Endosperm prevents toxic amounts of Zn from accumulating in the seed embryo – an adaptation to metalliferous sites in metal-tolerant Biscutella laevigata†
Alicja
Babst-Kostecka
 *a,
Wojciech J.
Przybyłowicz
*a,
Wojciech J.
Przybyłowicz
 bc,
Antony
van der Ent
bc,
Antony
van der Ent
 de,
Chris
Ryan
f,
Charlotte C.
Dietrich
de,
Chris
Ryan
f,
Charlotte C.
Dietrich
 a and
Jolanta
Mesjasz-Przybyłowicz
a and
Jolanta
Mesjasz-Przybyłowicz
 c
c
aW. Szafer Institute of Botany, Polish Academy of Sciences, Department of Ecology, Lubicz 46, 31-512 Krakow, Poland. E-mail: a.kostecka@botany.pl; Fax: +48 124219790; Tel: +48 124241704
bAGH University of Science and Technology, Faculty of Physics & Applied Computer Science, al. Mickiewicza 30, 30-059 Kraków, Poland
cDepartment of Botany and Zoology, Stellenbosch University, Private Bag X1, Matieland 7602, South Africa
dCentre for Mined Land Rehabilitation, Sustainable Minerals Institute, The University of Queensland, Australia
eLaboratoire Sols et Environnement, Université de Lorraine, France
fCSIRO, Mineral Resources, Australia
First published on 7th November 2019
Abstract
Seed germination represents the first crucial stage in the life cycle of a plant, and the seed must contain all necessary transition elements for the development and successful establishment of the seedling. Problematically, seed development and germination are often hampered by elevated metal(loid) concentrations in industrially polluted soils, making their revegetation a challenging task. Biscutella laevigata L. (Brassicaceae) is a rare perennial pseudometallophyte that can tolerate high concentrations of trace metal elements. Yet, the strategies of this and other plant species to ensure reproductive success at metalliferous sites are poorly understood. Here we characterized several parameters of germination and used synchrotron X-ray fluorescence microscopy to investigate the spatial distribution and concentration of elements within B. laevigata seeds from two metallicolous and two non-metallicolous populations. We find that average germination time was shorter and the seed weight was lower in the metallicolous compared to the non-metallicolous populations. By allowing for at least two generations within one growth season, relatively fast germination at metalliferous sites accelerates microevolutionary processes and likely enhances the potential of metallicolous accessions to adapt to environmental stress. We also identified different strategies of elemental accumulation within seed tissues between populations. Particularly interesting patterns were observed for zinc, which was found in 6-fold higher concentrations in the endosperm of metallicolous compared to non-metallicolous populations. This indicates that the endosperm protects the seed embryo from accumulating toxic concentrations of metal(loid)s, which likely improves reproductive success. Hence, we conclude that elemental uptake regulation by the seed endosperm is associated with enhanced metal tolerance and adaptation to metalliferous environments in B. laevigata.
Significance to metallomicsPlants can only establish in metalliferous environments, if their reproduction is successful. Seeds must therefore be protected from intoxication by excess metal(loid)s that are abundant in soils at industrial legacy sites. This study visualizes, quantifies, and compares the distribution of various elements in Biscutella laevigata seeds from metalliferous and natural habitats. Interestingly, we found that this species has developed a strategy to allocate zinc and other elements to non-harmful sections of the seed, using them as a barrier to prevent the intoxication of sensitive parts. This enhances our knowledge of how plants can adapt to and tolerate soil pollution. |
Introduction
The global industrial revolution has led to an unprecedented release of toxic substances into the environment.1 The far-reaching consequences of this pollution include soil contamination with hazardous waste, which threatens environmental and human health around the world. Among pollutants, trace metal elements (including arsenic, As, cadmium, Cd, zinc, Zn, lead, Pb, and thallium, Tl) are of major concern. Negative impacts can arise from direct contact with polluted soil or ground water, or from ingestion via the food chain (soil-plant-human or soil-plant-animal-human), reduction in food quality, and food insecurity resulting from reduced soil fertility and agricultural production.2–4 Unlike organic contaminants, trace metal elements do not undergo microbial or chemical degradation and may persist at elevated concentration in soils for a long time after their dissemination.5 This is particularly problematic in soils in the vicinity of metalliferous mining and smelter sites, where trace elements are continuously accumulated upon release.6 Hence, there is a growing demand for cost-effective and environmentally friendly technologies to remediate contaminated sites.7 Revegetation has drawn special attention as a promising “green and clean technology” for intervention to toxic exposures.8 Plant establishment on mine tailings not only mitigates hazards associated with wind dispersal of local contaminated dust, but every square meter of vegetation can effectively remove up to 1 kg of dust per year from the air that moves across the planted region.9 A lasting plant cover also helps immobilizing contaminants in the ground, with positive effects on ground water quality,10 and provides important ecosystem services such as carbon sequestration, intensified water cycling, and habitat for numerous species. Problematically, most plants are sensitive to high soil trace metal element concentrations, which often inhibit seed germination and plant growth on mine tailings.11–13Only a limited number of vascular plant species called ‘metallophytes’ have developed the ability to survive and reproduce in toxic metalliferous environments.14,15 This unusual characteristic has been defined as metal tolerance.16,17 While non-metallophyte species may to some extent also tolerate elevated metal concentrations in soils, only metallophytes possess physiological mechanisms that allow them to cope with very high concentrations that cause toxicity. Two contrasting physiological strategies have thereby evolved: plants are either ‘excluders’ that restrict trace metal element allocation to aerial parts by limiting root uptake and/or transport through the stem, or they are ‘(hyper)accumulators’ that allocate extraordinarily large amounts of trace metal elements to their shoots. Plants that pursue a third, intermediate strategy are called ‘indicators’ and seek a proportional relation between elements in soil and in plants.14,18
While trace metal element allocation to foliage has been extensively studied in metallophytes, only a very limited number of investigations have focussed on the elemental distribution in their seeds (e.g.ref. 19–24) and most studies to date have focussed on nickel (Ni), Zn and Cd hyperaccumulator plants.25–30 Yet, more research of this kind is urgently needed because plant establishment at post-mining sites (and elsewhere) critically depends on successful seed germination and early seedling growth.31
A general presumption is that metallophytes must keep their seeds free of toxic trace metal concentrations to provide their offspring with a ‘fresh start’ on metalliferous soils.32 However, some transition elements are necessary in the seed to ensure the development of the seedling. Trace metal element uptake, distribution and concentration must therefore be carefully regulated to reduce the toxicity risk. Importantly, it becomes more and more evident that not only environmental conditions, but also the species demographic history and pre-adaptation of plant populations play a role in plant tolerance and sensitivity to unfavourable soil conditions during these first two crucial life stages. Accordingly, plant populations originating from specific habitats may perform better or worse on a given metalliferous site than those from other habitats.33–36 This calls for comprehensive, quantitative investigations of the variation in the elemental distribution in the seeds of metallophytes to gain insight into plant adaptation to metal contaminated soils. Pseudometallophytes, i.e. taxa with populations both on and off metalliferous soils, are of particular research interest because extreme environmental conditions promote rapid differentiation between their metallicolous (M) and non-metallicolous (NM) populations.37–39
Biscutella laevigata L. (Brassicaceae) is a perennial pseudometallophyte, that is widespread across Central and Western Europe.40,41 Its distribution range reaches its northern limit in Poland, where the species is restricted to only a few known localities, mostly on non-metalliferous sites in the Western Tatra Mts, but also on calamine waste heaps in the Olkusz region. Metal tolerance is present in all known Polish B. laevigata populations and is further enhanced in metallicolous populations in response to stress from high trace metal element concentration in soils.42 Thus, populations from natural and anthropogenic locations from Southern Poland have adapted to different environmental conditions and have thereby genetically diverged.38 Due to these clear divergence patterns, Polish metallicolous and non-metallicolous populations of B. laevigata represent particularly interesting material to study plant adaptation to trace metal element polluted environments. In terms of elemental allocation patterns in B. laevigata seeds, metallicolous plants were reported to strictly and actively select elements and their amounts taken up by different seed tissues.20 However, because no comparison with seeds from unpolluted sites is yet available, the association of these interesting patterns to adaptation to metalliferous environments remains unclear.
In this study, we investigated two metallicolous and two non-metallicolous B. laevigata populations that have previously been identified as the most and the least trace metal element tolerant accessions from Southern Poland.42 We examined several reproductive traits (e.g. germination rate, average germination time, seed weight) and investigated the spatial distribution and concentration of elements within B. laevigata seeds from these four populations in the context of adaptation to metalliferous environments. For the latter, we employed synchrotron X-ray fluorescence microscopy (XFM). The XFM approach offers several unique capabilities that are of interest to plant scientists, including the highly sensitive detection of most trace elements and a fine spatial resolution.24,43 The specific aims of this study were to (i) characterize the parameters of germination, (ii) investigate the elemental composition of B. laevigata seeds, and (iii) compare patterns of elemental distribution and concentration among populations growing on anthropogenic and natural habitats. This is to verify, if metallicolous plants have evolved a strategy to combat trace metal element stress at the seed level. By addressing these points, our study provides new insight into plant adaptation to restrictive environments. At the same time, we aim to provide a better understanding of the mechanisms that underlie the physiology of trace metal element tolerance at the seed developmental stage.
