Open Access Article
Open Access ArticleNiS2 nanoparticles anchored on open carbon nanohelmets as an advanced anode for lithium-ion batteries†
D. D.
Yang
,
M.
Zhao
,
R. D.
Zhang
,
Y.
Zhang
,
C. C.
Yang
 * and
Q.
Jiang
* and
Q.
Jiang

Key Laboratory of Automobile Materials (Jilin University), Ministry of Education, School of Materials Science and Engineering, Jilin University, Changchun 130022, China. E-mail: ccyang@jlu.edu.cn; Fax: +86-431-85095876; Tel: +86-431-85095371
First published on 13th December 2019
Abstract
Low intrinsic conductivity and large volume expansion seriously restrict the efficient lithium storage performance of metal sulfides. Here, we fabricate a hybrid material of NiS2 nanoparticles/carbon nanohelmets (NiS2/CNHs) to address the above issues. As an anode material in lithium-ion batteries, NiS2/CNHs exhibit excellent cycling stability (490 mA h g−1 after 3000 cycles at 5 A g−1) and rate properties (412 mA h g−1 at 10 A g−1), outperforming other NiSx-based anode materials. These remarkable performances originate from the three-dimensional helmet-like integrated architecture of NiS2/CNHs, which reduces the electrode resistance due to the tight combination between NiS2 and CNHs, provides efficient diffusion paths for the electrolyte and Li+ owing to the amorphous nanoporous carbon structure, and significantly mitigates the aggregation and buffers the large volumetric expansion of NiS2 nanoparticles upon long-term cycling thanks to the open three-dimensional architecture and well-dispersed NiS2 nanoparticles on it.
Introduction
Lithium-ion batteries (LIBs), as efficient energy storage devices, have been widely used in consumer electronics and electric vehicles due to their high energy density, light weight, long life span and environmental benignity.1–5 In this context, developing advanced electrode materials with excellent electrochemical performance is of great importance to cater to the growing requirements of large-scale practical applications.6–9 To date, a large number of LIB anode materials, such as carbonaceous materials,10,11 alloys (NiSn, CuZn, SiCu, etc.),12–14 metal oxides (Fe2O3, SnO2, CoO2, TiO2, etc.),15–18 and metal nitrides and sulfides (Ni3N, Fe3N, MoS2, CoS2, SnS2, etc.),19–23 have been extensively studied. Among the various candidates, transition metal sulfides (TMSs) have enormous potential due to their higher electronic conductivity, much better cycling performance and higher theoretical capacity than those of their metal oxide counterparts.24 In particular, NiS2 attracted many researchers' attention owing to its high theoretical capacity (870 mA h g−1). Nevertheless, the inherent relatively low conductivity and huge volume change during the charge/discharge process restrict the electrochemical performance of NiS2 in LIBs.25,26To tackle the aforementioned issues, many effective strategies have been proposed to construct advanced NiS2 nanostructures and their hybrid materials. In this regard, the hybridization of NiS2 with carbon-based materials is an effective approach to improve the electrochemical properties.25–27 For example, Chen et al. reported that a composite of NiS2/graphene delivered a reversible capacity as high as 810 mA h g−1 at a current density of 500 mA g−1 after 1000 cycles.26 Lou et al. synthesized a NiS2@C hybrid material, which exhibits excellent rate performance and a reversible capacity of 577 mA h g−1 at 2000 mA g−1.27 However, achieving both superior cycle stability and rate performance is highly desirable. Recently, Yang et al. synthesized a hybrid structure by anchoring NiO nanoparticles on open helmet-like carbon skeletons (NiO/CNHs), which shows both excellent long-term cycling performance (424 mA h g−1 after 1500 cycles at 7.5C) and rate performance (450 mA h g−1 at 15C).28 Such a unique carbon nanoarchitecture is expected to also be applicable for NiS2 nanoparticles.
Herein, a hybrid material of NiS2 nanoparticles/carbon nanohelmets (NiS2/CNHs) was fabricated through the sulfurization of NiO/CNHs. The NiS2/CNH hybrid material has the following advantages: (1) the tight combination between NiS2 nanoparticles and CNHs can greatly improve the overall conductivity of the hybrid material; (2) the CNH structure can be used as a matrix for dispersing NiS2 nanoparticles uniformly, avoiding their self-aggregation and thus increasing the active sites for Li+ insertion/extraction; (3) the porous CNHs can provide efficient electrolyte pathways and accelerate carrier transfer; (4) the open CNH skeleton can effectively alleviate the internal stress caused by the large volume change of NiS2 nanoparticles during the charge/discharge process. As expected, the NiS2/CNH hybrid material exhibits superior rate performance (412 mA h g−1 at 10 A g−1) and excellent long-term cycling stability (490 mA h g−1 after 3000 cycles at 5 A g−1) as an ideal anode material in LIBs.
