Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
LC-ICP-MS method for the determination of “extractable copper” in serum†
C. Derrick
Quarles
Jr
 *a,
Marcel
Macke
*a,
Marcel
Macke
 b,
Bernhard
Michalke
b,
Bernhard
Michalke
 c,
Hans
Zischka
c,
Hans
Zischka
 de,
Uwe
Karst
de,
Uwe
Karst
 b,
Patrick
Sullivan
a and
M. Paul
Field
a
b,
Patrick
Sullivan
a and
M. Paul
Field
a
aElemental Scientific, Inc., 7277 World Communications Dr., Omaha, NE, USA. E-mail: derrick.quarles@icpms.com
bUniversity of Münster, Institute of Inorganic and Analytical Chemistry, Corrensstrasse 30, 48149 Münster, Germany
cResearch Unit Analytical BioGeoChemistry, Helmholtz Center Munich, German Research Center for Environmental Health, Ingolstaedter Landstrasse 1, 85764 Neuherberg, Germany
dInstitute of Molecular Toxicology and Pharmacology, Helmholtz Center Munich, German Research Center for Environmental Health, Ingolstaedter Landstrasse 1, 85764 Neuherberg, Germany
eTechnical University Munich, School of Medicine, Institute of Toxicology and Environmental Hygiene, Biedersteiner Strasse 29, 80802 Munich, Germany
First published on 5th August 2020
Abstract
Copper is an essential element for biological functions within humans and animals. There are several known diseases associated with Cu deficiency or overload, such as Menkes disease and Wilson disease, respectively. A common clinical method for determining extractable Cu levels in serum, which is thought to be potentially dangerous if in excess, is to subtract the value of tightly incorporated Cu in ceruloplasmin from total serum Cu. In this work, an automated sample preparation and liquid chromatography (LC) system was combined with inductively coupled plasma-mass spectrometry (ICP-MS) to determine bound Cu and extractable Cu in serum. This LC-ICP-MS method took 250 s for sample preparation and analysis, followed by a column recondition/system reset, thus, a 6 minute sample-to-sample time including sample preparation. The method was validated using serum collected from either control (Atp7b+/−) or Wilson disease rats (Atp7b−/−). The extractable Cu was found to be 4.0 ± 2.3 μM Cu in healthy control rats, but 2.1 ± 0.6 μM Cu in healthy Wilson rats, and 27 ± 16 μM Cu in diseased Wilson rats, respectively. In addition, the extractable Cu/bound Cu ratio was found to be 6.4 ± 3.5%, 38 ± 29%, and 34 ± 22%, respectively. These results suggest that the developed method could be of diagnostic value for Wilson disease, and possibly other copper related diseases.
Significance to metallomicsA direct and fast method for the determination of extractable Cu in serum samples is presented using an automated sample preparation LC-ICP-MS system. This method may provide additional, specific information in the diagnostic and examination steps required for the evaluation of Wilson disease patients. |
Introduction
Copper (Cu) is an essential trace element for both humans and animals. The main source of Cu is from dietary intake and the human body contains approximately 100 mg of Cu, with a majority of it found in muscle, skeletal, plasma, and the liver.1 Many enzymes require copper as a co-factor, including ceruloplasmin, ascorbic acid oxidase, cytochrome c oxidase, and superoxide dismutase.2 However, deficient or excess copper levels in the body can lead to adverse effects. For example, copper deficiency can lead to muscular or neurological issues during fetal development, and in adults, it can lead to altered cholesterol production.3–6 When copper is in excess it can become toxic to cells, resulting in cell damage and death.1,7–9Two well-known diseases are related to either copper deficiency or copper overload, Menkes disease and Wilson disease, respectively. Menkes disease was clinically reported in 1962,10 and in the early 1990's it was discovered to be caused by a genetic mutation in the copper transporting ATPase ATP7A.11 Serum Cu and ceruloplasmin levels are low in Menkes disease patients, resulting in Cu deficiency.2 Menkes disease patients typically do not survive and death is seen at a young age, typically ≤3 years of age.12 Wilson disease was first reported in 1912,13 and in the early 1990s, it was discovered that it is caused by a genetic mutation in ATP7B.14,15 This mostly liver-residing copper transporting ATPase either incorporates Cu into ceruloplasmin or aids in the excretion of Cu into the bile.16 Consequently, ATP7B mutations can lead to excess extractable Cu levels building up in the body, typically in liver and brain.
The screening and diagnosis for Wilson disease comprises multiple components: family history, signs or physical symptoms related to Wilson disease (e.g. Kayser–Fleischer rings), and laboratory studies. Ceruloplasmin (CP) is the major copper carrying protein in the blood and accounts for 85–95% of serum copper. Cu-containing CP oxidizes Fe(II) to Fe(III), that way maintaining iron redox balance and lowering risk for oxidative stress.17–19 Due to a lower Cu incorporation, Wilson disease patients generally have a lower ceruloplasmin level.20–22 However, in patients with inflammation from chronic active hepatitis, the ceruloplasmin levels may be normal.20 Currently, there is no established clinical method for the direct determination of extractable copper, instead serum Cu and serum ceruloplasmin are measured and the correlation of extractable copper is deduced from these tests.23 Elevated serum Cu or the correlated extractable copper levels can be a sign of Wilson disease. The ability to directly measure extractable Cu could not only aid in the diagnosis process, but also could provide important information on the disease status and in the treatment and drug development stages.