Materials and methods
Study sites and plant material
The sampling included two metalliferous (M_PL2 and M_PL6) and two non-metalliferous (NM_PL8 and NM_SK14) locations of B. laevigata at its north-eastern distribution limit. Both metalliferous sites are located on lowland waste heaps and dust deposits in the heavily contaminated post-mining area of Olkusz in Southern Poland. Total and extractable Zn soil concentration at site M_PL2 was 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 580 and 3420 μg g−1, respectively. At site M_PL6, the corresponding values were 51
580 and 3420 μg g−1, respectively. At site M_PL6, the corresponding values were 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140 and 5920 μg g−1 (Table 1). The NM_PL8 site is located in a meadow at the Tatra Mts foothills, whereas NM_SK14 grows on a calcareous slope of the Western Tatra Mountains. The metal concentration in non-metalliferous soils was considerably lower, ranging between 50 and 100 μg g−1 for total Zn and 20–70 μg g−1 for extractable Zn. At each site, ripe seeds from ten B. laevigata mother plants were sampled separately at a minimum distance of 3 m to avoid clonal repetition.44
140 and 5920 μg g−1 (Table 1). The NM_PL8 site is located in a meadow at the Tatra Mts foothills, whereas NM_SK14 grows on a calcareous slope of the Western Tatra Mountains. The metal concentration in non-metalliferous soils was considerably lower, ranging between 50 and 100 μg g−1 for total Zn and 20–70 μg g−1 for extractable Zn. At each site, ripe seeds from ten B. laevigata mother plants were sampled separately at a minimum distance of 3 m to avoid clonal repetition.44
| Site | Type | Location | Latitude N | Longitude E | Elevation (m a.s.l.) | pHa | ZnTa (μg g−1) | ZnEb (μg g−1) |
|---|---|---|---|---|---|---|---|---|
| a Adapted from Babst-Kostecka et al. 2016. b Adapted from Babst-Kostecka et al. 2014. | ||||||||
| M_PL2 | Metalliferous | Olkusz | 50°17′34.45′′ | 19°29′01.95′′ | 304 | 8.2 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 580 ± 2860 580 ± 2860 |
3420![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ± ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1130 1130 |
| M_PL6 | Metalliferous | Olkusz | 50°17′06.74′′ | 19°27′59.27′′ | 338 | 7.8 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140 ± 22 140 ± 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210 210 |
5920![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ± ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2320 2320 |
| NM_PL8 | Non-metalliferous | Tatra Mts | 49°15′05.63′′ | 19°54′37.62′′ | 1342 | 7.7 | 100 ± 40 | 70 ± 40 |
| NM_SK14 | Non-metalliferous | Tatra Mts | 49°13′57.22′′ | 20°16′24.10′′ | 1719 | 7.5 | 50 ± 25 | 20 ± 13 |
Germination test and reproductive traits
Thirty seeds were randomly selected from each population and germinated in plastic containers (25 × 15 × 5 cm) filled with a sterile, mixed perlite![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) vermiculite (4
vermiculite (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v
1, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) substrate. Seeds were sown to a depth of ∼1 cm and the distance among the seeds was 5 cm. Three containers per population were randomly arranged in a controlled growth chamber with the following conditions:42 photoperiod: 13 h day and 11 h night; temperature: 20 °C day and 15 °C night; relative humidity: 65%; and irradiance: 300 μmol photons m−2 s−1. Seed germination was determined by visual detection of seedling emergence and was recorded daily until no seeds germinated for 3 days, which was the case after 27 days. Additionally, from each population the seed weight [mg] was determined for 30 independent seeds from two randomly selected B. laevigata mother plants (n = 30 seeds × 8 plants). One seed from each of the eight mother plants was then selected for X-ray fluorescence microscopy and longitudinal sections of the middle parts of air-dried ripe seeds were adhered to Kapton (polyimide) tape mounted on specimen holders.
v) substrate. Seeds were sown to a depth of ∼1 cm and the distance among the seeds was 5 cm. Three containers per population were randomly arranged in a controlled growth chamber with the following conditions:42 photoperiod: 13 h day and 11 h night; temperature: 20 °C day and 15 °C night; relative humidity: 65%; and irradiance: 300 μmol photons m−2 s−1. Seed germination was determined by visual detection of seedling emergence and was recorded daily until no seeds germinated for 3 days, which was the case after 27 days. Additionally, from each population the seed weight [mg] was determined for 30 independent seeds from two randomly selected B. laevigata mother plants (n = 30 seeds × 8 plants). One seed from each of the eight mother plants was then selected for X-ray fluorescence microscopy and longitudinal sections of the middle parts of air-dried ripe seeds were adhered to Kapton (polyimide) tape mounted on specimen holders.
Synchrotron X-ray fluorescence microscopy (XFM)
The X-ray fluorescence microscopy (XFM) beamline of the Australian Synchrotron employs an in-vacuum undulator to produce a brilliant X-ray beam of 4.1–20 keV with a focus down to ∼1000 nm. An Si(111) monochromator and a Kirkpatrick–Baez (K/B) pair of mirrors delivers a monochromatic focused beam onto the specimen.45 The beamline is equipped with a Maia detector, which uses a large detector array to maximize the detected signal and count-rates for efficient imaging. Maia enables high overall count-rates and uses an annular detector geometry, where the beam passes though the detector and strikes the sample at normal incidence.46,47 This enables a large solid-angle (1.2 steradian) to be achieved in order to maximize the detected signal and consequently to reduce the dose and potential damage to a specimen.48 Maia is designed for event-mode data acquisition, where each detected X-ray event is recorded, tagged by detector number in the array, position in the scan, and other metadata (Ryan et al. 2014).Data processing and statistics
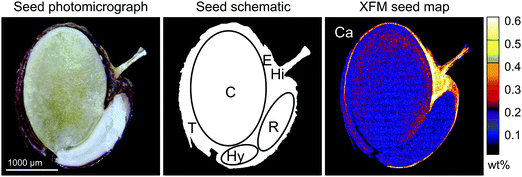
The differences in mean germination rate, germination time, and seed weight were assessed using the Kruskal–Wallis test. The XFM data was quantitatively processed using the Dynamic Analysis method.49 This method generates elemental images, which are (i) overlap-resolved, (ii) with subtracted background and (iii) quantitative, i.e. in μg g−1 dry weight units. The Compton scatter maps were used to correct for local areal density variations. Elemental concentrations from these areas are also reported in μg g−1 dry weight. The error estimates were extracted from the error matrix generated in the fit and the minimum detection limits (MDL) were calculated using the Currie equation.50 Maps were complemented by data extracted from arbitrarily selected regions of interest (ROIs) within scanned seeds, representing specific seed parts. Six ROIs were selected based on the seed morphological structures (Fig. 1). Accordingly, besides the whole seed section, we distinguished the testa, hilum, endosperm, radicle, hypocotyl, and cotyledon. For few results below the MDL, one-half of the reported limit was used for the purpose of statistical analysis.51 The differences in mean concentration of elements between ecotypes at the whole-seed cross-section level and for individual ROIs were assessed using the Wilcoxon signed-rank test. At the ecotype level, the differences in mean concentration of elements between seed tissues were assessed using the Kruskal–Wallis test.Results & discussion
