-host nanoprecipitate that will function as a highly surface-functional carrier of a payload molecule, and allow for either pH-triggered disassembly and release or disassembly, release and degradation to low molecular weight polymer fragments (Fig. 7). As discussed above, this may have important implications in the environment or within in vivo settings.Two materials, that vary solely in the brancher used, were selected for this demonstration, namely G2-p(DEAEMA50-co-EGDMA0.9) and G2-p(DEAEMA50-co-BDME2.0), and fluoresceinamine was selected as the guest molecule. Fluoresceinamine is a hydrophobic dye that is poorly water soluble, often used as a fluorescent tracer, and has good solubility in acetone. The two homo hyp-PDs were, therefore, individually dissolved in acetone (5 mg mL−1) and combined with an acetone solution of fluoresceinamine (1 mg mL−1) at a volume ratio allowing a dye-loading of 9 wt%. Each mixed acetone solution was nanoprecipitated into water (pH = 7.8) and yielded very similar orange/red, monomodal aqueous nanoparticle dispersions after evaporation of acetone (Dz = 45 or 40 nm and PDI = 0.197 or 0.190 for G2-p(DEAEMA50-co-EGDMA0.9) and G2-p(DEAEMA50-co-BDME2.0) respectively; see ESI Fig. S27†).
This slight decrease in hydrodynamic diameter relative to ‘blank’ nanoprecipitates implies a role for the hydrophobic fluorescer in modifying the early stages of the nanoprecipitation process. The smaller Dz value for the guest/host nanoprecipitate formed from G2-p(DEAEMA50-co-BDME2.0) may indicate the presence of a small number of methacrylic acid residues within the hyp-PD structure. These may have formed by undesired cleavage of the BDME acetal prior to nanoprecipitation and induce a fluorescer/polymer interaction that facilitates the particle formation. NMR studies were unable to identify carboxylic acid functionality at the possible low concentrations due to considerable resonance overlap.
The dye-loaded aqueous dispersions were transferred to dialysis bags (molecular weight cut-off = 2000 g mol−1) and left to stand in pure water for 48 hours after which the reservoir water of each sample showed a slight yellow colour that was quantified by UV-visible spectroscopy against a standard curve (ESI Fig. S28†). The encapsulation efficiency of each hyp-polydendron nanoprecipitate was, therefore, calculated as 74% and 99% for G2-p(DEAEMA50-co-EGDMA0.9) and G2-p(DEAEMA50-co-BDME2.0) respectively, suggesting again that the presence of BDME modifies the internal environment of the nanoparticles. The samples were transferred to fresh water and left to stand at ambient temperature for a further 24 hours after which the water remained clear and uncoloured (Fig. 7A(i)).
The addition of HCl to the reservoir water, to achieve a pH of approximately 2, led to an observable yellow colour leaching from the dialysis tubing within 30 minutes (Fig. 7A(ii)) which was monitored and quantified by UV-visible spectroscopy over a 60 hour period (Fig. 7B).
Although both hyp-PD nanoprecipitates demonstrated a pH-responsive release, the release from the BDME-containing nanoprecipitates was rapid and led to a greater cumulative mass of released fluorescer; this is interesting to note as the presence of BDME within the nanoparticle clearly led to a higher encapsulation efficiency, suggesting a more favourable environment within the nanoparticle. The addition of HCl would be expected to swell the nanoparticles formed from G2-p(DEAEMA50-co-EGDMA0.9) or G2-p(DEAEMA50-co-BDME2.0), leading to dissolution and disassembly, but in the case of G2-p(DEAEMA50-co-BDME2.0) an additional degradation of the linking BDME chemistry would be expected to further break down the protonated macromolecules into their respective LDH primary chains, as seen in acetone solution studies (Fig. 4).
In order to study the impact of the acid trigger on the molecular weight distribution of the hyp-PDs within the nanoprecipitates, the contents of the dialysis tubing were removed, dried and reprecipitated from THF solution into hexane, prior to filtration and drying in vacuo overnight. Despite the very low mass of the recovered samples, SEC analysis was possible (Fig. 7C and Table 5) and comparison with the corresponding LDH and hyp-PD was undertaken.