Results and discussion
Materials synthesis and characterization
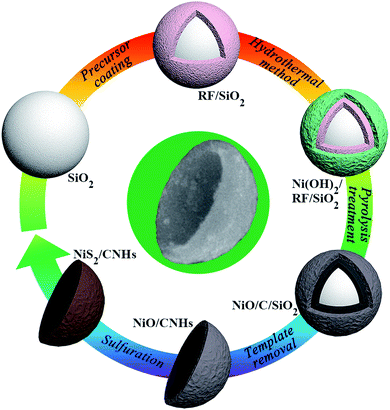
The fabrication procedure of the NiS2/CNH hybrid material is schematically illustrated in Fig. 1. The field-emission scanning electron microscope (FESEM) images of the corresponding products, including SiO2 spheres, resorcinol and formaldehyde resin/SiO2 spheres (RF/SiO2), Ni(OH)2/RF/SiO2, NiO/C/SiO2 and NiO/CNHs, are shown in Fig. S1–S5.† Firstly, RF resin shells were formed on the surface of the SiO2 template spheres with a polymerization process. Then, Ni(OH)2 was grown on the surface of RF/SiO2via a hydrothermal reaction followed by a pyrolysis treatment under nitrogen protection to obtain NiO/C/SiO2. Afterwards, NiO/CNH hybrids were obtained through a template removal method in NaOH solution. Here, the formation mechanism of the CNHs can be described as follows. During the carbonization process, some gaseous products (CO2, CO, H2O, CH4, H2, etc.) were generated from the pyrolysis of RF, which escaped from the system once their pressure became high enough. The plasticity of the carbon shell was weakened due to the pressure impact and the large number of pores created by the release of gaseous products. During the etching process of SiO2, the Si–O–C interconnections were destroyed, resulting in a decrease of the interfacial tension force. As a result, the shrinkage of carbon shells occurred under the influence of constrictive forces from NiO. With the continuous increase of the shrinkage degree, it was difficult for the highly porous hollow carbon skeleton to withstand the shrinkage force, and it eventually fractured to form the CNH structure.28 In addition, the intense agitation forces during the etching process also play an important role in accelerating the fracture of the hollow structure. Finally, NiO/CNHs were sulfurized with sublimed sulfur in a quartz tube reactor to obtain the NiS2/CNH composite. It is known that Ni-based oxides and sulfides undergo conversion in LIBs. In general, TMSs exhibit higher electronic conductivity than their metal oxide counterparts.24 Moreover, the metal–sulfur bonds in TMSs are relatively weaker than the metal–oxygen bonds in transition metal oxides (TMOs) due to the lower electronegativity of S compared with O, which is favorable for the conversion reaction for TMSs during the charge/discharge process in LIBs.29,30 Note that the sulfurization process of NiO/CNHs was conducted under conditions of nitrogen protection, high sulfur content and high temperature. The corresponding conversion reaction from NiO to NiS2 can be expressed using:| 2NiO (s) + 5S (g) → 2NiS2 (s) + SO2 (g). | (1) |
Such an approach has been reported in previous studies.31,32 For example, Wu et al. synthesized a hybrid of NiS2 nanosheets grown on a graphite substrate via the sulfidation of NiO nanosheets with S vapor at 350 °C under argon protection.31 Li et al. reported that NiO hollow spheres were sulfurized to NiS2 by using sulfur powder at 350 °C under an argon atmosphere.32 For comparison, a sample of pristine NiS2 was also fabricated by sulfurization of NiO without the CNH skeleton. All materials synthesis details are provided in the Experimental section (see the ESI† for details).
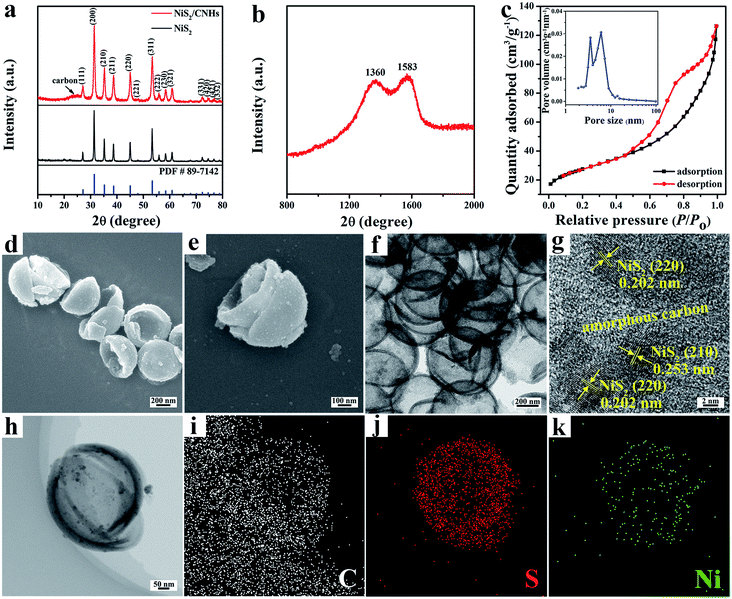
Fig. 2a compares the X-ray diffraction (XRD) patterns of NiS2/CNHs and pristine NiS2. For each sample, the characteristic peaks at 27.1°, 31.4°, 35.3°, 38.8°, 45.1° and 53.4° can be indexed to the (111), (200), (210), (211), (220) and (311) planes, respectively, of the cubic NiS2 phase with the Pa![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group (JCPDF no. 89-7142). Moreover, for NiS2/CNHs, the broad peak between 20° and 30° is assigned to the characteristic structure of amorphous carbon.28,33Fig. 2b shows the Raman spectrum of NiS2/CNHs, in which two typical peaks at 1360 and 1586 cm−1 are assigned to the D band and G band of carbon, respectively.34 The calculated value of ID/IG is 1.00, demonstrating the abundance of topological defects and disorders in CNHs, which are beneficial for lithium storage.35 The nitrogen adsorption/desorption isotherm (see Fig. 2c) of NiS2/CNHs exhibits a type-IV behavior with a representative hysteresis loop in the relative pressure range of 0.45–1 that can be linked to a large amount of mesopores in NiS2/CNHs.36,37 From the inset curve of Fig. 2c, the sizes of most pores are concentrated within 2–10 nm, while other pores exhibit a wide size distribution between 10 and 100 nm. Moreover, the NiS2/CNHs exhibit a relatively large specific surface area of 96.62 m2 g−1. The large specific surface area and the high porosity result in a large electrode–electrolyte contact area and fast Li+ transport, which are beneficial for high discharge capacity and excellent rate performance.38,39Fig. 2d and e present the FESEM images