The adaptation of plants to extremely harsh conditions at metalliferous sites affects reproductive traits and eventually leads to the evolution of metal tolerant ecotypes.52–54 Our study identified important differences between metallicolous and non-metallicolous B. laevigata ecotypes that may contribute to this process and greatly impact the colonization of and subsequent survival at metalliferous sites.The germination rate varied broadly between populations, with the lowest values in NM_SK14 (53.3%) and the highest values in M_PL6 (76.7%; Table 2 and Fig. 2). All populations showed average germination times above 180 hours (Table 2). Population M_PL2 was characterized by more rapid and uniform germination than the other three populations. The average germination time is considered to be a good measure of the speed with which a species can occupy a certain environment. It can be classified into three general categories: rapid (<120 hours), intermediate (between 120 and 240 hours), and slow (>240 hours).55 Accordingly, the germination time of B. laevigata seeds from metallicolous accessions falls into the intermediate category, whereas germination of seeds from non-metallicolous plants is slow. Recent studies have indicated that slow and heterogeneous germination times may be associated with species that occur in alpine environments with spatially variable and temporally unstable conditions.56,57 Specifically, seeds that are dispersed in such habitats do not always find favourable conditions immediately, due to e.g. low temperatures and short growing seasons. Thus, slow and temporally distributed germination of B. laevigata seeds in mountain habitats is likely part of a germination strategy that promotes successful recruitment of new individuals in an unpredictable climate. By contrast, relatively fast germination and more stable climatic conditions at the investigated metalliferous sites provide these lowland B. laevigata accessions with at least two generations within one growing season (A. Babst-Kostecka, personal observation). A similar strategy has been reported for Arabidopsis arenosa, Silene vulgaris, and Rumex dentatus, and can be associated with greater potential of metallicolous accessions to adapt to environmental stress at metalliferous sites, i.e. through an increased rate of microevolutionary processes.58–60
| Population | Germination rate (%) | Germination time (h) |
|---|---|---|
| M_PL2 | 60.0 a | 186.7 ± 65.6 a |
| M_PL6 | 76.7 b | 229.6 ± 93.2 a |
| NM_PL8 | 53.3 c | 342.0 ± 132.2 b |
| NM_SK14 | 70.0 d | 339.4 ± 98.2 b |
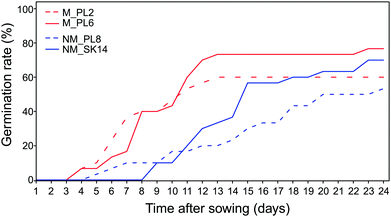 | ||
| Fig. 2 Cumulative germination rate over time for Biscutella laevigata seeds from two metallicolous (M, red) and two non-metallicolous (NM, blue) populations (30 seeds per population). | ||
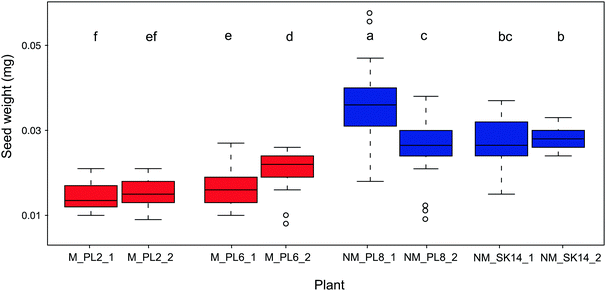
Seed mass is another important parameter that ensures effective and prolific reproduction of plants in metalliferous soils. Being positively correlated with seedling survival, this trait is considered to be critical for the adaption of plants to unfavorable environments.61 Indeed, seed mass is the most realistic measure of plant generative processes in response to metal exposure.62 In plants that colonize metalliferous sites, reproduction often increases at the expense of vegetative development.37,63,64 Thus, vegetative parts are minimized, but flowers and seeds are usually normal in size or even bigger than in plants at non-metalliferous sites. Accordingly, heavier seeds in metallicolous compared to non-metallicolous populations have been reported, e.g., in natural populations of A. arenosa58 and Nocceae caerulescens.65 In the present study, we found that B. laevigata populations significantly differed in seed weight, however, non-metallicolous plants had on average 1.7-fold heavier seeds (0.029 ± 0.007 mg) than metallicolous plants (0.017 ± 0.004 mg; Fig. 3). This lower reproductive biomass allocation in metallicolous B. laevigata can be associated with costs of tolerance. Indeed, given that metal tolerance in B. laevigata is constitutive,42 our findings are consistent with the theory that elevated concentration of trace metal elements in soil increases maintenance costs because an organism needs to spend energy to counterbalance their potentially toxic effects.66 Such a trade-off between trait and environment can increase survival under stress conditions, but leaves less energy for growth, reproduction and/or other processes.4,36,39 Importantly, B. laevigata can reproduce via vegetative propagation and thereby occupy habitats with genetically identical individuals that are best suited for local conditions. Our results suggest that the latter strategy may play an important role in the colonization of and establishment at unfavourable metalliferous sites by B. laevigata. While the trade-offs between investment in vegetative vs. sexual reproduction at metalliferous sites may limit resource allocation to seed production, new genotypes still need to be introduced into the population to ensure diversity of the genetic pool.67 Thus, a sufficient amount of seeds needs to be produced to enable populations to cope with environmental heterogeneity and ensure species survival. In this context, knowledge of the elemental composition of seeds is essential for better understanding the processes and strategies that underlie successful sexual reproduction in plants.68
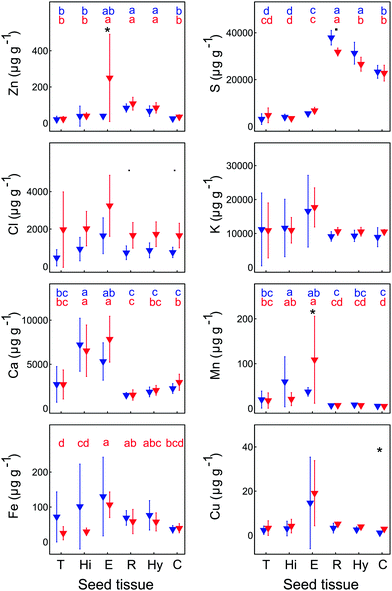
The XFM analysis detected the following macro- and micro-nutrients in B. laevigata seeds: Zn, sulphur (S), chlorine (Cl), potassium (K), calcium (Ca), manganese (Mn), iron (Fe) and copper (Cu) (Table 3). Other elements whose concentrations did not reach the minimum detection limit in some tissues, e.g. chromium (Cr), cobalt (Co), Ni, and As, are shown in ESI,† Table S1. Strong and diverse patterns of elemental distribution and concentrations were observed at the ecotype and population levels (Table S2, ESI†), and considerable differences were also observed between specific seed sections (Fig. 4, 5 and Table S3, ESI†). The most notable results were observed for Zn (Fig. 4). Zinc is an essential element that needs to be transported to the developing seeds; however, the mechanisms regulating its allocation to specific seed tissues are largely unknown. In our study, the highest Zn concentrations were found in the endosperm of seeds from metallicolous populations (up to 584 μg g−1 in M_PL6). These concentrations were significantly (P = 0.029; ESI,† Table S2) – on average 6-fold – higher than the concentrations in the endosperm of seeds from non-metallicolous populations. The second region that exhibited differences between the two ecotypes in terms of accumulated Zn was the hilum, with on average 4-fold higher Zn content in metallicolous than in non-metallicolous samples (except for one sample in NM_SK14). Other tissue types contained rather similar amounts of Zn in material from both edaphic origins, with the lowest values predominantly found in testa (2.2–38.0 μg g−1) and cotyledon (15.1–46.4 μg g−1). Within embryonic tissues, the highest Zn concentration was found in the radicle, independent of plant edaphic type. By preventing the accumulation of toxic concentrations of metal(loid)s within the seed embryo, mother plants ensure reproductive success.32 Accordingly, the uptake of Zn is known to be restricted and previous studies have shown species-specific Zn distribution patterns within seeds. For instance, in seeds of the metal hyperaccumulator Thlaspi praecox, Zn was mainly allocated to cotyledons and epidermis.26 This seems to be a protective strategy for the seeds, as cotyledons are rapidly discarded by most plants at an early developmental stage. By contrast, Mesjasz-Przybyłowicz et al. (2001) found the highest amount of Zn in the endosperm of metallicolous B. laevigata seeds. The authors suggested that the endosperm acts as a barrier against the transport of toxic amounts of elements into embryonic parts. In metalliferous environments, due to the potential toxicity of excess levels of metal(loid)s, carefully regulated delivery of elements from the mother plant into seeds is essential to prevent seed inhibition and subsequent negative effects on seed germination.69 The pronounced differences in Zn concentration between seeds from metallicolous and non-metallicolous B. laevigata populations revealed in our study – in particular primary Zn allocation to the endosperm in plants exposed to highly elevated Zn content in soil – further suggest that uptake regulation by the endosperm is indeed associated with metal tolerance and adaptation to metalliferous environments. Moreover, Zn acquisition by different seed tissues appears to be controlled in a population-specific manner, indicating the importance of local microevolutionary processes.
