Open Access Article
Open Access ArticleMn(II) chelate-coated superparamagnetic iron oxide nanocrystals as high-efficiency magnetic resonance imaging contrast agents†
Changqiang
Wu‡
 a,
Tianwu
Chen‡
a,
Lihua
Deng
ac,
Qian
Xia
a,
Chuan
Chen
b,
Mu
Lan
a,
Yu
Pu
a,
Hongjie
Tang
d,
Ye
Xu
e,
Jiang
Zhu
*ab,
Chenjie
Xu
a,
Tianwu
Chen‡
a,
Lihua
Deng
ac,
Qian
Xia
a,
Chuan
Chen
b,
Mu
Lan
a,
Yu
Pu
a,
Hongjie
Tang
d,
Ye
Xu
e,
Jiang
Zhu
*ab,
Chenjie
Xu
 f,
Chengyi
Shen
a and
Xiaoming
Zhang
*a
f,
Chengyi
Shen
a and
Xiaoming
Zhang
*a
aSichuan Key Laboratory of Medical Imaging and School of Medical Imaging, Affiliated Hospital of North Sichuan Medical College, Nanchong 637000, China. E-mail: zhujiang@nsmc.edu.cn; zhangxm@nsmc.edu.cn
bSchool of Pharmacy, North Sichuan Medical College, Nanchong 637000, China
cDepartment of Radiology, First People's Hospital of Neijiang, Neijiang 641000, China
dDepartment of Radiology, Nanchong Hospital of Traditional Chinese Medicine, Nanchong 637000, China
eDepartment of Radiology, Children's Hospital of Chongqing Medical University, Chongqing 401122, China
fSchool of Chemical and Biomedical Engineering, Nanyang Technological University, Singapore
First published on 17th June 2020
Abstract
In this communication, a paramagnetic bifunctional manganese(II) chelate ([Mn(Dopa-EDTA)]2−) containing a catechol group is designed and synthesized. The catechol can bind iron ions on the surface of superparamagnetic iron oxide (SPIO) nanocrystals to form core–shell nanoparticles. Both 4 and 7 nm SPIO@[Mn(Dopa-EDTA)]2− show good water solubility, single-crystal dispersion, and low cytotoxicity. The study of the interplay between the longitudinal and transverse relaxation revealed that 4 nm SPIO@[Mn(Dopa-EDTA)]2− with lower r2/r1 = 1.75 at 0.5 T tends to be a perfect T1 contrast agent while 7 nm SPIO@[Mn(Dopa-EDTA)]2− with a higher r2/r1 = 15.0 at 3.0 T tends to be a T2 contrast agent. Interestingly, 4 nm SPIO@[Mn(Dopa-EDTA)]2− with an intermediate value of r2/r1 = 5.26 at 3.0 T could act as T1–T2 dual-modal contrast agent. In vivo imaging with the 4 nm SPIO@[Mn(Dopa-EDTA)]2− nanoparticle shows unique imaging features: (1) long-acting vascular imaging and different signal intensity changes between the liver parenchyma and blood vessels with the CEMRA sequence; (2) the synergistic contrast enhancement of hepatic imaging with the T1WI and T2WI sequence. In summary, these Fe/Mn hybrid core–shell nanoparticles, with their ease of synthesis, good biocompatibility, and synergistic contrast enhancement ability, may provide a useful method for tissue and vascular MR imaging.