of NiS2/CNHs, where hollow CNHs are formed with heights of 200–300 nm and diameters of 400–600 nm. It is obvious that the NiS2 nanoparticles are uniformly dispersed on CNHs without the accumulation of many nanoparticles. In contrast, the NiS2 nanoparticles without CNHs as a carrier aggregated into a bulk one (see Fig. S6†), which demonstrates that CNHs can effectively inhibit the agglomeration of NiS2 nanoparticles. Fig. 2f shows a transmission electron microscopy (TEM) image of NiS2/CNHs, further confirming the uniform dispersion of NiS2 nanoparticles on the CNH skeleton. Such an open structure can greatly alleviate the huge volume change of NiS2 during the charge/discharge process and thus enhance the cycle stability of NiS2/CNHs. It should be mentioned that the inner carbon layer in the open NiS2/CNHs shows structural advantages for lithium storage by (i) providing a large number of active sites for Li+ insertion/extraction, which is beneficial for the enhancement of the specific capacity; (ii) providing efficient diffusion paths for the electrolyte and Li+; and (iii) significantly reducing the internal resistance contributed by the contact between NiS2 and the inner carbon layer. Fig. 2g presents a high-resolution TEM image of NiS2/CNHs, in which the interplanar spacings of 0.202 nm and 0.253 nm correspond to the (220) and (210) planes of NiS2, respectively. From the figure, NiS2 nanoparticles are all surrounded by amorphous carbon, indicating the excellent contact between them. Fig. S7† exhibits a TEM image and particle size distribution (the inset) of NiS2/CNHs, where the mean size of NiS2 nanoparticles is 8.10 nm. Such small nanoparticles provide abundant active sites for Li+ insertion/extraction, increasing the reversible capacity of the NiS2/CNHs. Moreover, the TEM and elemental mapping images display homogeneous distributions of S and Ni along the CNH skeleton (see Fig. 2h–k). To further determine the content of active material in NiS2/CNHs, a thermogravimetric analysis (TGA) test was carried out. As exhibited in Fig. S8,† the volatilization of water contained in the NiS2/CNHs occurred below the temperature of 250 °C. The mass loss above 250 °C was ascribed to the oxidation of NiS2 and carbon. Thus, the content of NiS2 in the NiS2/CNHs is calculated to be 83.8%, based on the equation in Fig. S8.†
space group (JCPDF no. 89-7142). Moreover, for NiS2/CNHs, the broad peak between 20° and 30° is assigned to the characteristic structure of amorphous carbon.28,33Fig. 2b shows the Raman spectrum of NiS2/CNHs, in which two typical peaks at 1360 and 1586 cm−1 are assigned to the D band and G band of carbon, respectively.34 The calculated value of ID/IG is 1.00, demonstrating the abundance of topological defects and disorders in CNHs, which are beneficial for lithium storage.35 The nitrogen adsorption/desorption isotherm (see Fig. 2c) of NiS2/CNHs exhibits a type-IV behavior with a representative hysteresis loop in the relative pressure range of 0.45–1 that can be linked to a large amount of mesopores in NiS2/CNHs.36,37 From the inset curve of Fig. 2c, the sizes of most pores are concentrated within 2–10 nm, while other pores exhibit a wide size distribution between 10 and 100 nm. Moreover, the NiS2/CNHs exhibit a relatively large specific surface area of 96.62 m2 g−1. The large specific surface area and the high porosity result in a large electrode–electrolyte contact area and fast Li+ transport, which are beneficial for high discharge capacity and excellent rate performance.38,39Fig. 2d and e present the FESEM images of NiS2/CNHs, where hollow CNHs are formed with heights of 200–300 nm and diameters of 400–600 nm. It is obvious that the NiS2 nanoparticles are uniformly dispersed on CNHs without the accumulation of many nanoparticles. In contrast, the NiS2 nanoparticles without CNHs as a carrier aggregated into a bulk one (see Fig. S6†), which demonstrates that CNHs can effectively inhibit the agglomeration of NiS2 nanoparticles. Fig. 2f shows a transmission electron microscopy (TEM) image of NiS2/CNHs, further confirming the uniform dispersion of NiS2 nanoparticles on the CNH skeleton. Such an open structure can greatly alleviate the huge volume change of NiS2 during the charge/discharge process and thus enhance the cycle stability of NiS2/CNHs. It should be mentioned that the inner carbon layer in the open NiS2/CNHs shows structural advantages for lithium storage by (i) providing a large number of active sites for Li+ insertion/extraction, which is beneficial for the enhancement of the specific capacity; (ii) providing efficient diffusion paths for the electrolyte and Li+; and (iii) significantly reducing the internal resistance contributed by the contact between NiS2 and the inner carbon layer. Fig. 2g presents a high-resolution TEM image of NiS2/CNHs, in which the interplanar spacings