| Region of interest (ROI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Element | Population | Sample | Whole section | Testa | Hilum | Endosperm | Radicle | Hypocotyl | Cotyledon |
| a Note that for results below errors of analysis one-half of the reported detection limit was used to calculate the mean. | |||||||||
| Zn | M_PL2 | 1 | 38.7 (0.3) | 10.1 (0.3) | 65.0 (0.9) | 266 (2) | 67.6 (0.4) | 47.0 (0.2) | 22.8 (0.2) |
| 2 | 57.4 (0.3) | 34.4 (0.6) | 37.9 (0.5) | 100 (0.7) | 154 (0.9) | 115 (1) | 43.7 (0.3) | ||
| M_PL6 | 1 | 31.9 (0.2) | 35.7 (0.5) | 24.1 (0.4) | 49.8 (0.5) | 106 (0.7) | 80.3 (0.7) | 25.9 (0.2) | |
| 2 | 74.7 (0.4) | 11.0 (0.7) | 32.4 (0.5) | 584 (4) | 103 (0.6) | 97.2 (0.8) | 46.4 (0.3) | ||
| Mean | 50.7 | 22.8 | 39.8 | 249.9 | 107.6 | 84.9 | 34.7 | ||
| NM_PL8 | 1 | 36.2 (0.2) | 38.0 (0.5) | 16.6 (0.4) | 35.3 (0.4) | 78.7 (0.5) | 46.8 (0.5) | 33.6 (0.2) | |
| 2 | 38.5 (0.2) | 33.6 (0.4) | 11.8 (0.4) | 48.7 (0.9) | 108.7 (0.8) | 99.2 (0.9) | 32.1 (0.2) | ||
| NM_SK14 | 1 | 22.0 (0.2) | 2.2 (0.1) | 3.8 (0.2) | 25.8 (0.4) | 54.6 (0.4) | 37.7 (0.4) | 15.1 (0.1) | |
| 2 | 30.9 (0.2) | 7.3 (0.4) | 122 (1) | 48.0 (0.5) | 93.1 (0.7) | 82.6 (0.5) | 19.6 (0.1) | ||
| Mean | 31.9 | 20.3 | 38.5 | 39.4 | 83.8 | 66.6 | 25.1 | ||
| M_PL2 | 1 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (160) 400 (160) |
2440 (290) | 4200 (330) | 8280 (310) | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (270) 200 (270) |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 (420) 350 (420) |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 170 (190) 170 (190) |
|
| 2 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 220 (90) 220 (90) |
8850 (460) | 2840 (310) | 4860 (230) | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 880 (300) 880 (300) |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 (440) 450 (440) |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 170 (90) 170 (90) |
||
| M_PL6 | 1 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (110) 400 (110) |
5650 (320) | 3660 (310) | 6630 (310) | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 170 (290) 170 (290) |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670 (400) 670 (400) |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560 (110) 560 (110) |
|
| 2 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 870 (110) 870 (110) |
2090 (710) | 3100 (310) | 7220 (260) | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 890 (220) 890 (220) |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 870 (390) 870 (390) |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160 (120) 160 (120) |
||
| S | Mean | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 472.5 472.5 |
4757.5 | 3450 | 6747.5 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 785 785 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 585 585 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 765 765 |
|
| NM_PL8 | 1 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 (140) 260 (140) |
4410 (300) | 3900 (330) | 5070 (290) | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 770 (300) 770 (300) |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 790 (410) 790 (410) |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230 (140) 230 (140) |
|
| 2 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 (130) 850 (130) |
5570 (270) | 5110 (340) | 5770 (530) | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110 (300) 110 (300) |
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 (440) 450 (440) |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 (130) 450 (130) |
||
| NM_SK14 | 1 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090 (150) 090 (150) |
2150 (210) | 2210 (260) | 4780 (340) | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (220) 500 (220) |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 710 (400) 710 (400) |
195![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 (170) 80 (170) |
|
| 2 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140 (150) 140 (150) |
<850a | 4660 (260) | 6450 (260) | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180 (240) 180 (240) |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180 (320) 180 (320) |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 970 (160) 970 (160) |
||
| Mean | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 585 585 |
3245 | 3970 | 5517.5 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 890 890 |
31282.5 | 23307.5 | ||
| Cl | M_PL2 | 1 | 1480 (18) | 740 (110) | 2400 (120) | 3510 (110) | 1540 (50) | 1590 (90) | 1550 (25) |
| 2 | 2130 (16) | 2250 (160) | 2310 (120) | 3330 (100) | 2160 (70) | 2230 (100) | 2100 (23) | ||
| M_PL6 | 1 | 2020 (14) | 4700 (130) | 2720 (120) | 5040 (120) | 2220 (60) | 2240 (110) | 2200 (18) | |
| 2 | 670 (16) | <400a | 670 (100) | 1090 (80) | 750 (50) | 850 (100) | 770 (23) | ||
| Mean | 1575 | 1972.5 | 2025 | 3242.5 | 1667.5 | 1727.5 | 1655 | ||
| NM_PL8 | 1 | 520 (19) | 870 (119) | 570 (100) | 1005 (86) | 490 (44) | 590 (83) | 600 (22) | |
| 2 | 660 (18) | 820 (86) | 1000 (110) | 1510 (180) | 690 (60) | 870 (91) | 720 (20) | ||
| NM_SK14 | 1 | 330 (18) | <110a | 378 (84) | 1030 (100) | 500 (35) | 580 (83) | 550 (21) | |
| 2 | 1290 (16) | <270a | 1790 (90) | 3040 (93) | 1270 (40) | 1430 (78) | 1160 (17) | ||
| Mean | 700 | 470 | 934.5 | 1646 | 737 | 867 | 757 | ||
| K | M_PL2 | 1 | 9520 (60) | 3040 (50) | 7180 (80) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (90) 500 (90) |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560 (53) 560 (53) |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 390 (73) 390 (73) |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 130 (62) 130 (62) |
| 2 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 770 (40) 770 (40) |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 710 (90) 710 (90) |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 020 (120) 020 (120) |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340 (80) 340 (80) |
9760 (46) | 9650 (50) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 590 (35) 590 (35) |
||
| M_PL6 | 1 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 920 (56) 920 (56) |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 (130) 840 (130) |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090 (150) 090 (150) |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 (120) 440 (120) |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280 (57) 280 (57) |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 390 (80) 390 (80) |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 420 (55) 420 (55) |
|
| 2 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 190 (40) 190 (40) |
7020 (120) | 9530 (100) | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480 (90) 480 (90) |
9800 (46) | 9670 (56) | 9900 (34) | ||
| Mean | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 350 |
10902.5 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 955 955 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 690 690 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 525 525 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 510 510 |
||
| NM_PL8 | 1 | 9570 (50) | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 470 (130) 470 (130) |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 430 (160) 430 (160) |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090 (130) 090 (130) |
9040 (50) | 8130 (55) | 9060 (40) | |
| 2 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 870 (60) 870 (60) |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480 (110) 480 (110) |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160 (190) 160 (190) |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 (170) 250 (170) |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 860 (50) 860 (50) |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 980 (67) 980 (67) |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 590 (52) 590 (52) |