Ultrafiltration with a 30 kDa weight cutoff of serum samples followed by inductively coupled plasma-mass spectrometry (ICP-MS) has been demonstrated as a potential method for determining extractable Cu in Wilson disease patients.24 Alternatively, a fractionation of Cu species can be carried out using chromatographic methods, which might be also able to provide more specific information. Sanz-Medel et al. presented the potential for liquid chromatography (LC) coupled to ICP-MS to provide important metal–protein related information for the bioinorganic and clinical fields.25 In this review, six methods in the literature reference the analysis of serum by either size exclusion chromatography (SEC) or anion exchange chromatography (AEC) for the detection of Cu and Cu related proteins. Muniz et al. presented an AE-based LC-ICP-MS method for the detection of Cu bound to ceruloplasmin. However, the method takes >22 minutes per sample.26 Inagaki et al. presented a method for the detection of Cu-bound albumin, Cu-bound ceruloplasmin, and total Cu by offline pre-treatment followed by SE-based LC-ICP-MS.27 In this method, a 5 h pre-treatment was necessary to allow for the exchange of Cu bound to albumin to Chelex-100 resin prior to the SEC method. Lopez-Avila et al. presented a novel two-step method for the determination of Cu bound ceruloplasmin using immunoaffinity chromatography followed by SEC-ICP-MS.28 The immunoaffinity chromatography took 30 minutes (sample-to-sample time) in addition to the 20 minute separation by SEC-ICP-MS for Cu bound ceruloplasmin and extractable copper. Most recently, Solovyev et al. presented the ability to determine the speciation of Cu-containing proteins in 10 minutes by AEC-ICP-MS29 or Cu(I) vs. Cu(II) and CP.30,31
These methods for the determination of Cu bound ceruloplasmin or extractable copper involve additional sample preparation, two dimensional methods, and/or lengthy analysis times. In this work, we present a fast and specific method for the determination of extractable Cu in serum using LC-ICP-MS. The validation of this method was carried out using samples obtained from either control (Atp7b+/−) or Wilson disease rats (Atp7b−/−), either being still healthy or diseased. Sample preparation is carried out using an inline sample dilution technique just prior to injecting the serum samples onto a Cu-specific column that separates bound Cu from extractable Cu.
Methods
Materials and reagents
All eluents, reagents and diluents were prepared in freshly deionized (DI) water from an Aquatron water still purification system (Barloworld Scientific, Nemours, France). Copper solution (1000 mg L−1 in 2% nitric acid) from Carl Roth GmbH + Co. KG (Karlsruhe, Germany) was used to prepare extractable copper standards for external calibration. Bovine serum albumin (Sigma-Aldrich), human ceruloplasmin (Sigma-Aldrich), apo-transferrin (Sigma-Aldrich), and holo-transferrin (Sigma-Aldrich) were prepared from powders and reconstituted at 50 μM concentrations in ammonium acetate buffer at pH 7.4.Animal study and sample preparation
Animals were maintained under the Guidelines for the Care and Use of Laboratory Animals of the Helmholtz Center Munich. Animal experiments were approved by the government authorities of the Regierung von Oberbayern, Munich, Germany. The rats with Atp7b−/− (Wilson disease rats) are affected by a progressive copper overload in their livers, whereas the Atp7b+/− rats are non-affected controls.32,33 Up to ∼90 days of age the Wilson disease rats are healthy (no elevated serum parameters such as alanine aminotransferase (ALT), serum aspartate aminotransferase (AST), or bilirubin which would indicate liver damage) and continue to put on weight. At around 90 days of age weight gain is halted and the animals begin to lose weight with progressively increasing ALT and AST levels, which is why such rats are considered as “disease onset”. The liver values get worse over the next week and the bilirubin levels become detectable, these rats are considered “diseased” at this point. Around two to three weeks later these rats will die. All of the rats with genotype Atp7b−/− show this highly reproducible disease progress, which is due to a constant delivery of copper (in this case 205 μM (13–14 ppm) Cu) through diet, which in return causes a steady increase in copper burden. This method of dietary intake leads to a 100% disease rate in these Atp7b−/− rats.Blood was taken from either control Atp7b+/− or WD Atp7b−/− rats, serum cleared by centrifugation (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 2 min), and stored at −80 °C in tubes made of polypropylene. AST was measured with a Reflotron system (Roche Diagnostics, Penzberg, Germany) and starting liver damage in animals was considered clinically apparent if serum AST was >200 U L−1.34 Serum copper levels were analyzed by ICP-OES (Ciros Vision, SPECTRO Analytical Instruments, Kleve, Germany) as previously described.32 Immediately prior to the analysis, the samples were heated up to ambient temperature and diluted manually by a factor of ten using DI water (100 μL serum:900 μL DI water).