Magnetic resonance imaging (MRI) is one of the most powerful non-invasive medical diagnostic imaging modalities which can offer high spatial resolution and tissue contrast imaging, without ionizing radiation.1 The nuclear magnetic resonance (NMR) signal of clinical MRI mainly comes from protons of water, which is about 65% of human body weight. The desired MR image contrast between different biological tissues could be created from the weighting image of their intrinsic physical parameters: longitudinal time (T1), transversal time (T2),
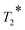 and proton density (ρ).2
and proton density (ρ).2
Despite the inherent contrast being obtained, there remains a need for contrast agents (CAs) in MRI to further delineate the structure, enhance tissue differentiation and monitor physiological functions. Now, more than one third (1/3) routine clinical MR scans are contrast-enhanced with the paramagnetic chelates of gadolinium-based contrast agents (GBCAs).3,4 MRI CAs can catalytically shorten the relaxation times of bulk water protons and their efficiency is measured by relaxivity (ri, i = 1, 2), which is defined as the change in the relaxation rate (Δ(R1, 2) = Δ(1/T1, 2), in units of s−1) of the water protons upon addition of 1 mM CAs.5
MR CAs can be categorized as either T1 or T2 CAs based on their effects being more pronounced for either T1 or T2. T1 CAs (positive contrast agents), such as the paramagnetic chelates of gadolinium (Gd3+), iron (Fe3+) or manganese (Mn2+) ions with low r2/r1 (approximately 1), can increase the signal intensity and produce bright images in a T1-weighted sequence.6 Typical T1 CAs such as the FDA approved nine GBCAs are small molecules with poor relaxation efficiency and short circulation time in the vascular system because of their fast tumbling in solution and rapid renal excretion.7
On the other hand, T2 CAs (negative contrast agents), such as magnetic nanoparticles with a very high r2 value (up to 500 mmol−1 s−1) and high r2/r1 ratio, can significantly decrease the signal intensity and produce dark images in a T2 or 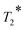 -weighted sequence.8 However, because the “black holes” effect produced by the large magnetic susceptibility of nanoparticles, the T2 dark images of nanoparticle labelled lesions sometimes will confused with low signal noise ratio (SNR) areas such as calcification, air, hemorrhage, and blood clots.9,10
-weighted sequence.8 However, because the “black holes” effect produced by the large magnetic susceptibility of nanoparticles, the T2 dark images of nanoparticle labelled lesions sometimes will confused with low signal noise ratio (SNR) areas such as calcification, air, hemorrhage, and blood clots.9,10
T 1 and T2 weighted imaging, with an accurate match of spatial and temporal imaging parameters can provide the cross-validation information in MRI, are routinely proceeded usually in clinical diagnostic work.11,12 Moreover, T1–T2 dual-modal CAs are necessary, which can produce effective contrast enhancement in both T1 and T2 weighted imaging.13 The ideal T1–T2 dual-modal CAs should have high T1 and T2 relaxivities (r1 and r2) and intermediate r2/r1 ratios (about 5–8).14 Recently, several attempts have been made to design T1–T2 dual-modal CAs based on magnetic nanoparticles, and its r1, r2 and r2/r1 ratio can be tuned by modulating their crystal size, composition, and surface state.15–17 For example, ultra-small superparamagnetic iron oxide (SPIO),18–21 paramagnetic metal (such as Gd and Mn) doping22–25 or paramagnetic metal oxide (such as MnO and Gd2O3) coating core/shell26–30 SPIO nanocrystals with a desired r2/r1 ratio have been reported. On the other hand, optimizing the rotational correlation time (τR) of small molecular gadolinium complexes by conjugating them onto the surface of SPIO nanoparticles is another effective approach to build T1–T2 dual-modal CAs.31–34
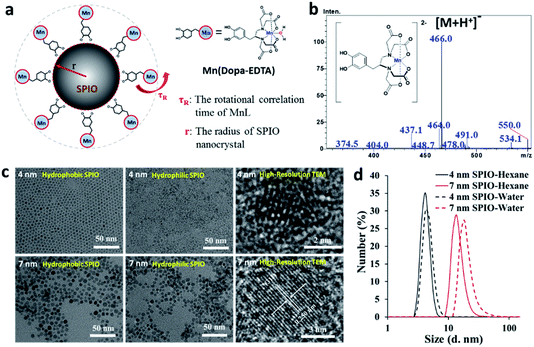
The design of CAs with clinical application prospects should take into account efficacy and biosafety simultaneously. Over the last decade, there is increasing concern about the safety of using GBCAs due to nephrogenic systemic fibrosis (NSF) and gadolinium retention,35–39 and much emphasis is now being put on alternative approaches based on non-lanthanide metals, in particular, more biocompatible manganese by scientists.40–44 However, the direct use of free Mn or manganese oxide also has neurotoxicity risks, and a stable Mn(II) chelate and low Mn dosages are needed. In our previous work, Mn based CAs with good stability and efficacy were developed by optimizing the ligand structure.7,45–50 In this paper, we seek to design a new bifunctional Mn(II) chelate containing a catechol group, which possesses a high affinity for iron(III)31,51–54, to coat on the surface of SPIO nanocrystals for forming non-gadolinium-based nano-scale CAs (Fig. 1a). To explore the performance in MRI, we studied its morphology, composition, aggregate states, relaxation properties and imaging effect in vivo.