of 0.202 nm and 0.253 nm correspond to the (220) and (210) planes of NiS2, respectively. From the figure, NiS2 nanoparticles are all surrounded by amorphous carbon, indicating the excellent contact between them. Fig. S7† exhibits a TEM image and particle size distribution (the inset) of NiS2/CNHs, where the mean size of NiS2 nanoparticles is 8.10 nm. Such small nanoparticles provide abundant active sites for Li+ insertion/extraction, increasing the reversible capacity of the NiS2/CNHs. Moreover, the TEM and elemental mapping images display homogeneous distributions of S and Ni along the CNH skeleton (see Fig. 2h–k). To further determine the content of active material in NiS2/CNHs, a thermogravimetric analysis (TGA) test was carried out. As exhibited in Fig. S8,† the volatilization of water contained in the NiS2/CNHs occurred below the temperature of 250 °C. The mass loss above 250 °C was ascribed to the oxidation of NiS2 and carbon. Thus, the content of NiS2 in the NiS2/CNHs is calculated to be 83.8%, based on the equation in Fig. S8.†
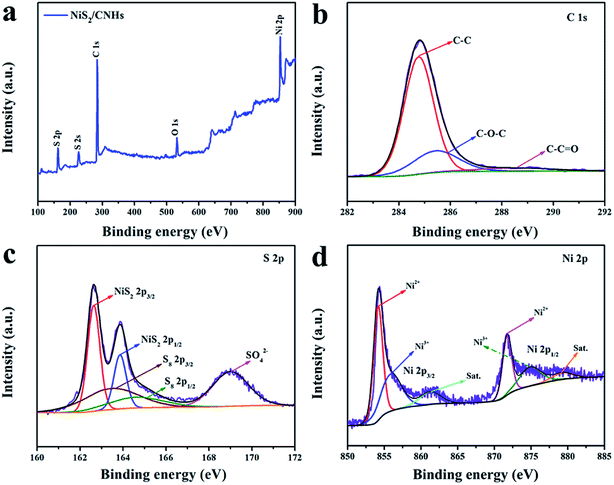
The chemical composition and surface electronic state of C, S and Ni elements in NiS2/CNHs were further investigated by X-ray photoelectron spectroscopy (XPS). The survey spectrum reveals that the primary elements in NiS2/CNHs are C, S, Ni and O (see Fig. 3a). As presented in Fig. 3b, the high resolution XPS spectrum of C 1s has three obvious peaks, which correspond to the strong C–C bond (284.8 eV), C–O bond (285.5 eV) and weaker C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond (288.8 eV), respectively.40,41 Five fitted peaks exist in the S 2p spectrum (see Fig. 3c) where the two strong peaks at 162.6 and 163.85 eV are attributed to 2p3/2 and 2p1/2 of the S element in the NiS2, indicating the presence of S–S bonds in the NiS2/CNHs. In addition, a pair of peaks around 163.55 eV and 164.7 eV are assigned to the residual S (α-S8) in NiS2/CNHs, which may be retained during the sulfurization process.42,43 The peak located at 169.0 eV is ascribed to the S–O covalent bond due to the surface oxidation of the NiS2/CNHs.41,44 Note that elemental sulfur is not detected in the XRD pattern, indicating its trace amount.45Fig. 3d shows the high resolution XPS spectrum of Ni 2p, which exhibits three pairs of peaks. The peaks situated at 854.2 and 871.75 eV are attributed to 2p3/2 and 2p1/2 of Ni2+, while the peaks located at 856.05 and 875.1 eV are assigned to 2p3/2 and 2p1/2 of Ni3+. In addition, the two other peaks at 861.35 and 879.5 eV are ascribed to the satellite peaks of Ni 2p3/2 and Ni 2p1/2, respectively.25,46
O bond (288.8 eV), respectively.40,41 Five fitted peaks exist in the S 2p spectrum (see Fig. 3c) where the two strong peaks at 162.6 and 163.85 eV are attributed to 2p3/2 and 2p1/2 of the S element in the NiS2, indicating the presence of S–S bonds in the NiS2/CNHs. In addition, a pair of peaks around 163.55 eV and 164.7 eV are assigned to the residual S (α-S8) in NiS2/CNHs, which may be retained during the sulfurization process.42,43 The peak located at 169.0 eV is ascribed to the S–O covalent bond due to the surface oxidation of the NiS2/CNHs.41,44 Note that elemental sulfur is not detected in the XRD pattern, indicating its trace amount.45Fig. 3d shows the high resolution XPS spectrum of Ni 2p, which exhibits three pairs of peaks. The peaks situated at 854.2 and 871.75 eV are attributed to 2p3/2 and 2p1/2 of Ni2+, while the peaks located at 856.05 and 875.1 eV are assigned to 2p3/2 and 2p1/2 of Ni3+. In addition, the two other peaks at 861.35 and 879.5 eV are ascribed to the satellite peaks of Ni 2p3/2 and Ni 2p1/2, respectively.25,46
 | ||
| Fig. 3 XPS analysis. (a) The survey XPS spectrum of NiS2/CNHs. (b–d) High-resolution XPS spectra of C 1s, S 2p and Ni 2p, respectively, of NiS2/CNHs. | ||
Electrochemical performance
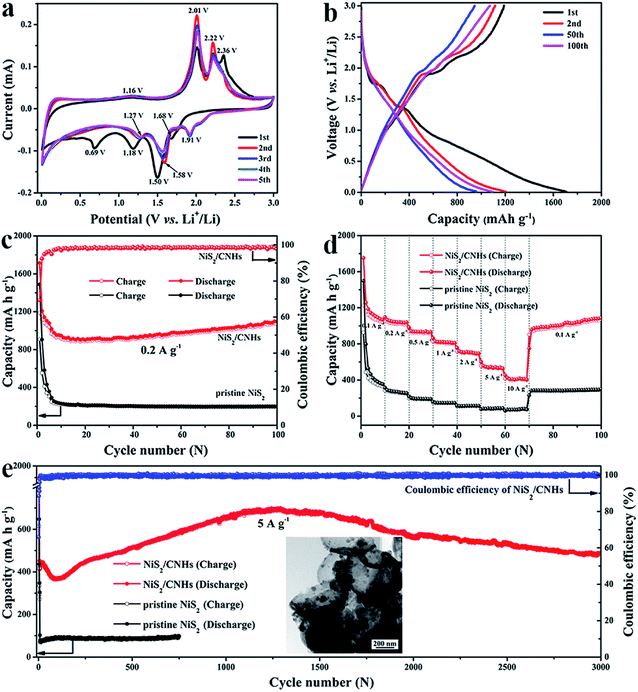
Fig. 4a presents the cyclic voltammetry (CV) curves of the NiS2/CNH electrode for the initial five cycles within a voltage window of 0.01–3.0 V (vs. Li+/Li) at a constant scan rate of 0.1 mV s−1. During the first cathodic scan process, two strong peaks located at 1.50 V and 1.18 V are attributed to the intercalation of Li+ into the NiS2 lattice to form LixNiS2 and the following conversion reaction process from LixNiS2 to Ni and Li2S, respectively.26,27 It is noteworthy that the pronounced cathodic peak at 0.69 V, which disappears in the subsequent cycles, corresponds to the formation of an irreversible solid–electrolyte interface (SEI) layer.26,27 Furthermore, a weak peak at 1.68 V can be attributed to the lithiation reaction of a trace amount of residual sulfur in the NiS2/CNHs.47 For the reverse anodic scan process, the part of lithium stored at the defects will be removed first, which generates a peak at about 1.16 V.26 Two remarkable peaks at 