| hyp-PD polymer & acid treatment | TD-SECa | Ð | |
|---|---|---|---|
| M n (g mol−1) | M w (gmol−1) | ||
| a Triple detection size exclusion chromatography using THF/2% TEA eluent; all. | |||
| G2-p(DEAEMA50-co-BDME2.0) | 157![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
321![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
2.04 |
| After acid addition (particle in water) | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.45 |
| After acid addition (solution in acetone) | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 950 |
51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 150 |
1.28 |
| G2-p(DEAEMA50-co-EGDMA0.9) | 125![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
341![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
2.72 |
| After acid addition (particle in water) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 513 513![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 938 938![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.60 |
| After acid addition (solution in acetone) | — | — | — |
The nanoparticle dispersion generated from G2-p(DEAEMA50-co-BDME2.0) showed a considerable decrease in molecular weight after triggered release using HCl, as would be expected from previous studies of fully dissolved hyp-PD in acetone. Noticeably, TD-SEC analysis does not show a complete degradation to primary LDH chains suggesting incomplete degradation; however, the molecular weight distribution overlays well with the corresponding G2-p(DEAEMA50) LDH (Fig. 7C) and the process of acid-degradation within the dialysis tubing and subsequent reprecipitation may well have led to removal of part of the low molecular weight fraction within the sample. In stark contrast, purification and analysis of the G2-p(DEAEMA50-co-EGDMA0.9) derived sample led to a considerable increase in observed molecular weight (see ESI Fig. S29†), also suggesting a removal of a portion of the lower molecular weight species and caution must be exercised with the characterisation of this sample. As mentioned earlier, the potential to encapsulate and release guest molecules, with subsequent triggered nanoparticle degradation has potential widespread application.
Conclusions
The hyperbranched-polydendron platform offers considerable flexibility to design complex macromolecules that are able to form uniform nanoparticles under the relatively facile process of rapid nanoprecipitation. The primary chains of the hyp-PDs may be varied considerably, allowing the tuning of behaviour that translates to control of the resulting nanoparticles including their response to nanoprecipitation and subsequent stimuli.Importantly, the balance of the branched hyp-PD structure, which is dominated by covalent bonding and chemical functionality, and the nanoprecipitation process, which is dominated by non-covalent self-assembly, may be subject to considerable tuning. Within the current study, the pH-response within aqueous nanoparticle dispersions was dictated by the design of the branched polymer building blocks with options ranging from dissolution to the constituent branched building blocks, simple swelling after protonation of tertiary amine functionality, or a combination of swelling, dissolution and branched polymer degradation to yield a solution of linear-dendritic hybrid primary chain components.
The versatility of this approach to designing nanoparticle behaviour through branched polymer chemistry and architecture offers considerable opportunities within future applications, including environment specific degradation routes such as enzymatic mechanisms.38 The range of dendron initiators that may be synthesised and combined with commercially available monomers and divinyl branchers is substantial and the ease of synthesis of these complex materials presents a facile route to new material study and optimisation.
Conflicts of interest
HER, PC, FYH, AO and SPR are coinventors of patented inventions related to hyperbranched-polydendrons.Acknowledgements
The authors acknowledge the University of Liverpool and EPSRC for funding (Grant number EP/I038721/1) and Doctoral Training Awards for HER and FYH.Notes and references
- M. Wei, Y. Gao, X. Li and M. J. Serpe, Polym. Chem., 2017, 8, 127–143 RSC.
- X. Fan, J. Y. Chung, Y. X. Lim, Z. Li and X. J. Loh, ACS Appl. Mater. Inter., 2016, 8, 33351–33370 CrossRef CAS.
- K. M. Herbert, S. Schrettl, S. J. Rowan and C. Weder, Macromolecules, 2017, 50, 8845–8870 CrossRef CAS.
- J. Niskanen and H. Tenhu, Polym. Chem., 2017, 8, 220–232 RSC.
- W. Li, Y. Kim, J. Li and M. Lee, Soft Matter, 2014, 10, 5231–5242 RSC.
- D. Wang, Y. Jin, X. Zhu and D. Yan, Prog. Polym. Sci., 2017, 64, 114–153 CrossRef CAS.
- R. G. Tuguntaev, C. I. Okeke, J. Xu, C. Li, P. C. Wang and X.-J. Liang, Curr. Pharm. Design, 2016, 22, 2857–2865 CrossRef CAS.
- Z. Su and X. Jiang, Polymer, 2016, 93, 221–239 CrossRef CAS.
- H. Che and J. C. M. van Hest, J. Mater. Chem B, 2016, 4, 4632–4647 RSC.
- J. Hu, G. Zhang, Z. Ge and S. Liu, Prog. Polym. Sci., 2014, 39, 1096–1143 CrossRef CAS.
- S. Liu and S. P. Armes, Angew. Chem. Int. Edit., 2002, 41, 1413–1416 CrossRef CAS.