The bifunctional ligand (named Dopa-EDTA) with a catechol group and EDTA structure was synthesized from Dopa, and the detailed synthesis steps and the characterization data of intermediates can be found in the ESI.† The chelate of [Mn(Dopa-EDTA)]2− was confirmed from the ESI Mass spectrum (Fig. 1b). Monodispersed SPIO nanocrystals were synthesized by thermal decomposition of iron acetylacetonate.55 Two hydrophobic SPIO nanoparticles with different crystal size distributions were obtained respectively in different reaction solvents and the corresponding water soluble SPIO@[Mn(Dopa-EDTA)]2− nanoparticles can be easily obtained by ligand exchange with [Mn(Dopa-EDTA)]2− (the synthetic procedure can be found in the ESI†). To study the crystal type, size and dispersity of nanoparticles, transmission electron microscopy (TEM) and the dynamic light scattering (DLS) technique were used before and after ligand exchange. As shown in Fig. 1c, both SPIO nanoparticles showed good monodispersion in TEM, and the lattice fringes are clearly visible in the high-resolution TEM (HRTEM) images. These results indicated that the particles are single crystals with monodispersion being maintained before and after ligand exchange. Crystal sizes were measured from select different representative areas under a TEM and were 3.7 ± 0.7 nm and 6.6 ± 1.0 nm respectively for the two sizes of SPIO nanoparticles. In the following text, the corresponding samples were named 4 nm SPIO and 7 nm SPIO. The distance between the two adjacent planes is measured to be 2.50 Å, corresponding to the (311) planes in the spinel-structured Fe3O4. Certainly, further the selected area electron diffraction (SAED) pattern was used to characterize the crystal structure of SPIO nanoparticles (Fig. S3†). The measured lattice spacing based on the rings in the diffraction pattern conformed to the known lattice spacing for bulk Fe3O4 along with their respective hkl indices from the PDF database (Fig. S3†). In DLS analysis (Fig. 1d), it can be observed that there is a slight increase in the number average size, from 4.2 ± 0.7 nm to 4.6 ± 0.9 nm for 4 nm SPIO and from 14.7 ± 3.8 nm to 20.5 ± 6.9 nm for 7 nm SPIO. These results depicted that SPIO nanocrystals maintained monodispersion after transfer to the water phase as a whole but a small amount of aggregation may exist which is unavoidable and often hard to find under a TEM. Zeta potential measurements in ultrapure water show −8.4 ± 2.6 mV and −27.0 ± 1.5 mV for 4 nm and 7 nm nanoparticles respectively, which ensured the stable dispersion of SPIO@[Mn(Dopa-EDTA)]2− nanoparticles in water. The good water solubility, monodispersion in TEM and negative zeta potentials of SPIO nanocrystals explain the successfull ligand exchange of hydrophilic [Mn(Dopa-EDTA)]2− with hydrophobic oleic acid on the surface of SPIO nanoparticles. Iron and manganese ion concentrations of SPIO@[Mn(Dopa-EDTA)]2− nanoparticle solutions were measured by inductively coupled plasma-mass spectrometry (ICP-MS), and Fe-to-Mn ratios (Fe/Mn) and the number of iron and manganese atoms per SPIO nanoparticle were calculated respectively (Table S1†). The Fe/Mn ratio depends on the specific surface area of nanoparticles, as [Mn(Dopa-EDTA)]2− is coated on the surface of SPIO nanocrystals. Deservedly, small size SPIO nanocrystals should have a larger specific surface, which would result in a lower Fe/Mn ratio. Fe/Mn ratios of 4 nm and 7 nm SPIO@[Mn(Dopa-EDTA)]2− are 5.3 and 17.4 respectively and in good accordance with theory.
In order to understand the MR contrast effect (r2/r1 ratio) of SPIO@[Mn(Dopa-EDTA)]2− nanoparticles, we measured their T1 and T2 relaxivities at three main common magnetic fields (0.5, 1.5 and 3.0 T) (Fig. S4 and S5†). The relaxivities were calculated with total paramagnetic metal ion concentration (total iron and manganese ions) of SPIO@[Mn(Dopa-EDTA)]2− nanoparticles in units of (Fe + Mn) mM−1 s−1 (Table 1). In both nanoparticle groups, r1 decreased with the increase of magnetic field (from 0.5 to 3.0 T). On the contrary, r2 gradually increased in the same field range, and resulted in the increase of r2/r1 ratio. This phenomenon indicated that T2 relaxation is more effective at higher fields which reflected the ability of the CA to produce local magnetic inhomogeneities. Overall, both the SPIO@[Mn(Dopa-EDTA)]2− nanoparticles have superior r1 and r2 values, and are typical T1–T2 dual-modal CAs in the appropriate magnetic field (3.0 T for 4 nm SPIO@[Mn(Dopa-EDTA)]2− and 1.5 T for 7 nm SPIO@[Mn(Dopa-EDTA)]2−). In addition, 4 nm SPIO@[Mn(Dopa-EDTA)]2− with smaller r2/r1 = 1.75 at 0.5 T tends to be a perfect T1 contrast agent and 7 nm SPIO@[Mn(Dopa-EDTA)]2− with higher r2/r1 = 15.0 at 3.0 T tends to be a T2 contrast agent. The relaxivities (r1 and r2) of [Mn(Dopa-EDTA)]2− were also measured as a control at corresponding fields, and are listed in Table S2.† The results show that [Mn(Dopa-EDTA)]2− is only a common small molecule T1 contrast agent in the three magnetic fields.