2.01 V and 2.22 V are associated with the formation of LixNiS2 and NiS2, respectively.25,27,48 The peak at 2.36 V, gradually weakened in the subsequent cycles, is ascribed to the conversion of Li2S to S. Moreover, in the following cathodic sweep, the peak at 1.91 V and the extremely slight peaks nearby are attributed to the multi-step lithiation of S.26 From the second cycle onward, the cathodic peaks at 1.50 V and 1.18 V slightly shift to 1.58 V and 1.27 V, respectively. Note that the CV profiles show little difference with increasing cycles from the third cycle, indicating the excellent cycling stability of the NiS2/CNH electrode. According to previous studies,26,27,48 the corresponding lithium storage conversion reactions can be expressed using the following equations:| NiS2 + xLi+ + xe− ↔ LixNiS2 | (2) |
| LixNiS2 + (4 − x)Li+ + (4 − x)e− ↔ Ni + 2Li2S. | (3) |
The galvanostatic charge/discharge profiles of the NiS2/CNH electrode for the 1st, 2nd, 50th and 100th cycles at a current density of 0.2 A g−1 are shown in Fig. 4b. The NiS2/CNH electrode delivers high first-cycle discharge and charge capacities of 1714.5 and 1189.2 mA h g−1, respectively, with an initial coulombic efficiency of 69.36%. The high irreversible capacity loss during the first cycle can be attributed to the formation of the SEI layer.49–52 The inconspicuous voltage plateaus in Fig. 4b may be attributed to the complicated multi-step reactions of NiS2, the pseudocapacitive behavior of nanosized-NiS2 particles, and the partial effect from the sloping voltage curve of carbonaceous material.53–57 Such a phenomenon has also been reported for various TMS anode materials in previous studies.27,51,58 In this work, the NiS2/CNH electrode material exhibits high specific capacity and excellent cycling stability and rate performance. In addition, most of the charge capacity is released below a voltage of 1.5 V, which is beneficial to improving the energy density of batteries.59,60 Thus, the NiS2/CNH hybrid has great potential for application in high-performance LIBs in the near future. Note that the active material is the NiS2/CNH hybrid and all the capacities recorded in this work are calculated on the basis of the total mass of NiS2/CNHs. The cycling performances of NiS2/CNH and pristine NiS2 electrodes at a current density of 0.2 A g−1 are shown in Fig. 4c. The NiS2/CNH hybrid electrode exhibits an excellent cycling performance with a specific capacity of 1096 mA h g−1 after 100 cycles, which is much higher than that of the pristine NiS2 electrode (only 199 mA h g−1). Moreover, the coulombic efficiency of the NiS2/CNH electrode is close to 100%, indicating its excellent reversibility during the Li+ insertion/extraction process. The discharge capacity of the NiS2/CNH electrode drops from 1714.5 mA h g−1 to 902.2 mA h g−1 in the first 20 cycles and then increases gradually to a value as high as 1096 mA h g−1 after 100 cycles. To the best of our knowledge, this phenomenon of capacity increase is common in metal oxide/sulfide composites and is normally ascribed to the improved charge transfer kinetics by the reversible reaction between metal nanoparticles and electrolytes and the gradual activation process during the cycling process.51,61 However, it is apparent that the capacity retention for the pristine NiS2 electrode is very poor and shows a monotonous decreasing trend. Fig. 4d compares the rate performance of the NiS2/CNH and pristine NiS2 electrodes. The excellent rate performance of NiS2/CNHs is evidenced by the average specific capacities of 1112, 1045, 935, 819, 706, 541 and 412 mA h g−1 at current densities of 0.1, 0.2, 0.5, 1, 2, 5 and 10 A g−1, respectively. Moreover, the reversible capacity of the NiS2/CNH electrode rapidly recovers to 1087.1 mA h g−1 in the 30th cycle when the current density is changed back to 0.1 A g−1 and then maintains a continuous increase during further cycling, while the pristine NiS2 electrode shows a much worse performance at each current density. The superior rate performance of NiS2/CNHs is attributed to the low internal resistance, which has been demonstrated by the electrochemical impedance spectroscopy (EIS) measurement results shown in Fig. S9.† The NiS2/CNH electrode displays a smaller intrinsic electrode/electrolyte resistance Rel (=1.9 Ω) and charge transfer resistance Rct (=57 Ω) than the pristine NiS2 electrode (2.5 Ω and 143 Ω, respectively). In addition, the large specific surface area and pore volume of NiS2/CNHs provide efficient diffusion pathways for Li+ and the electrolyte, which is also beneficial for achieving excellent rate performance. Furthermore, Fig. S10† displays the comparison of the rate performance between the NiS2/CNH electrode in this work and previously reported NiSx-based anode materials. It is obvious that NiS2/CNHs show the best rate performance among them.