- S. Liu, J. V. M. Weaver, Y. Tang, N. C. Billingham, S. P. Armes and K. Tribe, Macromolecules, 2002, 35, 6121–6131 CrossRef CAS.
- L. N. Pilon, S. P. Armes, P. Findlay and S. P. Rannard, Eur. Polym. J., 2006, 42, 1487–1498 CrossRef CAS.
- N. O'Brien, A. McKee, D. C. Sherrington, A. T. Slark and A. Titterton, Polymer, 2000, 41, 6027–6031 CrossRef.
- J. V. M. Weaver, R. T. Williams, B. J. L Royles, P. H. Findlay, A. I. Cooper and S. P. Rannard, Soft Matter, 2008, 4, 985–992 RSC.
- Y. Zhao, L.-P. Lv, S. Jiang, K. Landfester and D. Crespy, Polym. Chem., 2015, 6, 4197–4205 RSC.
- C. Ergene and E. F. Palermo, Biomacromolecules, 2017, 18, 3400–3409 CrossRef CAS.
- A. Sagi, R. Weinstain, N. Karton and D. Shabat, J. Am. Chem. Soc., 2008, 130, 5434–5435 CrossRef CAS.
- N. Chan, N. R. Ko, S. Y. An, B. Khorsand and J. K. Oh, ACS Symp. Ser., 2015, 1188, 273–291 CrossRef CAS.
- K. K. Bawa and J. K. Oh, Mol. Pharm., 2017, 14, 2460–2474 CrossRef CAS.
- N. Vanparijs, L. Nuhn and B. G. De Geest, Chem. Soc. Rev., 2017, 46, 1193–1239 RSC.
- J. F. Quinn, M. R. Whittaker and T. P. Davis, Polym. Chem., 2017, 8, 97–126 RSC.
- S. Chatani, C. J. Kloxin and C. N. Bowman, Polym. Chem., 2014, 5, 2187–2201 RSC.
- I. Gitsov, J. Polym. Sci. Pol. Chem., 2008, 46, 5295–5314 CrossRef CAS.
- T. Das, S. Sengupta, U. K. Ghorai, A. Dey and A. Bandyopadhyay, RSC Adv., 2015, 5, 102932–102941 RSC.
- F. L. Hatton, P. Chambon, T. O. McDonald, A. Owen and S. P. Rannard, Chem. Sci., 2014, 5, 1844–1853 RSC.
- F. Y. Hern, S. E. R. Auty, O. C. J. Andrén, M. Malkoch and S. P. Rannard, Polym. Chem., 2017, 8, 1644–1653 RSC.
- F. L. Hatton, L. M. Tatham, L. R. Tidbury, P. Chambon, T. He, A. Owen and S. P. Rannard, Chem. Sci., 2015, 6, 326–334 RSC.
- F. L. Hatton, P. Chambon, A. C. Savage and S. P. Rannard, Chem. Commun., 2016, 52, 3915–3918 RSC.
- R. A. Slater, T. O. McDonald, D. J. Adams, E. R. Draper, J. V. M. Weaver and S. P. Rannard, Soft Matter, 2012, 8, 9816–9827 RSC.
- F. Y. Hern, A. Hill, A. Owen and S. Rannard, Polym. Chem., 2018, 9, 1767–1771 RSC.
- H. E. Rogers, P. Chambon, S. E. R. Auty, F. Y. Hern, A. Owen and S. P. Rannard, Soft Matter, 2015, 11, 7005–7015 RSC.
- E. Ruckenstein and H. Zhang, Macromolecules, 1999, 32, 3979–3983 CrossRef CAS.
- J. Rosselgong, S. P. Armes, W. R. S. Barton and D. Price, Macromolecules, 2010, 43, 2145–2156 CrossRef CAS.
- T. Alhilfi, P. Chambon and S. P. Rannard, J. Polym. Sci. Pol. Chem., 2020, 58, 1426–1438 CAS.
- Y. Li and S. P. Armes, Macromolecules, 2005, 38, 8155–8162 CrossRef CAS.
- Y.-J. Sheng, T.-Y. Wang, W. M. Chen and H.-K. Tsao, J. Phys. Chem. B, 2007, 111, 10938–10945 CrossRef CAS.
- S. K. Pramanik, S. Sreedharan, H. Singh, M. Khan, K. Tiwari, A. Shiras, C. Smythe, J. A. Thomas and A. Das, Bioconjugate Chem., 2018, 29, 1520–4812 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterisation, NMR spectra, SEC chromatograms, dynamic light scattering data. See DOI: 10.1039/d0na00696c |
| This journal is © The Royal Society of Chemistry 2020 |