000g, 2 min), and stored at −80 °C in tubes made of polypropylene. AST was measured with a Reflotron system (Roche Diagnostics, Penzberg, Germany) and starting liver damage in animals was considered clinically apparent if serum AST was >200 U L−1.34 Serum copper levels were analyzed by ICP-OES (Ciros Vision, SPECTRO Analytical Instruments, Kleve, Germany) as previously described.32 Immediately prior to the analysis, the samples were heated up to ambient temperature and diluted manually by a factor of ten using DI water (100 μL serum:900 μL DI water).
LC-ICP-MS analysis
Liquid chromatography for speciation analysis was performed using a prepFAST IC (Elemental Scientific Inc., Omaha, NE, USA). The system is equipped with metal-free syringe pumps for gradient elution and a prepFAST M5 module that allows inline dilutions of standards and samples. Using syringe loading, the samples and standards were drawn up into a 500 μL sample loop. Then, the solutions were transferred into a 500 μL dilution loop and mixed with adjustable amounts of DI water to create exact dilutions, before they were injected onto the column. It must be noted that there is an option to analyze the samples using a 50 μL sample loop and perform the sample prep into a 500 μL loop. However, since the rat serum samples were limited in volume and it was unsure at that time if multiple analyses would be needed an offline dilution was performed for these experiments to provide a larger sample volume.Chromatographic separations of bound and extractable Cu species were carried out using an Elemental Scientific CF-Cu-02 column (200 μL resin volume, 50 × 4 mm). The CF-Cu-02 column is packed with resin that has negatively charged functional groups at pHs of 6.0–8.0 which allows Cu (it must be noted that this method is not selective for Cu(I) or Cu(II)) to bind tightly to the resin. When the pH is lowered to ∼1, the resin will exchange Cu for H resulting in Cu eluting off the column to the ICP-MS for detection. Samples were diluted inline by a factor of two and injected onto the column for 18 s at a flow rate of 1 mL min−1 (equivalent to 300 μL injection volume). Typically, the dilution of the serum samples would be performed completely inline, but sample volume was limited, thus a 10× offline dilution was performed prior to the 2× inline dilution. Ammonium acetate (NH4OAc, ≥99.99% trace metal basis, Sigma-Aldrich, St. Louis, MO, USA) and ammonia solution (25–30%, puriss., Honeywell Fluka, Seelze, Germany) were used to prepare eluent A (100 mM NH4OAc, pH 7.4) whereas a second eluent B consisted of 10% nitric acid (v/v) (HNO3, prepared from 69%, EMSURE, Merck KGaA, Darmstadt, Germany). Applying a two-step gradient, bound Cu species were eluted first using 100% eluent A. Mobile phase composition was changed to 100% eluent B 80 s after injection to allow the elution of extractable copper. The sample-to-sample time was 6 minutes 10 s per sample, the elution of extractable Cu was completed in under 4 minutes with a total run time of 250 s, and an additional 120 s for column conditioning and reset of the prepFAST IC for the next sample.
The prepFAST IC was coupled to an iCAP TQ ICP-MS (Thermo Fisher Scientific, Bremen, Germany) that was equipped with a MicroFlow PFA ST nebulizer (Elemental Scientific), a Peltier-cooled quartz cyclonic spray chamber and a 2.5 mm quartz injector. Sampler and skimmer cones consisted of nickel. The plasma was operated with an RF power of 1.55 kW and the argon flow for the coolant, auxiliary, and nebulizer gases were set to 14, 0.8, and 1.19 L min−1, respectively. All analyses were performed in triple quadrupole mode using an oxygen flow of 0.23 mL min−1 and a helium flow of 0.80 mL min−1. The ions 56Fe16O+, 63Cu+, 65Cu+ and 66Zn+ were monitored with individual dwell times of 100 ms in time resolved acquisition mode. Only the 63Cu+ was reported in this work, however, the other isotopes were investigated for possible trends but not reported in this work. The total run time per chromatogram was 250 s, sample uptake was set to 0 s, and a rinsing time of 120 s.
Extractable copper was determined by external calibration using a 1.6 μM (100 μg L−1) stock solution. Utilizing the prepFAST M5 module, inline dilutions were performed to generate a calibration range between 0.016–1.6 μM (1 and 100 μg L−1).
Data evaluation was carried out using Qtegra 2.10 (Thermo Fisher Scientific). Prior to integration, the obtained chromatograms were smoothed with the moving mean filter over 15 data points.