||
| NM_SK14 | 1 | 5100 (34) | 1380 (27) | 1740 (36) | 3590 (40) | 7390 (40) | 7610 (65) | 5770 (40) | |
| 2 | 8410 (52) | 2500 (55) | 8140 (90) | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 (95) 350 (95) |
9260 (43) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480 (55) 480 (55) |
8270 (52) | ||
| Mean | 8987.5 | 11207.5 | 11617.5 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 570 570 |
9137.5 | 9300 | 8922.5 | ||
| Ca | M_PL2 | 1 | 3240 (15) | 1600 (24) | 7830 (57) | 9210 (49) | 2200 (12) | 2570 (25) | 3160 (14) |
| 2 | 3875 (9) | 3080 (34) | 9980 (73) | 5660 (26) | 1060 (9) | 1890 (17) | 4170 (11) | ||
| M_PL6 | 1 | 1864 (8) | 4890 (32) | 3550 (42) | 5670 (28) | 1010 (7) | 1350 (15) | 2100 (8) | |
| 2 | 2628 (7) | 1290 (40) | 4790 (41) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 830 (47) 830 (47) |
1830 (11) | 2410 (18) | 2430 (6) | ||
| Mean | 2901 | 2715 | 6537 | 7842 | 1525 | 2055 | 2965 | ||
| NM_PL8 | 1 | 2380 (8) | 5530 (34) | 4220 (40) | 5590 (32) | 1430 (7) | 1350 (14) | 2470 (7) | |
| 2 | 2216 (7) | 2770 (26) | 8650 (70) | 2270 (38) | 1860 (10) | 2300 (17) | 2180 (6) | ||
| NM_SK14 | 1 | 1725 (10) | 1750 (20) | 5270 (60) | 7100 (46) | 1120 (7) | 1430 (16) | 1530 (10) | |
| 2 | 2790 (13) | 850 (30) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 730 (80) 730 (80) |
6320 (30) | 1570 (8) | 2400 (18) | 2850 (13) | ||
| Mean | 2278 | 2725 | 7217.5 | 5320 | 1495 | 1870 | 2257 | ||
| Mn | M_PL2 | 1 | 8.2 (0.1) | 5.1 (0.6) | 42 (1) | 68 (1) | 9.5 (0.3) | 9.0 (0.5) | 3.7 (0.1) |
| 2 | 10.2 (0.1) | 14.9 (0.8) | 12.4 (0.6) | 57.9 (0.7) | 7.4 (0.4) | 7.3 (0.6) | 6.8 (0.1) | ||
| M_PL6 | 1 | 6.48 (0.08) | 42.7 (0.9) | 18.8 (0.8) | 54.5 (0.9) | 6.4 (0.3) | 6.2 (0.6) | 4.4 (0.1) | |
| 2 | 13.8 (0.1) | 8 (1) | 10.1 (0.7) | 254 (2) | 5.1 (0.3) | 5.5 (0.5) | 5.21 (0.1) | ||
| Mean | 9.67 | 17.7 | 21 | 108 | 7.1 | 7.0 | 5.0 | ||
| NM_PL8 | 1 | 7.23 (0.07) | 26.7 (0.8) | 15.4 (0.7) | 27.8 (0.7) | 5.6 (0.3) | 4.2 (0.5) | 5.6 (0.1) | |
| 2 | 11.2 (0.1) | 43.6 (0.8) | 108 (2) | 42 (2) | 8.3 (0.4) | 12.3 (0.6) | 7.8 (0.1) | ||
| NM_SK14 | 1 | 5.0 (0.1) | 5.2 (0.4) | 7.2 (0.6) | 30.4 (1.0) | 5.4 (0.2) | 5.7 (0.6) | 3.7 (0.1) | |
| 2 | 8.0 (0.1) | 3.7 (0.9) | 108 (1) | 48.3 (1.0) | 7.1 (0.3) | 10.2 (0.5) | 4.4 (0.1) | ||
| Mean | 7.86 | 19.8 | 59.6 | 37.1 | 6.6 | 8.1 | 5.4 | ||
| Fe | M_PL2 | 1 | 31.7 (0.3) | 10.4 (0.5) | 44.6 (0.9) | 139 (1) | 42.0 (0.4) | 32.7 (0.6) | 29.6 (0.3) |
| 2 | 64.1 (0.3) | 25.3 (0.8) | 28.1 (0.7) | 111 (1) | 104.8 (0.8) | 88 (1) | 58.7 (0.3) | ||
| M_PL6 | 1 | 33.9 (0.2) | 51.9 (0.9) | 23.7 (0.7) | 54.2 (0.8) | 63.2 (0.5) | 69.8 (0.8) | 35.4 (0.2) | |
| 2 | 32.5 (0.2) | 13 (1) | 20.7 (0.8) | 122 (1) | 23.8 (0.3) | 39.5 (0.6) | 33.9 (0.2) | ||
| Mean | 40.5 | 25.1 | 29.3 | 106.5 | 58.4 | 57.5 | 39.4 | ||
| NM_PL8 | 1 | 54.3 (0.3) | 114 (1) | 26.1 (0.7) | 90.5 (1.0) | 85.8 (0.6) | 36.9 (0.7) | 49.3 (0.3) | |
| 2 | 53.3 (0.3) | 151 (2) | 81 (1) | 297 (3) | 86.9 (0.7) | 136 (1) | 41.1 (0.3) | ||
| NM_SK14 | 1 | 26.1 (0.2) | 11.2 (0.4) | 19.3 (0.6) | 50.5 (1.0) | 44.0 (0.4) | 70 (1) | 25.7 (0.2) | |
| 2 | 37.5 (0.3) | 10.9 (0.8) | 279 (3) | 82 (1) | 58.8 (0.5) | 62.6 (0.8) | 29.4 (0.2) | ||
| Mean | 42.8 | 71.8 | 101.3 | 130 | 68.9 | 76.4 | 36.4 | ||
| Cu | M_PL2 | 1 | 4.82 (0.07) | 0.8 (0.2) | 8.7 (0.4) | 39.6 (0.6) | 5.6 (0.1) | 3.9 (0.2) | 2.95 (0.07) |
| 2 | 3.37 (0.05) | 2.2 (0.3) | 1.7 (0.2) | 5.8 (0.2) | 6.2 (0.2) | 5.1 (0.3) | 3.14 (0.06) | ||
| M_PL6 | 1 | 2.55 (0.04) | 8.2 (0.3) | 3.1 (0.3) | 11.7 (0.3) | 4.7 (0.1) | 3.5 (0.3) | 2.28 (0.05) | |
| 2 | 4.05 (0.07) | 2.0 (0.6) | 3.3 (0.3) | 19.2 (0.6) | 4.3 (0.2) | 3.3 (0.3) | 3.2 (0.06) | ||
| Mean | 3.70 | 3.3 | 4.2 | 19.1 | 5.2 | 3.9 | 2.89 | ||
| NM_PL8 | 1 | 0.82 (0.03) | 2.4 (0.3) | 1.0 (0.2) | 2.8 (0.2) | 1.2 (0.1) | 0.9 (0.3) | 0.79 (0.04) | |
| 2 | 1.81 (0.03) | 5.0 (0.2) | 2.3 (0.3) | 5.1 (0.5) | 3 (0.2) | 2.8 (0.2) | 1.43 (0.04) | ||
| NM_SK14 | 1 | 1.44 (0.04) | 1.0 (0.2) | 1.1 (0.2) | 5.1 (0.3) | 2.6 (0.1) | 2.4 (0.3) | 1.07 (0.04) | |
| 2 | 3.44 (0.05) | 0.9 (0.4) | 8.0 (0.3) | 45.7 (0.5) | 6.4 (0.1) | 4.2 (0.3) | 1.45 (0.05) | ||
| Mean | 1.88 | 2.3 | 3.1 | 14.7 | 3.3 | 2.6 | 1.18 | ||
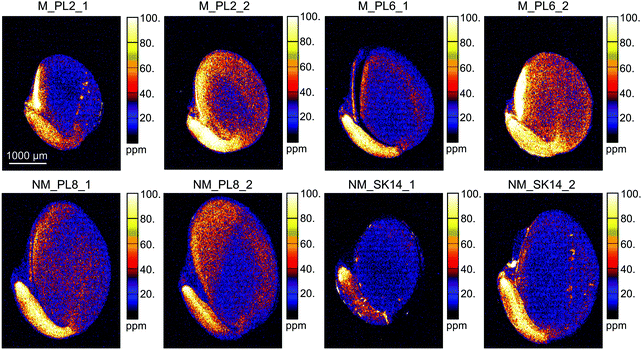 | ||
| Fig. 4 Quantitative elemental maps showing the distribution of Zn in Biscutella laevigata seed cross-sections. Seeds originated from two metallicolous (M) and two non-metallicolous (NM) populations. | ||
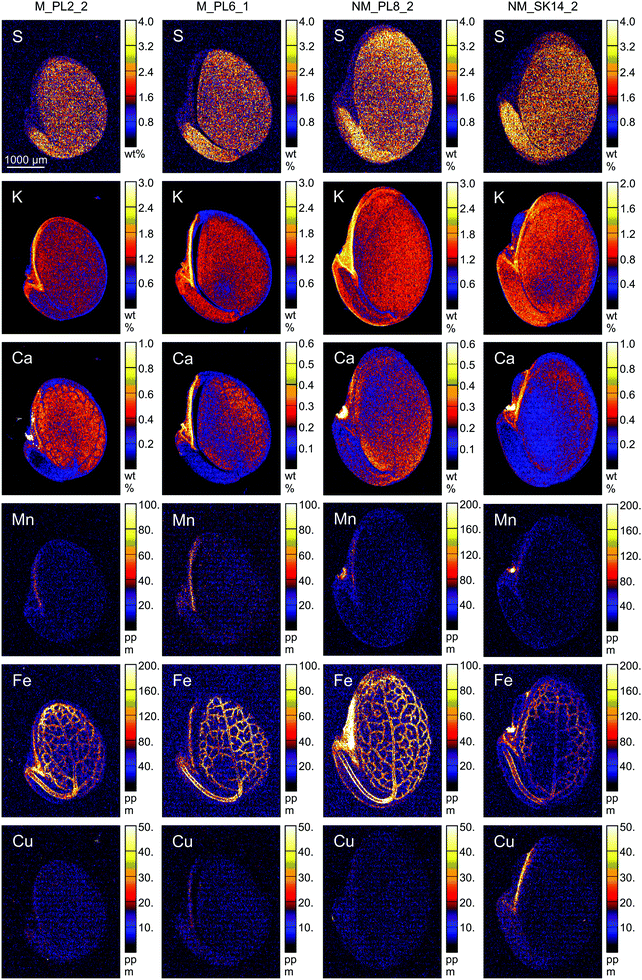 | ||
| Fig. 5 Quantitative elemental maps in Biscutella laevigata seed cross-sections. Seeds originated from two metallicolous (M) and two non-metallicolous (NM) populations. | ||
The endosperm was also a primary region of accumulation for K, Ca, Cl, Mn, Fe and Cu in the investigated B. laevigata seeds (Fig. 6). Furthermore, we observed element-specific allocation patterns to other tissues as described in the following paragraphs:
The range of observed K and Ca concentrations was rather narrow in radicle, hypocotyl and cotyledon, whereas it was much broader in testa, hilum, and endosperm. For these two elements, we did not observe any ecotype-specific trends. Limited differences in K and Ca concentrations between seed tissues were previously shown for A. thaliana.70 Moreover, a remarkably small influence of plant type (e.g. wild type vs. mutant plants) on K and Ca allocation patterns emphasized a common trend towards abundant storage of both elements in seeds in preparation for germination.70
Independent of plant origin, a clear and unique pattern was found for S, which was predominantly allocated to the radicle (29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 880–40
880–40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 770 μg g−1), hypocotyl (22
770 μg g−1), hypocotyl (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670–36
670–36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 μg g−1) and cotyledon (19
450 μg g−1) and cotyledon (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560–26
560–26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230 μg g−1). Considerably lower S concentrations (<850–8850 μg g−1) were found in testa, hilum and endosperm. Accordingly, S was the only element that formed two distinct groups of ROIs, with no overlap between those groups. Its primary storage in embryonic tissues supports successful seedling establishment and early plant development.71
230 μg g−1). Considerably lower S concentrations (<850–8850 μg g−1) were found in testa, hilum and endosperm. Accordingly, S was the only element that formed two distinct groups of ROIs, with no overlap between those groups. Its primary storage in embryonic tissues supports successful seedling establishment and early plant development.71
Several other elements revealed ecotype-specific allocation patterns. The concentration of Cl was on average twice higher in all specific ROIs of metallicolous compared to non-metallicolous seeds, except for testa where it was even 4-times higher. Due to relatively high variability among samples, the differences between ecotypes were slightly above the significance level of 0.05 (P = 0.057 for the whole seed section, radicle and cotyledon; ESI,† Table S2). In terms of Cu, concentrations were also higher in samples from metallicolous compared to non-metallicolous origin. The biggest differences between ecotypes appeared in endosperm, radicle, and cotyledon, where Cu was on average twice higher in metallicolous plants (except for one NM_SK14 sample that showed very high Cu in hilum, endosperm and radicle). As for Mn, we found the most interesting pattern in endosperm, where its concentration was 3-fold higher in metallicolous compared to non-metallicolous samples (p = 0.029). One particularly high value (254 μg g−1) emerged from a sample from the M_PL6 population. Interestingly, other anomalously high values of 108 μg g−1 characterized also the hilum of single samples from both non-metallicolous populations. These latter concentrations were 5-fold higher than the average Mn concentration in hilum of metallicolous samples. Manganese storage in seed tissues is interlinked with normal seedling growth, development, and its vigour index.72 By increasing the Mn content in seeds, plants are able to increase grain yield.73