| Sample name | Relaxivities | |||
|---|---|---|---|---|
| B 0 [T] | r 1 [mM−1 s−1]a | r 2 [mM−1 s−1]a | r 2/r1 | |
| a The mM represents concentrations of the total iron and manganese ions. | ||||
| 4 nm SPIO@[Mn(Dopa-EDTA)]2− | 0.5 | 8.4 | 14.7 | 1.75 |
| 1.5 | 7.1 | 24.2 | 3.41 | |
| 3.0 | 5.4 | 28.4 | 5.26 | |
| 7 nm SPIO@[Mn(Dopa-EDTA)]2− | 0.5 | 25.6 | 99.6 | 3.89 |
| 1.5 | 16.2 | 145.7 | 8.99 | |
| 3.0 | 10.0 | 150.0 | 15.00 | |
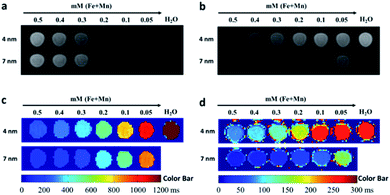
MRI CAs only work well with suitable imaging sequences and parameters. Herein, four types of imaging modes based on the spin-echo sequence (T1WI, T2WI, T1-MAP, and T2-MAP) carried out using a 1.5 T clinical MRI scanner to study the image quality of SPIO@[Mn(Dopa-EDTA)]2− nanoparticles in vitro. Fig. 2a and c show T1WI and T1-MAP of SPIO@[Mn(Dopa-EDTA)]2− aqueous solutions with different concentrations. T1WI imaging (TR = 200 ms, TE = 9 ms) depicted that signal intensity (SI) increased with the concentration of SPIO@[Mn(Dopa-EDTA)]2− nanoparticles, but there was no visual difference in the variation trend between 4 nm and 7 nm SPIO@[Mn(Dopa-EDTA)]2− nanoparticles, though the r1 of 7 nm SPIO@[Mn(Dopa-EDTA)]2− is apparently higher than that of 4 nm SPIO@[Mn(Dopa-EDTA)]2− (Table 1). This is because 7 nm SPIO@[Mn(Dopa-EDTA)]2− solutions show shorter T1 and T2 simultaneously than 4 nm SPIO@[Mn(Dopa-EDTA)]2− solutions at the same concentration, which produce similar SI in this T1WI imaging. To achieve distinct T1WI imaging, much lower TE values must be set, like ultra-short TE.56,57 However, the variation trend of enhanced relaxation can be recognized in T1-MAP more obviously (Fig. 2c). Fig. 2b and d show T2WI and T2-MAP of SPIO@[Mn(Dopa-EDTA)]2− aqueous solution, and SI is obviously varied. These results show that the 7 nm system is more effective than the 4 nm one in both T1-MAP and T2-MAP imaging modes, but the contrast enhancement in T1WI or T2WI imaging depends also on imaging parameters (TE and TR), especially for T1WI imaging.
The biodistribution, clearance and imaging effect of MRI CAs were heavily determined from their stability and the interactions with proteins in plasma once entering the bloodstream. SPIO@[Mn(Dopa-EDTA)]2− nanoparticles show good stability in plasma with their surface coated with negative charge [Mn(Dopa-EDTA)]2−. To further evaluate their serum stability, we monitored the size changes of SPIO@[Mn(Dopa-EDTA)]2− in DLS after being incubated with 20% (v/v) fetal bovine serum (FBS) solution at 37 °C for 5 h. The size increase with incubation time is observed in the presence of FBS (Fig. S6†), though visible precipitation did not occur in the whole experiment. The results state that nonspecific adsorption still exists on the surface of SPIO@[Mn(Dopa-EDTA)]2− after long time incubation in serum proteins. The cytotoxicity of SPIO@[Mn(Dopa-EDTA)]2− nanoparticles was evaluated by CCK-8 assay on mouse macrophage cell line Raw 264.7. Fig. S7† shows the cytotoxicity of 4 nm and 7 nm SPIO@[Mn(Dopa-EDTA)]2− after incubation for 24 h. The results indicate that low concentration SPIO@[Mn(Dopa-EDTA)]2− (below 10 μg mL−1) promoted growth of Raw 264.7 cells, and cell growth was restrained with increasing SPIO@[Mn(Dopa-EDTA)]2− nanoparticle concentration. This phenomenon states that Fe and Mn are necessary trace elements in the living body and benefit cell reproduction at suitable concentrations. 4 nm SPIO@[Mn(Dopa-EDTA)]2− shows more toxic effects than 7 nm SPIO@[Mn(Dopa-EDTA)]2− at high concentrations, maybe the result of its high [Mn(Dopa-EDTA)]2− content.