Results and discussion
Method development
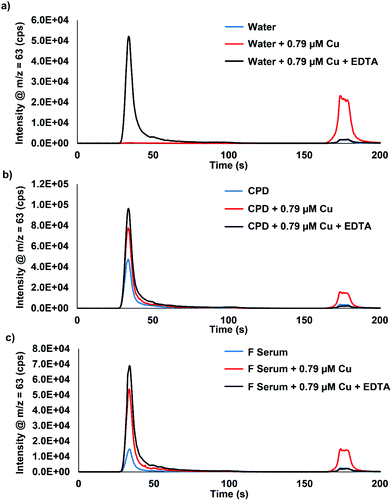
Fig. 1 displays the comparison of water, citrate-phosphate-dextrose (CPD) bovine blood, and bovine fetal serum (f serum) analyzed with 0.79 μM Cu spikes and ethylenediaminetetraacetic acid (EDTA). The bound Cu in this example has no interaction with the column and elutes at approximately 30 s, whereas the extractable Cu binds to the column and is eluted with 10% HNO3 at approximately 170 s. The ability to selectively bind extractable Cu to the chromatographic column while allowing the bound Cu to pass through is demonstrated. Fig. 1a shows no bound Cu for water or for extractable Cu, however, when 0.79 μM Cu is added into the sample, a large increase in the extractable Cu peak is displayed. When EDTA is added, all of the extractable Cu is shifted to the bound Cu peak, demonstrating that all of the Cu is bound to EDTA and does not interact with the column. Fig. 1b and c show this same phenomenon with CPD blood and fetal serum. However, when 0.79 μM Cu is added to the samples, an increase in both the bound Cu and extractable Cu is observed. This increase is most likely albumin and ceruloplasmin binding Cu as the natural equilibrium is changed with the spikes of extractable Cu into the serum samples. Table 1 displays the peak area responses for the chromatograms displayed in Fig. 1. Fig. S1 (ESI†) displays the results from bovine blood plasma and serum samples that were collected/stored using different anti-coagulant agents. Three serum samples that used EDTA as an anticoagulant showed identical results to what was seen in Fig. 1 when EDTA was added to the samples.| Extractable Cu (×103) | Bound Cu (×103) | Extractable Cu/total Cu (%) | ||
|---|---|---|---|---|
| Water | No addition | — | — | — |
| +0.79 μM Cu | 344 | 3.2 | 99 | |
| +0.79 μM Cu + EDTA | 4.8 | 530 | 1 | |
| CPD | No addition | 31 | 475 | 6 |
| +0.79 μM Cu | 226 | 779 | 23 | |
| +0.79 μM Cu + EDTA | 11 | 976 | 1 | |
| Fetal serum | No addition | 12 | 145 | 8 |
| +0.79 μM Cu | 210 | 510 | 29 | |
| +0.79 μM Cu + EDTA | 8.9 | 705 | 1 | |
The validation of this method is shown in Fig. S2 (ESI†), where 0.016–0.79 μM Cu was spiked into fetal serum, heparin blood, and EDTA blood. The fetal serum, heparin blood, and EDTA blood displayed the same trends as seen for the studies shown in Fig. 1. Spiking real serum samples was not an option for validation/recovery of extractable Cu since the serum proteins were also binding the Cu.35,36 To determine the precision of the method, a stock amount of fetal serum was spiked with 0.16 μM Cu and analyzed as 125 repeat samples (∼15 h of continuous analysis time). Over the span of 125 samples, there was a 2.0% RSD for the extractable Cu and 4.2% RSD for the bound Cu. In addition, blood samples preserved using EDTA cannot be analyzed with the method presented here. EDTA has a strong binding constant for Cu as compared to other anticoagulants such as heparin whose binding with Cu is very weak.37,38 When EDTA is present in the blood sample, the extractable Cu is bound more tightly to EDTA and passes through the column as a bound Cu species. In contrast, none of the other anticoagulants demonstrated such issues, therefore, should be straightforward for real samples analysis.