Overall, the three elements addressed above (Cu, Mn, and Cl) were predominantly allocated to the endosperm, with considerably lower (on average 5-, 4- and 2-fold, respectively) concentrations in the remaining ROIs. Exceptions were one metallicolous plant with an equally high Cl concentration in testa as in endosperm, and two non-metallicolous plants for which Mn concentrations in the hilum exceeded those in endosperm). By comparison, in T. praecox and A. thaliana seeds, Cu was most abundant in radicle, cotyledon and seed coat26,74,75
Distinct distribution patterns were observed for Fe. While in radicle, hypocotyl and cotyledon, Fe concentration was rather similar in all samples, extremely high values were observed in testa (114.0 and 151.0 μg g−1), hilum (279 μg g−1) and endosperm (297 μg g−1) of individual seeds from non-metallicolous populations. Overall, this element was present in all samples and structures, mostly in the embryonic parts, and its distribution was clearly linked to the provascular network (Fig. 5). Similar Fe distribution patterns were reported for S. vulgaris21 and A. thaliana seeds.75–77 Transport of Fe into the provascular network has been shown to be mediated by VIT1, an ortholog of the vacuolar metal transporter CCC1 in yeast, based on complete loss of Fe enrichment around provascular strands in vit1 knock out mutants.78 Allocation of Fe to provascular tissues allows rapid mobilization of this element for the growing parts of the seedling during germination, thus it supports metabolic processes such as photosynthesis.79,80 In germinating seeds, the staining of Fe around provascular strands disappears during the first three days and thereafter, root Fe acquisition takes over.81
Conclusion
The seed is simultaneously the final sink, as well as the first source of nutrients in the life cycle of annual plants and their offspring. Whereas roots and foliage of mature plants have been widely studied, elemental transportation to the seed and allocation patterns within the seed are still poorly understood. In this study, we visualized and quantified a range of elemental allocation patterns in the metal-tolerant pseudometallophyte B. laevigata from both metalliferous and non-metalliferous sites. The patterns that we observed – especially the enhanced function of endosperm as a barrier against Zn excess in the embryonic parts of seeds on polluted sites – provides deep insight into plant adaptation to metalliferous environments and, by extent, the evolution of metal tolerance at the seed developmental stage. Yet, several open questions remain regarding the selective pressure(s) that drive the evolution of metal tolerance. Answering these questions will require transplant experiments to investigate, how tolerance affects the fitness in heterogeneous environments. The mechanistic understanding of processes underlying the optimization of metal(loid) concentration and localization in seeds is relevant for further investigations on the remediation of polluted mine tailings, and for the improvement of plant stress tolerance in general.Author contributions
ABK planned and designed the research. AVDE, JM-P and WJP performed the measurements. CR, JM-P, WJP, CD processed the image analyses. ABK, JM-P and CD analysed the data. ABK wrote the manuscript with contributions from all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was undertaken on the X-Ray Fluorescence Microscopy (XFM) beamline at the Australian Synchrotron, part of ANSTO. We thank Martin de Jonge (ANSTO) and Hugh Harris (University of Adelaide) for support during the synchrotron experiment, Aneta Słomka (Jagiellonian University) for discussions on seed anatomy, and Kamila Murawska and Szymon Miszczak (Institute of Botany PAS) for technical support. This work was supported by the Multi-modal Australian ScienceS Imaging and Visualisation Environment (MASSIVE), the POWROTY/REINTEGRATION programme of the Foundation for Polish Science co-financed by the European Union under the European Regional Development Fund (POIR.04.04.00-00-1D79/16-00), and statutory funds from the W. Szafer Institute of Botany PAS. W. J. Przybylowicz and J. Mesjasz-Przybylowicz are recipients of the South African National Foundation incentive grants no. 114693 and 114694, respectively. The Tatra National Park granted permits to collect samples within the park boundaries.References
- Millennium Ecosystem Assessment, Ecosystems and Human Well-being: Synthesis, Island Press, Washington, DC, 2005 Search PubMed.
- M. J. McLaughlin, B. A. Zarcinas, D. P. Stevens and N. Cook, Soil testing for heavy metals, Commun. Soil Sci. Plant Anal., 2000, 31, 1661–1700 CrossRef CAS.
- W. Ling, Q. Shen, Y. Gao, X. Gu and Z. Yang, Use of bentonite to control the release of copper from contaminated soils, Soil Res., 2007, 45, 618–623 CrossRef CAS.
- A. Kabata-Pendias, Trace elements in soils and plants, CRC Press, Boca Raton, Florida, 4th edn, 2011 Search PubMed.
- P. Maslin and R. M. Maier, Rhamnolipid-Enhanced Mineralization of Phenanthrene in Organic-Metal Co-Contaminated Soils, Biorem. J., 2000, 4, 295–308 CrossRef CAS.
- H. B. Bradl, Heavy metals in the environment: origin, interaction and remediation, Elsevier Academic Press, Neubrucke, Germany, 2005 Search PubMed.
- G. M. Pierzynski, J. T. Sims and G. F. Vance, Soils and environmental quality, Lewis Publishers, Boca Raton, 1994 Search PubMed.
- J.-L. Morel, G. Echevarria and N. Goncharova, Phytoremediation of metal-contaminated soils, Springer Science & Business Media, 2006 Search PubMed.
- J. Gil-Loaiza, J. P. Field, S. A. White, J. Csavina, O. Felix, E. A. Betterton, A. E. Sáez and R. M. Maier, Phytoremediation Reduces Dust Emissions from Metal(loid)-Contaminated Mine Tailings, Environ. Sci. Technol., 2018, 52, 5851–5858 CrossRef CAS.
- M. T. Gómez-Sagasti, I. Alkorta, J. M. Becerril, L. Epelde, M. Anza and C. Garbisu, Microbial monitoring of the recovery of soil quality during heavy metal phytoremediation, Water, Air, Soil Pollut., 2012, 223, 3249–3262 CrossRef.
- E. Gatliff, P. J. Linton, D. J. Riddle and P. R. Thomas, Bioremediation and Bioeconomy, Elsevier, 2016, pp. 589–608 Search PubMed.
- J. G. Alday, R. H. Marrs and C. Martínez-Ruiz, Vegetation succession on reclaimed coal wastes in Spain: the influence of soil and environmental factors, Appl. Veg. Sci., 2011, 14, 84–94 CrossRef.
- M. Mench, J. Vangronsveld, P. Bleeker, A. Ruttens, W. Geebelen and N. Lepp, Phytoremediation of metal-contaminated soils, Springer, 2006, pp. 109–190 Search PubMed.
- A. J. M. Baker, Accumulators and Excluders – Strategies in the response of Plants to heavy metals, J. Plant Nutr., 1981, 3, 643–654 CrossRef CAS.
- A. J. Baker, W. H. Ernst, A. van der Ent, F. Malaisse and R. Ginocchio, Metallophytes: the unique biological resource, its ecology and conservational status in Europe, central Africa and Latin America, Ecology of industrial pollution, 2010, pp. 7–40 Search PubMed.
- J. Antonovics, A. D. Bradshaw and R. G. Turner, Heavy metal tolerance in plants, Adv. Ecol. Res., 1971, 7, 1–85 Search PubMed.
- M. R. Macnair and J. M. Baker, Plants and the chemical elements, in Biochemistry, uptake, tolerance and toxicity, ed. M. E. Farago, VCH, NY, 1994, pp. 68–83 Search PubMed.
- A. J. M. Baker and R. R. Brooks, Terrestrial higher plants which hyperaccumulate metallic elements – a review of their distribution, ecology and phytochemistry, Biorecovery, 1989, 1, 81–126 CAS.