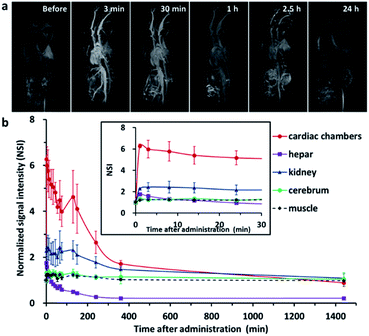
Contrast enhanced MRA (CEMRA) is an important T1 weighted imaging method to detect cardiovascular system diseases in clinics, such as arterial aneurysm, vascular malformation and tumor angiogenesis. Based on the excellent T1 relaxivity of SPIO@[Mn(Dopa-EDTA)]2− nanoparticles, CEMRA was performed on SD rats to evaluate their vascular structure, contrast enhancement, and tissue distribution in vivo. Fig. 3a shows the MR images of SD rats before and after intravenous injection of 4 nm SPIO@[Mn(Dopa-EDTA)]2− nanoparticles with the CEMRA sequence. The cardiac and vascular lumen can be enhanced over a long period time with these nanoparticles (lasting about 2.5 h) (Fig. S8†). The signal intensities (SI) in the cardiac chamber, liver parenchyma, renal parenchyma, cerebrum and muscles at different time points were measured and were normalized to SI prior to contrast agent injection. The change of normalized signal intensity (NSI) with time is depicted in Fig. 3b. From the NSI-time curves, it can be observed that the signal intensities in the cardiac chamber, liver parenchyma and renal parenchyma reached the peak quickly within minutes and then present different variation tendency with time. For the cardiac chamber and renal parenchyma, the signal intensities maintain a consistently high value up to 2 hours, and then decrease slowly, and finally return to the level before administration at 24 hours. However, the signal intensity in the liver parenchyma shows obviously different changes. After reaching the peak in one minute, the signal intensity constantly decreases with time, and maintains a very low signal for more than 24 hours. In addition, the cerebrum and muscles maintain a constant slightly enhancement for several hours after administration. These results show that SPIO@[Mn(Dopa-EDTA)]2− nanoparticles are an excellent MRA agent with a very long imaging time window, owing to their high longitudinal relaxivity (r1) and nano-size effect, which cannot be achieved by a small molecular contrast agent, like GdDTPA (Fig. S9†). The signal intensity change in the liver parenchyma is another interesting feature. The initial signal intensity increase is the result of the SPIO@[Mn(Dopa-EDTA)]2− nanoparticle distribution in the hepatic microvessel. The following intensity decrease gradually may be the result of SPIO@[Mn(Dopa-EDTA)]2− nanoparticle accumulation in the hepatic parenchyma, which may cause aggregation of SPIO@[Mn(Dopa-EDTA)]2− nanoparticles and lead to a strong T2 relaxation effect. These imaging features indicate that SPIO@[Mn(Dopa-EDTA)]2− nanoparticles are stable and single-dispersed in the circulating blood (induce a bright T1 contrast) and are gradually captured and confined by phagocytes in the liver tissue (induce a dark T2 contrast). This conclusion was further confirmed by pathological histology of the liver (Fig. S10†). Prussian blue staining showed plenty of blue iron particles located in the hepatic tissue, which were extracted from rats at 24 hours after administration of SPIO@[Mn(Dopa-EDTA)]2− nanoparticles. Similar results can also be found in previous reports.18,21
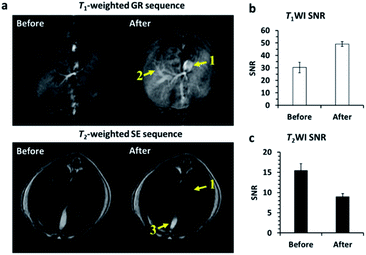
At 3.0 T, 4 nm SPIO@[Mn(Dopa-EDTA)]2− nanoparticles with an intermediate r2/r1 ratio (5.26) serve as T1–T2 dual-modal CAs. For further verification of the T1–T2 dual-modal enhancement of SPIO@[Mn(Dopa-EDTA)]2− nanoparticles in vivo, T1WI and T2WI sequences were used to study the hepatic imaging of BALB/c mice simultaneously. Fig. 4a shows the imaging results before and after administration of 4 nm SPIO@[Mn(Dopa-EDTA)]2− nanoparticles. The liver tissue area reveals hyperintensity in T1WI and hypointensity in T2WI after administration, and the hepatic vessels and gallbladder are visible in the T1–T2 dual-modal imaging. Fig. 4b and c show that the signal changes are obvious in the liver tissues before and after administration, and the SNR increase is 64.6 ± 18.7% for T1WI and the decrease is 41.7 ± 4.2% for T2WI respectively. These results indicate that SPIO@[Mn(Dopa-EDTA)]2− has potential to serve as a T1–T2 dual-modal MRI CA for liver imaging, which may be helpful for accurate diagnosis of liver lesions, like tumors and nodules.