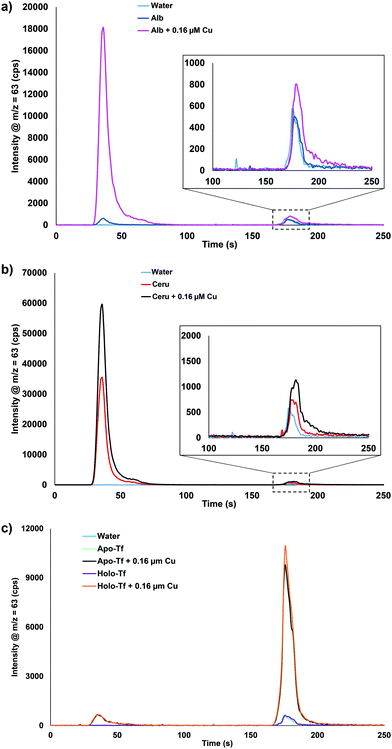
Albumin, ceruloplasmin, and transferrin (Tf) samples were prepared and spiked with 0.16 μM Cu to further understand which serum proteins were binding the spiked copper. Fig. 2 displays the results for water, protein, and protein + 0.16 μM Cu. Fig. 2a shows that there is some Cu bound to the neat albumin, but after addition of 0.16 μM Cu, a significant increase is seen in the bound Cu fraction with only a small increase in the extractable Cu portion displayed. Fig. 2b shows that the neat ceruloplasmin has mainly bound Cu but a small amount of extractable Cu was also detected. With the addition of 0.16 μM Cu, there is almost a twofold increase in the bound Cu and extractable Cu portions observed. Fig. 2c shows the results for neat apo-Tf and holo-Tf: as expected, no bound Cu or extractable Cu was observed. However, with the addition of 0.16 μM Cu, there was a small peak observed for the bound Cu portion but the majority was seen in the extractable Cu portion. These results suggest that when spiking Cu into the serum samples, albumin and ceruloplasmin are binding the Cu. Other proteins or peptides in blood/plasma could also be playing a role but were not tested in these experiments. To our knowledge, there are no known reference materials for extractable and bound Cu in serum. Since spike recovery studies are not possible based on the data presented here, the next plausible way to determine the method accuracy was to analyze real samples with known information.
Analysis of rat serum samples
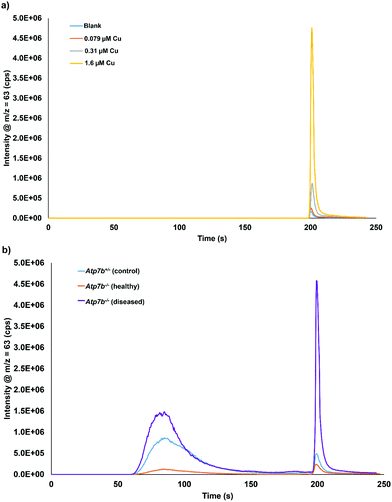
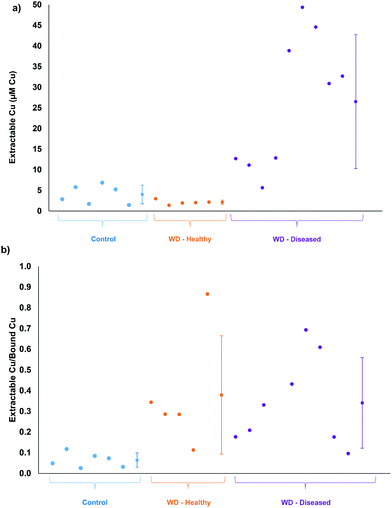
Calibration of the extractable Cu was carried out using DI water as the matrix, since matrix matching using plasma or serum was shown to be not possible. Chromatograms of 0.079, 0.31, and 1.6 μM Cu in DI water is displayed in Fig. 3a. A typical calibration curve for the extractable Cu response can be seen in Fig. S3 (ESI†). Fig. 3b displays example chromatograms for the serum samples analyzed from control and Wilson disease rats.Based on total Cu concentrations, the control group average is approximately 5.8 times greater than the healthy Wilson disease genotype (Atp7b−/−). The average values for the Wilson disease genotype group with symptoms (Atp7b−/−) is 2× greater than the control group, however, the spread of data points from the Wilson disease group with symptoms covers both the control and the healthy range of results. The total Cu concentration of each serum sample grouped by genotype and stage of disease can be found in Fig. S4 (ESI†). Fig. 4a displays the extractable Cu concentrations from each group of samples. The healthy Wilson disease group had the lowest extractable Cu concentration (2.1 ± 0.6 μM Cu), but statistically cannot be distinguished from the control group (4.0 ± 2.3 μM Cu). The Wilson disease group with symptoms had much higher extractable Cu values (27 ± 16 μM Cu) compared to the control group, approximately 6.6× higher. Fig. 4b displays the extractable Cu/bound Cu ratio for each of the sample groups. There is a significant difference between the control group (6.4%) and the Wilson disease groups (37.9% healthy and 34.1% with symptoms). In healthy Wilson disease rats, the total Cu levels are lower compared to the controls as no copper is incorporated into ceruloplasmin and although Cu accumulates in the liver only minor parts may be expelled into plasma due to minor hepatocyte death. This copper leakage from the liver plausibly accounts for the change in ratio of extractable Cu to bound Cu in comparison to the control group. When rats become diseased, i.e. their livers disintegrate, accumulated hepatocyte copper that may be partially bound to intracellular proteins (e.g. metallothionein) is expelled into serum. This causes a massive increase in both, bound and extractable copper (Fig. 3b). Interestingly, the ratio of extractable Cu to bound Cu for the Wilson disease carrying genotypes is the same whether healthy or diseased state. We therefore suggest to consider this ratio as a diagnostic value to distinguish controls from the Wilson disease rats. Furthermore, the extractable Cu levels can be used to determine disease status (see below).