To evaluate the long-term cycling stability of the NiS2/CNH electrode, it was galvanostatically discharged and charged at 0.1 A g−1 for the first five cycles and then at 5 A g−1 for 3000 cycles. The corresponding results are shown in Fig. 4e. The hybrid electrode suffers from capacity fluctuations during long-term cycling. After the first 100 cycles, the specific capacity of the hybrid electrode drops from 1790 mA h g−1 to 370 mA h g−1, resulting from the formation of the SEI film and irreversible transformation from NiS2 to metallic Ni nanoparticles.28,62 Then, the specific capacity increases gradually to the maximum capacity (699.4 mA h g−1) in the 1292nd cycle. Such a capacity increase can be attributed to the improved charge transfer kinetics during the gradual activation process and reversible reaction between Ni nanoparticles and electrolytes. This phenomenon has also been observed in other composites of TMOs/TMSs and carbon.51,63,64 In addition, electrochemical impedance spectroscopy (EIS) of the NiS2/CNH electrode after the 100th and 350th cycles was performed and the corresponding results are shown in Fig. S11.† It is found that the electrode/electrolyte resistance Rel (=3.2 Ω) and charge transfer resistance Rct (=34.3 Ω) values in the 350th cycle are smaller than those in the 100th cycle (Rel = 3.7 Ω and Rct = 44.7 Ω). Note that the impedance decreases gradually with the increasing cycles and the Rct values after cycling are lower than that of the fresh cell (Rct = 57 Ω). This phenomenon should be attributed to the in situ generated Ni from the incomplete conversion reactions which could enhance the electronic conductivity of the electrode.28,64 As shown in Fig. S11,† there exist two semicircles in the high-frequency region, suggesting the formation of a new phase (Ni nanoparticles), which is also consistent with the above analysis results.28,65 Finally, the specific capacity decreases gradually to 490 mA h g−1 after 3000 cycles. This may be ascribed to the falling off of some active materials caused by the volume expansion during the long-term continuous charge/discharge process.50 In contrast, the specific capacity of the pristine NiS2 electrode is only 97.6 mA h g−1 after 750 cycles. As exhibited in the inset TEM image in Fig. 4e, the NiS2/CNH hybrid retains its original helmet-like skeleton after 2000 cycles, which indicates its excellent structural stability. Besides, Fig. S12† shows a FESEM image of the NiS2/CNH electrode after 2000 cycles, further demonstrating the good integrity of the electrode material after long-term cycling.
As listed in Table S1,† the NiS2/CNH electrode exhibits the best cycling stability and rate performance among currently reported NiSx-based anode materials.24–26,39,46,48,49,66–68 The excellent electrochemical performance of NiS2/CNHs can be attributed to their characteristic structure with the following advantages: (1) well-dispersed NiS2 nanoparticles on CNHs can effectively mitigate the aggregation and buffer the volumetric expansion of NiS2 nanoparticles upon long-term cycling; (2) the tight combination between NiS2 nanoparticles and CNHs can greatly improve the overall conductivity of the NiS2/CNH electrode; and (3) the three-dimensional nanoporous carbon architecture can provide efficient diffusion paths for the electrolyte and Li+ to fully come into contact with electrochemically active NiS2 nanoparticles.
Conclusion
In summary, we have successfully fabricated a hybrid material of NiS2/CNHs, which combines the advantages of the high conductivity and good stability of the carbon material and the high theoretical capacity of NiS2. Benefiting from the above superior properties, when tested as an anode in LIBs, the NiS2/CNH electrode exhibits superior rate performance (412 mA h g−1 at 10 A g−1) and excellent cycling stability (490 mA h g−1 after 3000 cycles at 5 A g−1), outperforming other NiSx-based materials and making it a promising candidate as an anode material for highly reversible lithium storage.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project is financially supported by National Natural Science Foundation of China (No. 51671092 and 51631004), Project of Talent Development in Jilin Province, Program for JLU Science and Technology Innovative Research Team (No. 2017TD-09), and the Fundamental Research Funds for the Central Universities.References
- D. Larcher and J.-M. Tarascon, Nat. Chem., 2015, 7, 19–29 CrossRef CAS PubMed.
- Y.-M. Chiang, Science, 2010, 330, 1485–1486 CrossRef CAS PubMed.
- B. Dunn, H. Kamath and J.-M. Tarascon, Science, 2011, 334, 928–935 CrossRef CAS PubMed.
- X. Hu, J. Jia, G. Wang, J. Chen, H. Zhan and Z. Wen, Adv. Energy Mater., 2018, 8, 1801452 CrossRef.
- X. Su, Q. Wu, J. Li, X. Xiao, A. Lott, W. Lu, B. W. Sheldon and J. Wu, Adv. Energy Mater., 2014, 4, 1300882 CrossRef.
- Y. Zhao, L. P. Wang, M. T. Sougrati, Z. Feng, Y. Leconte, A. Fisher, M. Srinivasan and Z. Xu, Adv. Energy Mater., 2017, 7, 1601424 CrossRef.
- X. Feng, M. Ouyang, X. Liu, L. Lu, Y. Xia and X. He, Energy Storage Materials, 2018, 10, 246–267 CrossRef.
- X. Hu, Y. Li, G. Zeng, J. Jia, H. Zhan and Z. Wen, ACS Nano, 2018, 12, 1592–1602 CrossRef CAS PubMed.
- J. Xu, F. Lin, M. M. Doeff and W. Tong, J. Mater. Chem. A, 2017, 5, 874–901 RSC.