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of North Sichuan Medical College and experiments were approved by the Animal Ethics Committee of North Sichuan Medical College.
Conclusions
We have developed a new non-gadolinium-based nano-scale MRI contrast agent SPIO@[Mn(Dopa-EDTA)]2− nanoparticles based on the catechol chemistry strategy. Our study has shown that 4 and 7 nm SPIO@[Mn(Dopa-EDTA)]2− nanoparticles show excellent MRI relaxation performance and different relaxation behavior at 0.5, 1.5 and 3.0 T to be fit for use as T1, T2 or T1–T2 dual-modal contrast agents. 4 nm SPIO@[Mn(Dopa-EDTA)]2− nanoparticles with an appropriate value of r2/r1 (5.26) at 3.0 T can serve as T1–T2 dual-modal MRI contrast agents to produce the synergistic tissue contrast enhancement and long-acting vascular imaging in vivo. These nanoparticles may provide a useful method for human organ and vascular MR imaging with their ease of synthesis, good biocompatibility, and unique imaging features in vivo.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by the National Natural Science Foundation of China (81601490 and 81671675), Science and Technology Project of Sichuan, China (2016JY0172), Science and Technology Project of Municipal School Strategic Cooperation, Nanchong (NSMC20170101, NSMC20170430 and 18SXHZ0091), Scientific Research Start-up Fund of North Sichuan Medical College (CBY15-QD02), and Pre-research Project of North Sichuan Medical College (CBY19-YZ05).Notes and references
- M. Rudin and R. Weissleder, Nat. Rev. Drug Discovery, 2003, 2, 123–131 CrossRef CAS PubMed.
- G. Yves, H. Aline, G. Pierre and V. Q. Lam, J. Phys. D: Appl. Phys., 2010, 43, 213001 CrossRef.
- P. Caravan, J. J. Ellison, T. J. McMurry and R. B. Lauffer, Chem. Rev., 1999, 99, 2293–2352 CrossRef CAS PubMed.
- J. Tang, Y. Sheng, H. Hu and Y. Shen, Prog. Polym. Sci., 2013, 38, 462–502 CrossRef CAS.
- R. B. Lauffer, Chem. Rev., 1987, 87, 901–927 CrossRef CAS.
- V. M. Runge and J. T. Heverhagen, Invest. Radiol., 2018, 53, 381–389 CrossRef CAS PubMed.
- H. Su, C. Wu, J. Zhu, T. Miao, D. Wang, C. Xia, X. Zhao, Q. Gong, B. Song and H. Ai, Dalton Trans., 2012, 14480–14483 RSC.
- H. Ai, C. Flask, B. Weinberg, X.-T. Shuai, M. D. Pagel, D. Farrell, J. Duerk and J. Gao, Adv. Mater., 2005, 17, 1949–1952 CrossRef CAS.
- N. Lee, D. Yoo, D. Ling, M. H. Cho, T. Hyeon and J. Cheon, Chem. Rev., 2015, 115, 10637–10689 CrossRef CAS PubMed.
- J. W. Bulte and D. L. Kraitchman, NMR Biomed., 2004, 17, 484–499 CrossRef CAS PubMed.
- Z. Zhou, R. Bai, J. Munasinghe, Z. Shen, L. Nie and X. Chen, ACS Nano, 2017, 11, 5227–5232 CrossRef CAS PubMed.
- T.-H. Shin, J.-s. Choi, S. Yun, I.-S. Kim, H.-T. Song, Y. Kim, K. I. Park and J. Cheon, ACS Nano, 2014, 8, 3393–3401 CrossRef CAS PubMed.
- M. Moseley, Stroke, 2004, 35, 2632–2634 CrossRef.
- Z. Zhou, Z. Zhao, H. Zhang, Z. Wang, X. Chen, R. Wang, Z. Chen and J. Gao, ACS Nano, 2014, 8, 7976–7985 CrossRef CAS PubMed.