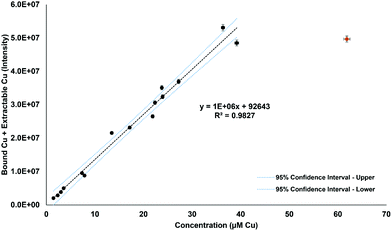
For further validation, the total Cu values determined by ICP-OES were compared to the bound Cu and extractable Cu peaks from the chromatographic method. Fig. 5 displays the comparison of the total Cu concentration versus bound Cu + extractable Cu peak areas. The results are in good agreement (R2 = 0.9827), except for one sample that was considered an outlier and excluded from the statistics, but left on the plot in Fig. 5. The final comparison for this study was to compare the serum aspartate aminotransferase (AST) values of the studied control and Wilson disease rats to the bound Cu and extractable Cu results. Elevated and increasing AST levels typically indicate progressive liver damage in these animals but also humans. The AST values may fluctuate upon disease onset and rise over 200 U L−1 once they hit diseased state, however the healthy rats are consistently below 200 U L−1.32 The three groups were plotted and grouped based on color (for visual aid). The bound Cu compared to the aspartate aminotransferase (Fig. 6a) showed that the control and healthy Wilson disease rats were distinguishable, however, when adding Wilson disease rats with symptoms, there is clear overlap with the controls. Using the aspartate aminotransferase data in combination with the bound Cu may therefore offer an identifier for early stage Wilson disease. In the early stages of Wilson disease, the serum Cu values will decrease as a result of the liver no longer excreting ceruloplasmin bound Cu. This finding is of potential clinical relevance, as if this stage can be identified, then treatment could be started before liver damage takes place. When comparing the extractable Cu to the aspartate aminotransferase (Fig. 6b) there are similar trends visualized for controls and healthy Wilson disease rats, importantly, however, a progression in disease (as indicated by increasing AST values) is best seen at the level of free serum copper (Fig. 6b).
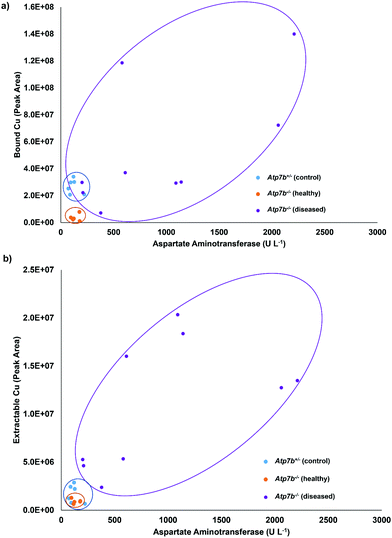 | ||
| Fig. 6 Comparison of the (a) bound Cu and (b) extractable Cu to the amount of aspartate aminotransferase. | ||
Conclusion
A fast method for the direct determination of extractable Cu in serum has been developed. The sample-to-sample time is just around 6 minutes, which is faster than what has been reported thus far in the literature. This method only reports bound and extractable Cu, whereas other methods do report the ability to quantify ceruloplasmin, Cu(I), and/or Cu(II) which is not possible with this method. Regardless, providing a simple and faster clinical method would allow for higher testing frequency (more patients diagnosed) in an automated and straightforward way. This approach would allow equipped laboratories to follow an individual patient from diagnosis to treatment, thus providing valuable information for patient and doctor that is not easily obtained with the given testing procedures. The method can be used for the analysis of blood samples provided EDTA was not used as an anticoagulant. Samples obtained from controls or Wilson disease rats were clearly distinguishable based on the ratio of extractable Cu/bound Cu. In addition, Wilson disease stages were mirrored by increasing serum extractable copper values. These initial data presented here, suggest that this method may possibly be used for diagnostic purposes, in both, identifying Wilson disease patients and also in monitoring their disease status. Future experiments will involve the quantification and identification of the proteins and elements that are part of the bound Cu elution in this work.Conflicts of interest
Authors C. Derrick Quarles Jr., Patrick Sullivan, and Paul Field are employed by Elemental Scientific, Inc., which developed the method used in this research.References
- M. Bost, S. Houdart, M. Oberli, E. Kalonji, J.-F. Huneau and I. Margaritis, Dietary copper and human health: Current evidence and unresolved issues, J. Trace Elem. Med. Biol., 2016, 35, 107–115 CrossRef CAS PubMed.
- M. T. Lorincz, in Handbook of Clinical Neurology, ed. D. H. Geschwind, H. L. Paulson and C. Klein, Elsevier BV, 2018, ch. 18, vol. 147, pp. 279–292 Search PubMed.
- L. Gambling and H. J. McArdle, Iron, copper, and fetal development, Proc. Nutr. Soc., 2004, 63, 553–562 CrossRef CAS PubMed.
- M. K. Georgieff, Nutrition and the developing brain: nutrient priorities and measurement, Am. J. Clin. Nutr., 2007, 85, 614S–620S CAS.