- L. Qie, W.-M. Chen, Z.-H. Wang, Q.-G. Shao, X. Li, L.-X. Yuan, X.-L. Hu, W.-X. Zhang and Y.-H. Huang, Adv. Mater., 2012, 24, 2047–2050 CrossRef PubMed.
- X. Liu, D. Chao, Y. Li, J. Hao, X. Liu, J. Zhao, J. Lin, H. J. Fan and Z. X. Shen, Nano Energy, 2015, 17, 43–51 CrossRef CAS.
- J. Hassoun, S. Panero, P. Simon, P. L. Taberna and B. Scrosati, Adv. Mater., 2007, 19, 1632–1635 CrossRef CAS.
- A. Varzi, L. Mattarozzi, S. Cattarin, P. Guerriero and S. Passerini, Adv. Energy Mater., 2018, 8, 1701706 CrossRef.
- H. Song, H. X. Wang, Z. Lin, X. Jiang, L. Yu, J. Xu, Z. Yu, X. Zhang, Y. Liu, P. He, L. Pan, Y. Shi, H. Zhou and K. Chen, Adv. Funct. Mater., 2016, 26, 524–531 CrossRef CAS.
- B. Wang, J. S. Chen, H. B. Wu, Z. Wang and X. W. Lou, J. Am. Chem. Soc., 2011, 133, 17146–17148 CrossRef CAS PubMed.
- X. W. Lou, Y. Wang, C. Yuan, J. Y. Lee and L. A. Archer, Adv. Mater., 2006, 18, 2325–2329 CrossRef CAS.
- S. Zhu, J. Li, X. Deng, C. He, E. Liu, F. He, C. Shi and N. Zhao, Adv. Funct. Mater., 2017, 27, 1605017 CrossRef.
- Z. Wang and X. W. Lou, Adv. Mater., 2012, 24, 4124 CrossRef CAS PubMed.
- C. Chen, X. Xie, B. Anasori, A. Sarycheva, T. Makaryan, M. Zhao, P. Urbankowski, L. Miao, J. Jiang and Y. Gogotsi, Angew. Chem., Int. Ed., 2018, 57, 1846–1850 CrossRef CAS PubMed.
- L. Yu, J. F. Yang and X. W. Lou, Angew. Chem., Int. Ed., 2016, 55, 1–5 CrossRef.
- J. Seo, J. Jang, S. Park, C. Kim, B. Park and J. Cheon, Adv. Mater., 2008, 20, 4269–4273 CrossRef CAS.
- M.-S. Balogun, Y. Zeng, W. Qiu, Y. Luo, A. Onasanya, T. K. Olaniyi and Y. Tong, J. Mater. Chem. A, 2016, 4, 9844–9849 RSC.
- H. Huang, S. Gao, A.-M. Wu, K. Cheng, X.-N. Li, X.-X. Gao, J.-J. Zhao, X.-L. Dong and G.-Z. Cao, Nano Energy, 2017, 31, 74–83 CrossRef CAS.
- Z. Gao, N. Song, Y. Zhang and X. Li, Nano Lett., 2015, 15, 8194–8203 CrossRef CAS PubMed.
- Y. Zhang, F. Lu, L. Pan, Y. Xu, Y. Yang, Y. Bando, D. Golberg, J. Yao and X. Wang, J. Mater. Chem. A, 2018, 6, 11978–11984 RSC.
- Q. Chen, W. Chen, J. Ye, Z. Wang and J. Y. Lee, J. Power Sources, 2015, 294, 51–58 CrossRef CAS.
- P. Lou, Y. Tan, P. Lu, Z. Cui and X. Guo, J. Mater. Chem. A, 2016, 4, 16849–16855 RSC.
- C. C. Yang, W. T. Jing, C. Li and Q. Jiang, J. Mater. Chem. A, 2018, 6, 3877–3883 RSC.
- X. Hu, Y. Liu, J. Chen, J. Jia, H. Zhan and Z. Wen, J. Mater. Chem. A, 2019, 7, 1138–1148 RSC.
- Y. Xiao, S. H. Lee and Y.-K. Sun, Adv. Energy Mater., 2017, 7, 1601329 CrossRef.
- X. Wu, B. Yang, Z. Li, L. Lei and X. Zhang, RSC Adv., 2015, 5, 32976–32982 RSC.
- D. Li, X. Zhao, R. Yu, B. Wang, H. Wang and D. Wang, Inorg. Chem. Front., 2018, 5, 535–540 RSC.
- C. C. Yang, D. M. Zhang, L. Du and Q. Jiang, J. Mater. Chem. A, 2018, 6, 12663–12671 RSC.
- R. Bi, C. Zeng, H. Huang, X. Wang and L. Zhang, J. Mater. Chem. A, 2018, 6, 14077–14082 RSC.
- H.-G. Wang, Y. Wang, Y. Li, Y. Wan and Q. Duan, Carbon, 2015, 82, 116–123 CrossRef CAS.
- J. Li, D. Yan, X. Zhang, S. Hou, T. Lu, Y. Yao and L. Pan, J. Mater. Chem. A, 2017, 5, 20428–20438 RSC.
- W. T. Jing, Y. Zhang, Y. Gu, Y. F. Zhu, C. C. Yang and Q. Jiang, Matter, 2019, 1, 720–733 CrossRef.
- C. Li, M. Zhao, C. N. Sun, B. Jin, C. C. Yang and Q. Jiang, J. Power Sources, 2018, 397, 162–169 CrossRef CAS.
- A. A. AbdelHamid, X. Yang, J. Yang, X. Chen and J. Y. Ying, Nano Energy, 2016, 26, 425–437 CrossRef CAS.
- W. Zhao, S. Ci, X. Hu, J. Chen and Z. Wen, Nanoscale, 2019, 11, 4688–4695 RSC.