- A. van der Ent, W. J. Przybyłowicz, M. D. de Jonge, H. H. Harris, C. G. Ryan, G. Tylko, D. J. Paterson, A. D. Barnabas, P. M. Kopittke and J. Mesjasz-Przybyłowicz, X-ray elemental mapping techniques for elucidating the ecophysiology of hyperaccumulator plants, New Phytol., 2018, 218, 432–452 CrossRef CAS.
- J. Mesjasz-Przybyłowicz, K. Grodzińska, W. Przybyłowicz, B. Godzik and G. Szarek-Łukaszewska, Nuclear microprobe studies of elemental distribution in seeds of Biscutella laevigata L. from zinc wastes in Olkusz, Poland, Nucl. Instrum. Methods Phys. Res., Sect. B, 2001, 181, 634–639 CrossRef.
- J. Mesjasz-Przybyłowicz, K. Grodzińska, W. Przybyłowicz, B. Godzik and G. Szarek-Łukaszewska, Micro-PIXE studies of elemental distribution in seeds of Silene vulgaris from a zinc dump in Olkusz, southern Poland, Nucl. Instrum. Methods Phys. Res., Sect. B, 1999, 158, 306–311 CrossRef.
- J. Mesjasz-Przybyłowicz, K. Grodzińska, W. Przybyłowicz, B. Godzik and G. Szarek-Łukaszewska, Comparison between Zn distribution in seeds from a zinc dump in Olkusz, Southern Poland, Proc. – Microsc. Soc. South. Afr., 1998, 28, 61 Search PubMed.
- J. Mesjasz-Przybyłowicz and W. J. Przybyłowicz, Micro-PIXE in plant sciences: present status and perspectives, Nucl. Instrum. Methods Phys. Res., Sect. B, 2002, 189, 470–481 CrossRef.
- G. Sarret, E. P. Smits, H. C. Michel, M. Isaure, F. Zhao and R. Tappero, Advances in agronomy, Elsevier, 2013, vol. 119, pp. 1–82 Search PubMed.
- S. Groeber, W. Przybyłowicz, G. Echevarria, E. Montarges-Pelletier, A. Barnabas and J. Mesjasz-Przybyłowicz, Fate of nickel and calcium in seedlings of the hyperaccumulator Berkheya coddii during germination, Biol. Plant., 2015, 59, 560–569 CrossRef CAS.
- K. Vogel-Mikus, P. Pongrac, P. Kump, M. Necemer, J. Simcic, P. Pelicon, M. Budnar, B. Povh and M. Regvar, Localisation and quantification of elements within seeds of Cd/Zn hyperaccumulator Thlaspi praecox by micro-PIXE, Environ. Pollut., 2007, 147, 50–59 CrossRef CAS.
- A. G. Kachenko, N. P. Bhatia, R. Siegele, K. B. Walsh and B. Singh, Nickel, Zn and Cd localisation in seeds of metal hyperaccumulators using μ-PIXE spectroscopy, Nucl. Instrum. Methods Phys. Res., Sect. B, 2009, 267, 2176–2180 CrossRef CAS.
- J. Mesjasz-Przybyłowicz and W. Przybyłowicz, PIXE and metal hyperaccumulation: from soil to plants and insects, X-Ray Spectrom., 2011, 40, 181–185 CrossRef.
- N. P. Bhatia, I. Orlic, R. Siegele, N. Ashwath, A. J. Baker and K. B. Walsh, Elemental mapping using PIXE shows the main pathway of nickel movement is principally symplastic within the fruit of the hyperaccumulator Stackhousia tryonii, New Phytol., 2003, 479–488 CrossRef CAS.
- W. Przybyłowicz, C. Pineda, V. Prozesky and J. Mesjasz-Przybyłowicz, Investigation of Ni hyperaccumulation by true elemental imaging, Nucl. Instrum. Methods Phys. Res., Sect. B, 1995, 104, 176–181 CrossRef.
- F. Aflaki, M. Sedghi, A. Pazuki and M. Pessarakli, Investigation of seed germination indices for early selection of salinity tolerant genotypes: A case study in wheat, Emir. J. Food Agric., 2017, 29, 222–226 CrossRef.
- H. Bothe and A. Słomka, Divergent biology of facultative heavy metal plants, J. Plant Physiol., 2017, 219, 45–61 CrossRef CAS PubMed.
- C. Gonneau, N. Noret, C. Godé, H. Frérot, C. Sirguey, T. Sterckeman and M. Pauwels, Demographic history of the trace metal hyperaccumulator Noccaea caerulescens (J. Presl and C. Presl) F. K. Mey in Western Europe, Mol. Ecol., 2017, 26, 904–922 CrossRef.
- A. Babst-Kostecka, H. Schat, P. Saumitou-Laprade, K. Grodzińska, A. Bourceaux, M. Pauwels and H. Frérot, Evolutionary dynamics of quantitative variation in an adaptive trait at the regional scale: The case of zinc hyperaccumulation in Arabidopsis halleri, Mol. Ecol., 2018, 27, 3257–3273 CrossRef.
- J. Nowak, H. Frérot, N. Faure, C. Glorieux, C. Liné, B. Pourrut and M. Pauwels, Can zinc pollution promote adaptive evolution in plants? Insights from a one-generation selection experiment, J. Exp. Bot., 2018, ery327, DOI:10.1093/jxb/ery327.
- C. C. Dietrich, K. Bilnicki, U. Korzeniak, C. Briese, K. A. Nagel and A. Babst-Kostecka, Does slow and steady win the race? Root growth dynamics of Arabidopsis halleri ecotypes in soils with varying trace metal element contamination, Environ. Exp. Bot., 2019, 167, 103862 CrossRef CAS.
- G. Jimenez-Ambriz, C. Petit, I. Bourrié, S. Dubois, I. Olivieri and O. Ronce, Life history variation in the heavy metal tolerant plant Thlaspi caerulescens growing in a network of contaminated and noncontaminated sites in southern France: role of gene flow, selection and phenotypic plasticity, New Phytol., 2007, 173, 199–215 CrossRef CAS.
- A. A. Babst-Kostecka, C. Parisod, C. Gode, P. Vollenweider and M. Pauwels, Patterns of genetic divergence among populations of the pseudometallophyte Biscutella laevigata from southern Poland, Plant Soil, 2014, 383, 245–256 CrossRef CAS.
- C. Dechamps, C. Lefèbvre, N. Noret and P. Meerts, Reaction norms of life history traits in response to zinc in Thlaspi caerulescens from metalliferous and nonmetalliferous sites, New Phytol., 2007, 173, 191–198 CrossRef CAS.
- J. Jalas, J. Suominen and R. e. Lampinen, Atlas Florae Europaeae. Distribution of Vascular Plants in Europe. 11. Cruciferae (Ricotia to Raphanus), The Committee for Mapping the Flora of Europe & Societas Biologica Fennica Vanamo, Helsinki, 1996 Search PubMed.
- K. Tremetsberger, C. König, R. Samuel, W. Pinsker and T. F. Stuessy, Infraspecific genetic variation in Biscutella laevigata (Brassicaceae): new focus on Irene Manton's hypothesis, Plant Syst. Evol., 2002, 163–181, DOI:10.1007/s00606-002-0189-x.
- A. A. Babst-Kostecka, P. Waldmann, H. Frérot and P. Vollenweider, Plant adaptation to metal polluted environments – physiological, morphological, and evolutionary insights from Biscutella laevigata, Environ. Exp. Bot., 2016, 127, 1–13 CrossRef CAS.
- P. M. Kopittke, T. Punshon, D. J. Paterson, R. V. Tappero, P. Wang, F. P. C. Blamey, A. van der Ent and E. Lombi, Synchrotron-based X-ray fluorescence microscopy as a technique for imaging of elements in plants, Plant Physiol., 2018, 178, 507–523 CrossRef CAS.
- I. Manton, The Problem of Biscutella Laevigata L, Z. Indukt. Abstamm.– Vererbungsl., 1934, 67, 41–57 Search PubMed.
- D. Paterson, M. D. de Jonge, D. L. Howard, W. Lewis, J. McKinlay, A. Starritt, M. Kusel, C. G. Ryan, R. Kirkham, G. Moorhead and D. P. Siddons, The X-ray Fluorescence Microscopy Beamline at the Australian Synchrotron, AIP Conf. Proc., 2011, 1365, 219–222 CrossRef.
- R. Kirkham, P. A. Dunn, A. Kuczewski, D. P. Siddons, R. Dodanwela, G. Moorhead, C. G. Ryan, G. De Geronimo, R. Beuttenmuller, D. Pinelli, M. Pfeffer, P. Davey, M. Jensen, D. Paterson, M. D. de Jonge, M. Kusel and J. McKinlay, The Maia Spectroscopy Detector System: Engineering For Integrated Pulse Capture, Low-Latency Scanning And Real-Time Processing, AIP Conf. Proc., 2010, 1234, 240–243 CrossRef CAS.