- L. M. Klevay, L. Inmann, L. K. Johnson, M. Lawler, J. R. Mahalko, D. B. Milne, H. C. Lukaski, W. Bolonchuk and H. H. Sandstead, Increased cholesterol in plasma in a young man during experimental copper depletion, Metab., Clin. Exp., 1984, 33, 1112–1118 CrossRef CAS PubMed.
- S. Reiser, A. Powell, C. Y. Yang and J. Canary, Effect of copper intake on blood cholestrol and its lipoprotein distribution in men, Nutr. Rep. Int., 1987, 36, 641–649 CAS.
- G. Crisponi, V. M. Nurchi, D. Fanni, C. Gerosa, S. Nemolato and G. Faa, Copper-related diseases: From chemistry to molecular pathology, Coord. Chem. Rev., 2010, 254, 876–889 CrossRef CAS.
- H. Zischka and C. Einer, Mitochondrial copper homeostasis and its derailment in Wilson disease, Int. J. Biochem. Cell Biol., 2018, 102, 71–75 CrossRef CAS PubMed.
- C. M. Saporito-Magrina, R. N. Musacco-Sebio, G. Andrieux, L. Kook, M. T. Orrego, M. V. Tuttolomondo, M. F. Desimone, M. Boerries, C. Borner and M. G. Repetto, Copper-induced cell death and the protective role of glutathione: the implication of impaired protein folding rather than oxidative stress, Metallomics, 2018, 10, 1743–1754 RSC.
- J. H. Menkes, M. Alter, G. K. Steigleder, D. R. Weakley and J. H. Sung, A sex-linked recessive disorder with retardation of growth, peculiar hair, and focal cerebral and cerebellar degeneration, Pediactrics, 1962, 29, 764–779 CAS.
- C. Vulpe, B. Levinson, S. Whitney, S. Packman and J. Gitschier, Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase, Nat. Genet., 1993, 3, 7–13 CrossRef CAS PubMed.
- S. G. Kaler, C. S. Holmes, D. S. Goldstein, J. Tang, S. C. Godwin, A. Donsante, C. J. Liew, S. Sato and N. Patronas, Neonatal diagnosis and treatment of Menkes disease, N. Engl. J. Med., 2008, 358, 605–614 CrossRef CAS PubMed.
- S. A. K. Wilson, Progressive lenticular degeneration: a familial nervous disease associated with cirrhosis of the liver, Brain, 1912, 34, 295–509 CrossRef.
- P. C. Bull, G. R. Thomas, J. M. Rommens, J. R. Forbes and D. W. Cox, The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene, Nat. Genet., 1993, 5, 327–337 CrossRef CAS PubMed.
- R. E. Tanzi, K. Petrukhin, I. Chernov, J. L. Pellegquer, W. Wasco, B. Ross, D. M. Romano, E. Parano, L. Pavone, L. M. Brzustowicz, M. Devoto, J. Peppercorn, A. I. Bush, I. Sternlieb, M. Pirastu, J. F. Gusella, O. Evgravfov, G. K. Penchaszadeh, B. Honig, I. S. Edelman, M. B. Soares, I. H. Scheinberg and T. C. Gilliam, The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene, Nat. Genet., 1993, 5, 344–350 CrossRef CAS PubMed.
- S. L. Fontaine, M. L. Ackland and J. F. B. Mercer, Mammalian copper-transporting P-type ATPases, ATP7A and ATP7B: Emerging Roles, Int. J. Biochem. Cell Biol., 2010, 42, 206–209 CrossRef PubMed.
- H. P. Roeser, G. R. Lee, S. Nacht and G. E. Cartwright, The role of ceruloplasmin in iron metabolism, J. Clin. Invest., 1970, 49, 2408–2417 CrossRef CAS PubMed.
- J. P. Kehrer, The Haber-Weiss reaction and mechanism of toxicity, Toxicology, 2000, 149, 43–50 CrossRef CAS PubMed.
- H. Sies, Oxidative stress: a concept in redox biology and medicine, Redox Biol., 2015, 4, 180–183 CrossRef CAS PubMed.
- J. D. Gitlin, Wilson Disease, Gastroenterology, 2003, 125, 1868–1877 CrossRef PubMed.
- R. Squitti, R. Ghidoni, I. Simonelli, I. D. Ivanova, N. A. Colabufo, M. Zuin, L. Benussi, G. Binetti, E. Cassetta, M. Rongioletti and M. Siotto, Copper dyshomeostasis in Wilson disease and Alzheimer's disease as shown by serum and urine copper indicators, J. Trace Elem. Med. Biol., 2018, 45, 181–188 CrossRef CAS PubMed.
- E. A. Roberts and M. L. Schilsky, Diagnosis and treatment of Wilson disease: An update, Hepatology, 2008, 47, 2089–2111 CrossRef CAS PubMed.