- Z. Zhou, C. Wu, H. Liu, X. Zhu, Z. Zhao, L. Wang, Y. Xu, H. Ai and J. Gao, ACS Nano, 2015, 9, 3012–3022 CrossRef CAS PubMed.
- H. Jung, B. Park, C. Lee, J. Cho, J. Suh, J. Park, Y. Kim, J. Kim, G. Cho and H. Cho, Nanomedicine, 2014, 10, 1679–1689 CrossRef CAS PubMed.
- G. Huang, H. Li, J. Chen, Z. Zhao, L. Yang, X. Chi, Z. Chen, X. Wang and J. Gao, Nanoscale, 2014, 6, 10404–10412 RSC.
- L. Wang, J. Huang, H. Chen, H. Wu, Y. Xu, Y. Li, H. Yi, Y. A. Wang, L. Yang and H. Mao, ACS Nano, 2017, 11, 4582–4592 CrossRef CAS PubMed.
- C. Bai, Z. Jia, L. Song, W. Zhang, Y. Chen, F. Zang, M. Ma, N. Gu and Y. Zhang, Adv. Funct. Mater., 2018, 28, 1802281 CrossRef.
- Z. Li, P. W. Yi, Q. Sun, H. Lei, H. Li Zhao, Z. H. Zhu, S. C. Smith, M. B. Lan and G. Q. M. Lu, Adv. Funct. Mater., 2012, 22, 2387–2393 CrossRef CAS.
- J. Huang, L. Wang, X. Zhong, Y. Li, L. Yang and H. Mao, J. Mater. Chem. B, 2014, 2, 5344–5351 RSC.
- X. L. Liu, C. T. Ng, P. Chandrasekharan, H. T. Yang, L. Y. Zhao, E. Peng, Y. B. Lv, W. Xiao, J. Fang, J. B. Yi, H. Zhang, K.-H. Chuang, B. H. Bay, J. Ding and H. M. Fan, Adv. Healthcare Mater., 2016, 5, 2092–2104 CrossRef CAS PubMed.
- X. Wang, Z. Zhou, Z. Wang, Y. Xue, Y. Zeng, J. Gao, L. Zhu, X. Zhang, G. Liu and X. Chen, Nanoscale, 2013, 5, 8098 RSC.
- N. Xiao, W. Gu, H. Wang, Y. Deng, X. Shi and L. Ye, J. Colloid Interface Sci., 2014, 417, 159–165 CrossRef CAS PubMed.
- Z. Zhou, D. Huang, J. Bao, Q. Chen, G. Liu, Z. Chen, X. Chen and J. Gao, Adv. Mater., 2012, 24, 6223–6228 CrossRef CAS PubMed.
- H. Cai, X. An, S. Wen, J. Li, G. Zhang, X. Shi and M. Shen, Part. Part. Syst. Charact., 2015, 32, 934–943 CrossRef CAS.
- J.-s. Choi, J.-H. Lee, T.-H. Shin, H.-T. Song, E. Y. Kim and J. Cheon, J. Am. Chem. Soc., 2010, 132, 11015–11017 CrossRef CAS PubMed.
- M.-H. Kim, H.-Y. Son, G.-Y. Kim, K. Park, Y.-M. Huh and S. Haam, Biomaterials, 2016, 101, 121–130 CrossRef CAS PubMed.
- F. Li, D. Zhi, Y. Luo, J. Zhang, X. Nan, Y. Zhang, W. Zhou, B. Qiu, L. Wen and G. Liang, Nanoscale, 2016, 8, 12826–12833 RSC.
- Y.-K. Peng, C. N. P. Lui, Y.-W. Chen, S.-W. Chou, E. Raine, P.-T. Chou, K. K. L. Yung and S. C. E. Tsang, Chem. Mater. , 2017, 29, 4411–4417 CrossRef CAS.
- K. H. Bae, Y. B. Kim, Y. Lee, J. Hwang, H. Park and T. G. Park, Bioconjugate Chem., 2010, 21, 505–512 CrossRef CAS PubMed.
- H. Guo, H. Sun, H. Zhu, H. Guo and H. Sun, New J. Chem., 2018, 42, 7119–7124 RSC.
- A. Szpak, S. Fiejdasz, W. Prendota, T. Strączek, C. Kapusta, J. Szmyd, M. Nowakowska and S. Zapotoczny, J. Nanopart. Res., 2014, 16, 2678 CrossRef PubMed.
- H. Yang, Y. Zhuang, Y. Sun, A. Dai, X. Shi, D. Wu, F. Li, H. Hu and S. Yang, Biomaterials, 2011, 32, 4584–4593 CrossRef CAS PubMed.