- D. P. Siddons, R. Kirkham, C. G. Ryan, G. De Geronimo, A. Dragone, A. J. Kuczewski, Z. Y. Li, G. A. Carini, D. Pinelli, R. Beuttenmuller, D. Elliott, M. Pfeffer, T. A. Tyson, G. F. Moorhead and P. A. Dunn, Maia X-ray Microprobe Detector Array System, J. Phys.: Conf. Ser., 2014, 499, 012001 CrossRef.
- C. Ryan, R. Kirkham, R. Hough, G. Moorhead, D. Siddons, M. De Jonge, D. Paterson, G. De Geronimo, D. Howard and J. Cleverley, Elemental X-ray imaging using the Maia detector array: The benefits and challenges of large solid-angle, Nucl. Instrum. Methods Phys. Res., Sect. A, 2010, 619, 37–43 CrossRef CAS.
- C. Ryan and D. Jamieson, Dynamic analysis: on-line quantitative PIXE microanalysis and its use in overlap-resolved elemental mapping, Nucl. Instrum. Methods Phys. Res., Sect. B, 1993, 77, 203–214 CrossRef.
- L. A. Currie, Limits for qualitative detection and quantitative determination. Application to radiochemistry, Anal. Chem., 1968, 40, 586–593 CrossRef CAS.
- D. R. Helsel, Nondetects and data analysis. Statistics for censored environmental data, Wiley-Interscience, 2005 Search PubMed.
- M. Pietrzykowski, J. Antonkiewicz, P. Gruba and M. Pająk, Content of Zn, Cd and Pb in purple moor-grass in soils heavily contaminated with heavy metals around a zinc and lead ore tailing landfill, Open Chem., 2018, 16, 1143–1152 CAS.
- C. Sailer, A. Babst-Kostecka, M. C. Fischer, S. Zoller, A. Widmer, P. Vollenweider, F. Gugerli and C. Rellstab, Transmembrane transport and stress response genes play an important role in adaptation of Arabidopsis halleri to metalliferous soils, Sci. Rep., 2018, 8, 16085 CrossRef.
- A. Słomka, M. Jędrzejczyk-Korycińska, A. Rostański, J. Karcz, P. Kawalec and E. Kuta, Heavy metals in soil affect reproductive processes more than morphological characters in Viola tricolor, Environ. Exp. Bot., 2012, 75, 204–211 CrossRef.
- P. Saboya and F. Borghetti, Germination, initial growth, and biomass allocation in three native Cerrado species, Braz. J. Bot., 2012, 35, 129–135 CrossRef.
- C. Körner, Alpine plant life: functional plant ecology of high mountain ecosystems; with 47 tables, Springer Science & Business Media, 2003 Search PubMed.
- G. L. Hoyle, K. J. Steadman, R. B. Good, E. J. McIntosh, L. M. Galea and A. B. Nicotra, Seed germination strategies: an evolutionary trajectory independent of vegetative functional traits, Front. Plant Sci., 2015, 6, 731 Search PubMed.
- E. Przedpelska and M. Wierzbicka, Arabidopsis arenosa (Brassicaceae) from a lead-zinc waste heap in southern Poland – a plant with high tolerance to heavy metals, Plant Soil, 2007, 299, 43–53 CrossRef CAS.
- M. Wierzbicka and D. Panufnik, The adaptation of Silene vulgaris to growth on a calamine waste heap, Environ. Pollut., 1998, 101, 415–426 CrossRef CAS.
- W.-X. Huang, Y. Huang, F.-Y. Ye, S. Shan and Z.-T. Xiong, Effects of copper on phenology and reproduction in Rumex dentatus from metalliferous and non-metalliferous sites, Ecotoxicol. Environ. Saf., 2011, 74, 1043–1049 CrossRef CAS.
- G. Pan, H. Zhang, P. Liu, Z. Xiao, X. Li and W. Liu, Effects of manganese stress on phenology and biomass allocation in Xanthium strumarium from metalliferous and non-metalliferous sites, Ecotoxicol. Environ. Saf., 2019, 172, 308–316 CrossRef CAS.
- W. H. O. Ernst and H. J. M. Nelissen, Life-cycle phases of a zinc- and cadmium-resistant ecotype of Silene vulgaris in risk assessment of polymetallic mine soils, Environ. Pollut., 2000, 107, 329–338 CrossRef CAS.
- Z. Podbielkowski and M. Podbielkowska, Plant environmental adaptation, Wydawnictwa Szkolne I Pedagogiczne, Warsaw (in Polish), 1992 Search PubMed.
- E. L. Zvereva and M. V. Kozlov, Growth and reproduction of dwarf shrubs, Vaccinium myrtillus and V. vitis-idaea, in a severely polluted area, Basic Appl. Ecol., 2005, 6, 261–274 CrossRef.
- N. Basic, C. Keller, P. Fontanillas, P. Vittoz, G. Besnard and N. Galland, Cadmium hyperaccumulation and reproductive traits in natural Thlaspi caerulescens populations, Plant Biol., 2006, 8, 64–72 CrossRef CAS.
- E. Maestri, M. Marmiroli, G. Visioli and N. Marmiroli, Metal tolerance and hyperaccumulation: Costs and trade-offs between traits and environment, Environ. Exp. Bot., 2010, 68, 1–13 CrossRef CAS.
- W. E. Van Drunen and M. E. Dorken, Trade-offs between clonal and sexual reproduction in Sagittaria latifolia (Alismataceae) scale up to affect the fitness of entire clones, New Phytol., 2012, 196, 606–616 CrossRef PubMed.
- S. C. Barrett, Influences of clonality on plant sexual reproduction, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 8859–8866 CrossRef CAS PubMed.
- I. Kranner and L. Colville, Metals and seeds: biochemical and molecular implications and their significance for seed germination, Environ. Exp. Bot., 2011, 72, 93–105 CrossRef CAS.
- J. N. Lott and M. M. West, Elements present in mineral nutrient reserves in dry Arabidopsis thaliana seeds of wild type and pho 1, pho 2, and man 1 mutants, Can. J. Bot., 2001, 79, 1292–1296 CrossRef CAS.
- M. J. Hawkesford and L. J. De Kok, Managing sulphur metabolism in plants, Plant, Cell Environ., 2006, 29, 382–395 CrossRef CAS.
- S. Mondal and B. Bose, Impact of micronutrient seed priming on germination, growth, development, nutritional status and yield aspects of plants, J. Plant Nutr., 2019, 1–23 Search PubMed.
- N. Longnecker, N. Marcar and R. Graham, Increased manganese content of barley seeds can increase grain yield in manganese-deficient conditions, Aust. J. Agric. Res., 1991, 42, 1065–1074 CrossRef CAS.
- L. I. Olsen, T. H. Hansen, C. Larue, J. T. Østerberg, R. D. Hoffmann, J. Liesche, U. Krämer, S. Surblé, S. Cadarsi and V. A. Samson, Mother-plant-mediated pumping of zinc into the developing seed, Nat. Plants, 2016, 2, 16036 CrossRef CAS PubMed.
- M. Schnell Ramos, H. Khodja, V. Mary and S. Thomine, Using μPIXE for quantitative mapping of metal concentration in Arabidopsis thaliana seeds, Front. Plant Sci., 2013, 4, 168 Search PubMed.
- L. Young, N. Westcott, C. Christensen, J. Terry, D. Lydiate and M. Reaney, Inferring the geometry of fourth-period metallic elements in Arabidopsis thaliana seeds using synchrotron-based multi-angle X-ray fluorescence mapping, Ann. Bot., 2007, 100, 1357–1365 CrossRef CAS.
- T. Punshon, K. Hirschi, J. Yang, A. Lanzirotti, B. Lai and M. L. Guerinot, The role of CAX1 and CAX3 in elemental distribution and abundance in Arabidopsis seed, Plant Physiol., 2012, 158, 352–362 CrossRef CAS PubMed.
- S. A. Kim, T. Punshon, A. Lanzirotti, L. Li, J. M. Alonso, J. R. Ecker, J. Kaplan and M. L. Guerinot, Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1, Science, 2006, 314, 1295–1298 CrossRef CAS PubMed.
- E. L. Bastow, V. S. G. De La Torre, A. E. Maclean, R. T. Green, S. Merlot, S. Thomine and J. Balk, Vacuolar iron stores gated by NRAMP3 and NRAMP4 are the primary source of iron in germinating seeds, Plant Physiol., 2018, 177, 1267–1276 CrossRef CAS PubMed.
- V. Lanquar, F. Lelièvre, S. Bolte, C. Hamès, C. Alcon, D. Neumann, G. Vansuyt, C. Curie, A. Schröder and U. Krämer, Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron, EMBO J., 2005, 24, 4041–4051 CrossRef CAS PubMed.
- H. Roschzttardtz, G. Conéjéro, C. Curie and S. Mari, Identification of the endodermal vacuole as the iron storage compartment in the Arabidopsis embryo, Plant Physiol., 2009, 151, 1329–1338 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9mt00239a |
| This journal is © The Royal Society of Chemistry 2020 |