- R. Squitti, M. Ventriglia, G. Barbati, E. Cassetta, F. Ferreri, G. D. Forno, S. Ramires, F. Zappasodi and P. M. Rossini, “Free” copper in serum of Alzheimer's disease patients correlates with markers of liver function, J. Neural Transm., 2007, 114, 1589–1594 CrossRef CAS PubMed.
- G. A. McMillin, J. J. Travis and J. W. Hunt, Direct measurement of free copper in serum or plasma ultrafiltrate, Am. J. Clin. Pathol., 2009, 131, 160–165 CrossRef CAS PubMed.
- A. Sanz-Medel, M. Montes-Bayon and M. L. F. Sanchez, Trace element speciation by ICP-MS in large biomolecules and its potential for proteomics, Anal. Bioanal. Chem., 2003, 377, 236–247 CrossRef CAS PubMed.
- C. S. Muniz, J. M. M. Gayon, J. I. G. Alonso and A. Sanz-Medel, Speciation of essential elements in human serum using anion-exchange chromatography coupled to post-column isotope dilution analysis with double focusing ICP-MS, J. Anal. At. Spectrom., 2001, 16, 587–592 RSC.
- K. Inagaki, N. Mikuriya, S. Morita, H. Haraguchi, Y. Nakahara, M. Hattori, T. Kinosita and H. Saito, Speciation of protein-binding zinc and copper in human blood serum by chelating resin pre-treatment and inductively coupled plasma mass spectrometry, Analyst, 2000, 125, 197–203 RSC.
- V. Lopez-Avila, O. Sharpe and W. H. Robinson, Determination of ceruloplasmin in human serum by SEC-ICPMS, Anal. Bioanal. Chem., 2006, 386, 180–187 CrossRef CAS PubMed.
- N. Solovyev, A. Ala, M. L. Schilsky, C. Mills, K. Willis and C. Harrington, Biomedical copper speciation in relation to Wilson's disease using strong anion exchange chromatography coupled to triple quadrupole inductively coupled plasma mass spectrometry, Anal. Chim. Acta, 2020, 1098, 27–36 CrossRef CAS PubMed.
- N. Solovyev, M. Vinceti, P. Grill, J. Mandrioli and B. Michalke, Redox speciation of iron, manganese, and copper in cerebrospinal fluid by strong cation exchange chromatography – sector field inductively coupled plasma mass spectrometry, Anal. Chim. Acta, 2017, 973, 25–33 CrossRef CAS PubMed.
- V. Federica, N. Solovyev, M. Vinceti, J. Mandrioli, M. Lucio and B. Michalke, The study of levels from redox-active elements in cerebrospinal fluid of amyotrophic lateral sclerosis patients carrying disease-related gene mutations shows potential copper dyshomeostasis, Metallomics, 2020, 12, 668–681 RSC.
- H. Zischka, et al., Liver mitochondrial membrane crosslinking and destruction in a rat model of Wilson Disease, J. Clin. Invest., 2011, 121, 1508–1518 CrossRef PubMed.
- J. Lichtmannegger, C. Leitzinger, R. Wimmer, S. Schmitt, S. Schulz, Y. Kabiri, C. Eberhagen, T. Rieder, D. Janik, F. Neff, B. K. Straub, P. Schirmacher, A. A. DiSpirito, N. Bandow, B. S. Baral, A. Flatley, E. Kremmer, G. Denk, F. P. Reiter, S. Hohenester, F. Eckardt-Schupp, N. A. Dencher, J. Adamski, V. Sauer, C. Niemietz, H. H. J. Schmidt, U. Merle, D. N. Gotthardt, G. Kroemer, K. H. Weiss and H. Zischka, Methanobactin reverses acute liver failure in a rat model of Wilson disease, J. Clin. Invest., 2016, 126, 2721–2735 CrossRef PubMed.
- C. Einer, et al., A high-calorie diet aggravates mitochondrial dysfunction and triggers severe liver damage in Wilson Disease rats, Cell. Mol. Gastroentrerol. Hepatol., 2018, 7, 571–596 CrossRef PubMed.
- T. Kirsipuu, A. Zadoroznaja, J. Smirnova, M. Friedemann, T. Plitz, V. Tougu and P. Palumaa, Copper(II)-binding equilibria in human blood, Sci. Rep., 2020, 10, 5686 CrossRef CAS PubMed.
- A. Zgirski and E. Frieden, Binding of Cu(II) to non-prosthetic sites in ceruloplasmin and bovine serum albumin, J. Inorg. Chem., 1990, 39, 137–148 CAS.
- H. W. Richardson, Handbook of copper compounds and applications, Marcel Dekker, Inc., New York, NY, USA, 1997 Search PubMed.
- I. Stevic, N. Parmar, N. Paredes, L. R. Berry and A. K. C. Chan, Binding of heparin to metals, Cell Biochem. Biophys., 2011, 59, 171–178 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0mt00132e |
| This journal is © The Royal Society of Chemistry 2020 |