- T. Tian, L. Huang, L. Ai and J. Jiang, J. Mater. Chem. A, 2017, 5, 20985–20992 RSC.
- Q. Chen, S. Sun, T. Zhai, M. Yang, X. Zhao and H. Xia, Adv. Energy Mater., 2018, 8, 1800054 CrossRef.
- Y. Lu, X. Li, J. Liang, L. Hu, Y. Zhu and Y. Qian, Nanoscale, 2016, 8, 17616–17622 RSC.
- K. Liang, K. Marcus, S. Zhang, L. Zhou, Y. Li, S. T. D. Oliveira, N. Orlovskaya, Y.-H. Sohn and Y. Yang, Adv. Energy Mater., 2017, 7, 1701309 CrossRef.
- G. Zhao, Y. Zhang, L. Yang, Y. Jiang, Y. Zhang, W. Hong, Y. Tian, H. Zhao, J. Hu, L. Zhou, H. Hou, X. Ji and L. Mai, Adv. Funct. Mater., 2018, 28, 1803690 CrossRef.
- Y. Lin, Z. Qiu, D. Li, S. Ullah, Y. Hai, H. Xin, W. Liao, B. Yang, H. Fan, J. Xu and C. Zhu, Energy Storage Materials, 2018, 11, 67–74 CrossRef.
- J. Ruan, T. Yuan, Y. Pang, S. Luo, C. Peng, J. Yang and S. Zheng, Carbon, 2018, 126, 9–16 CrossRef CAS.
- W. Pi, T. Mei, J. Li, J. Wang, J. Li and X. Wang, Chem. Eng. J., 2018, 335, 275–281 CrossRef CAS.
- J. Zhu, Y. Li, S. Kang, X.-L. Wei and P. K. Shen, J. Mater. Chem. A, 2014, 2, 3142–3147 RSC.
- P. Fan, H. Liu, L. Liao, J. Fu, Z. Wang, G. Lv, L. Mei, H. Hao, J. Xing and J. Dong, RSC Adv., 2017, 7, 49739–49744 RSC.
- R. Wu, D. Wang, X. Rui, B. Liu, K. Zhou, A. W. K. Law, Q. Yan, J. Wei and Z. Chen, Adv. Mater., 2015, 27, 3038–3044 CrossRef CAS PubMed.
- H. Li, X. Wang, Z. Zhao, Z. Tian, D. Zhang and Y. Wu, ChemElectroChem, 2019, 6, 404–412 CrossRef CAS.
- Y. F. Yuan, L. W. Ye, D. Zhang, F. Chen, M. Zhu, L. N. Wang, S. M. Yin, G. S. Cai and S. Y. Guo, Electrochim. Acta, 2019, 299, 289–297 CrossRef CAS.
- H.-S. Kim, J. B. Cook, S. H. Tolbert and B. Dunn, J. Electrochem. Soc., 2015, 162, 5083–5090 CrossRef.
- V. Augustyn, P. Simonbc and B. Dunn, Energy Environ. Sci., 2014, 7, 1597–1614 RSC.
- Y. Chen, X. Li, K. Park, J. Song, J. Hong, L. Zhou, Y.-W. Mai, H. Huang and J. B. Goodenough, J. Am. Chem. Soc., 2013, 135, 16280–16283 CrossRef CAS PubMed.
- Q. Pan, J. Xie, S. Liu, G. Cao, T. Zhu and X. Zhao, RSC Adv., 2013, 3, 3899–3906 RSC.
- H. Jiang, D. Ren, H. Wang, Y. Hu, S. Guo, H. Yuan, P. Hu, L. Zhang and C. Li, Adv. Mater., 2015, 27, 3687–3695 CrossRef CAS PubMed.
- C. Wu, P. Kopold, P. A. van Aken, J. Maier and Y. Yu, Adv. Mater., 2017, 29, 1604015 CrossRef PubMed.
- L. Xiao, Y. Cao, W. A. Henderson, M. L. Sushko, Y. Shao, J. Xiao, W. Wang, M. H. Engelhard, Z. Nie and J. Liu, Nano Energy, 2016, 19, 279–288 CrossRef CAS.
- Y. Du, Z. Yin, J. Zhu, X. Huang, X.-J. Wu, Z. Zeng, Q. Yan and H. Zhang, Nat. Commun., 2012, 3, 1177 CrossRef PubMed.
- C. Peng, B. Chen, Y. Qin, S. Yang, C. Li, Y. Zuo, S. Liu and J. Yang, ACS Nano, 2012, 6, 1074–1081 CrossRef CAS PubMed.
- H. Li, Y. Su, W. Sun and Y. Wang, Adv. Funct. Mater., 2016, 26, 8345–8353 CrossRef CAS.
- J. Luo, J. Liu, Z. Zeng, C. F. Ng, L. Ma, H. Zhang, J. Lin, Z. Shen and H. J. Fan, Nano Lett., 2013, 13, 6136–6143 CrossRef CAS PubMed.
- S. Venkatachalam, H. Zhu, C. Masarapu, K. Hung, Z. Liu, K. Suenaga and B. Wei, ACS Nano, 2009, 3, 2177–2184 CrossRef CAS.
- X. Wang, X. Liu, G. Wang, Y. Zhou and H. Wang, J. Power Sources, 2017, 342, 105–115 CrossRef CAS.
- L. Zhang, Y. Huang, Y. Zhang, H. Gu, W. Fan and T. Liu, Adv. Mater. Interfaces, 2016, 3, 1500467 CrossRef.
- X. Wu, S. Li, Y. Xu, B. Wang, J. Liu and M. Yu, Chem. Eng. J., 2019, 356, 245–254 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9na00661c |
| This journal is © The Royal Society of Chemistry 2020 |