- S. Fingerhut, M. Sperling, M. Holling, T. Niederstadt, T. Allkemper, A. Radbruch, W. Heindel, W. Paulus, A. Jeibmann and U. Karst, Acta Neuropathol., 2018, 136, 127–138 CrossRef CAS PubMed.
- T. Kanda, T. Fukusato, M. Matsuda, K. Toyoda, H. Oba, J. i. Kotoku, T. Haruyama, K. Kitajima and S. Furui, Radiology, 2015, 276, 228–232 CrossRef PubMed.
- M. A. Perazella, Clin. J. Am. Soc. Nephrol., 2007, 2, 200–202 CrossRef CAS PubMed.
- M. A. Perazella, Curr. Opin. Nephrol. Hypertens., 2009, 18, 519–525 CrossRef PubMed.
- V. M. Runge, Invest. Radiol., 2017, 52, 317–323 CrossRef CAS PubMed.
- D. Pan, S. D. Caruthers, A. Senpan, A. H. Schmieder, S. A. Wickline and G. M. Lanza, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2011, 3, 162–173 CAS.
- B. Drahoš, I. Lukeš and É. Tóth, Eur. J. Inorg. Chem., 2012, 2012, 1975–1986 CrossRef.
- D. Pan, A. H. Schmieder, S. A. Wickline and G. M. Lanza, Tetrahedron, 2011, 67, 8431–8444 CrossRef CAS PubMed.
- J. Wahsner, E. M. Gale, A. Rodríguez-Rodríguez and P. Caravan, Chem. Rev., 2019, 119, 957–1057 CrossRef CAS PubMed.
- E. M. Gale, H.-Y. Wey, I. Ramsay, Y.-F. Yen, D. E. Sosnovik and P. Caravan, Radiology, 2018, 286, 865–872 CrossRef PubMed.
- C. Wu, L. Yang, Z. Chen, H. Zhang, D. Li, B. Lin, J. Zhu, H. Ai and X. Zhang, RSC Adv., 2017, 7, 54603–54609 RSC.
- E. M. Gale, I. P. Atanasova, F. Blasi, I. Ay and P. Caravan, J. Am. Chem. Soc., 2015, 137, 15548–15557 CrossRef CAS PubMed.
- C. Wu, D. Li, L. Yang, B. Lin, H. Zhang, Y. Xu, Z. Cheng, C. Xia, Q. Gong, B. Song and H. Ai, J. Mater. Chem. B, 2015, 1470–1473 RSC.
- E. M. Gale, S. Mukherjee, C. Liu, G. S. Loving and P. Caravan, Inorg. Chem., 2014, 53, 10748–10761 CrossRef CAS PubMed.
- A. Forgács, R. Pujales-Paradela, M. Regueiro-Figueroa, L. Valencia, D. Esteban-Gómez, M. Botta and C. Platas-Iglesias, Dalton Trans., 2017, 46, 1546–1558 RSC.
- J. Zhu, E. M. Gale, I. Atanasova, T. A. Rietz and P. Caravan, Chem. – Eur. J., 2014, 20, 14507–14513 CrossRef CAS PubMed.
- J. A. Davies, S. G. Dutremez, C. M. Hockensmith, R. Keck, N. Richardson, S. Selman, D. A. Smith, C. W. Ulmer, L. S. Wheatley and J. Zeiss, Acad. Radiol., 1996, 3, 936–945 CrossRef CAS PubMed.
- Q. Ye, F. Zhou and W. Liu, Chem. Soc. Rev., 2011, 40, 4244–4258 RSC.
- C. Xu, K. Xu, H. Gu, R. Zheng, H. Liu, X. Zhang, Z. Guo and B. Xu, J. Am. Chem. Soc., 2004, 126, 9938–9939 CrossRef CAS PubMed.
- R. C. Hider and X. Kong, Nat. Prod. Rep., 2010, 27, 637–657 RSC.
- S. Sun, H. Zeng, D. B. Robinson, S. Raoux, P. M. Rice, S. X. Wang and G. Li, J. Am. Chem. Soc., 2004, 126, 273–279 CrossRef CAS PubMed.
- O. M. Girard, J. Du, L. Agemy, K. N. Sugahara, V. R. Kotamraju, E. Ruoslahti, G. M. Bydder and R. F. Mattrey, Magn. Reson. Med., 2011, 65, 1649–1660 CrossRef CAS PubMed.
- L. Wang, X. Zhong, W. Qian, J. Huang, Z. Cao, Q. Yu, M. Lipowska, R. Lin, A. Wang, L. Yang and H. Mao, J. Magn. Reson. Imaging, 2014, 40, 1071–1081 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0na00117a |
| ‡ C. W. and T. C. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |