Selenomethionine alleviates LPS-induced chicken myocardial inflammation by regulating the miR-128-3p-p38 MAPK axis and oxidative stress
Jing
Liu
,
Shengchen
Wang
,
Qiaojian
Zhang
,
Xiaojing
Li
and
Shiwen
Xu
 *
*
College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, P. R. China. E-mail: shiwenxu@neau.edu.cn; Tel: +86 0451 55190407
First published on 6th November 2019
Abstract
Selenium is closely related to the occurrence of heart disease, and an appropriate amount of selenium can alleviate inflammatory changes caused by various factors. Lipopolysaccharide (LPS), as a specific component of the cell wall of Gram-negative bacteria, is often used to construct various inflammatory models. In order to explore the effect of selenium on LPS-induced myocardial inflammation in chickens, we chose 4-month-old laying hens to be fed with a selenium-rich diet containing 0.5 g kg−1 Se, and injected LPS into the abdominal cavity at the age of 8 months to establish an inflammation model. We observed the myocardial tissue lesions by light microscopy, and detected miR-128-3p, p38MAPK, and NF-κB pathway-associated inflammatory factors and Th1/Th2 related factors by qRT-PCR and Western blot. The results showed that LPS stimulation inhibited miR-128-3p, which increased the expression of p38MAPK and NF-κB, while the expression of TNF-α, IL-1, PTGE, COX-2 and iNOS increased. Additionally, the expression of IL-4 and IL-6 increased and IFN-γ decreased, suggesting an imbalance of Th1/Th2. We also found that LPS treatment not only increased the content of H2O2 and MDA in the myocardium, but also increased the expression of HSP60, HSP70 and HSP90, while the activity of SOD, GPX and CAT and the content of GSH decreased. Interestingly, the addition of selenium can alleviate the changes in the above indicators. Finally, we concluded that selenium inhibits the occurrence of oxidative stress and ultimately alleviates myocardial inflammation induced by LPS through the miR-128-3p-p38MAPK-NF-κB pathway.
Significance to metallomicsSelenium, as one of the essential trace elements in human and animal bodies, has certain protective effects on pathological damage of various organs in chickens. Biochemical and genetic studies have shown that microRNAs play an important role in the inflammatory response. This work demonstrates the protection role of selenomethionine by regulating miR-128-3p and its target p38MAPK on myocardial inflammation of chickens, and demonstrates that this mechanism is realized through the NF-κB pathway. The information is helpful in providing clues to explain the protective effects of organic selenium on myocardial damage. More importantly, this research is of great importance in the clinical field and can be used to study novel targeted therapies. |
1. Introduction
Selenium(Se) is an essential trace element in human and animal life. Many physiological functions of humans and animals are extremely sensitive to changes of Se, such as antioxidant,1 growth and reproduction2 and immune response.3 Se deficiency can cause many pathological reactions, including autophagy, apoptosis and necrosis.4–6 Se deficiency can induce oxidative stress, lipid peroxidation and cell apoptosis related to the gene expression of selenoprotein residing in the endoplasmic reticulum in chicken skeletal muscle.7 Inflammation is also a major pathological response to Se deficiency. Se deficiency will induce and aggravate the inflammatory response through HSP60 and TLR2-MAPK signaling pathways in the liver of carp.8 In Se-deficient carp head kidney, reduced miR-146a promotes ROS production and induces inflammation through the MAPK pathway.9 Properly increasing the content of organic Se can treat a variety of diseases. Se has an antagonistic effect on cadmium-induced apoptosis of the PPAR-γ/PI3K/Akt pathway in the chicken pancreas,10 while 3-((4-chlorophenyl)seleno)-1-methyl-1H-indole (CMI) may be helpful in treating depression associated with inflammation and oxidative stress.11 Se can improve lead-induced inflammation of chicken testis by reducing the expression of inflammatory factors and heat shock proteins.12 Se supplementation can counteract the negative effects of Cd on chicken liver, including reducing Cd-induced HSP stress and the inflammatory response.13MicroRNAs (miRNAs) are a class of endogenous non-coding RNAs with regulatory functions found in eukaryotes, which can degrade target mRNA or block their translation. Various pathophysiological processes such as apoptosis, autophagy and necrosis require the participation of miRNAs.14 miR-327 reduces myocardial damage caused by inflammation through its target gene RP105 in myocardial ischemia/reperfusion injury.15 miR-495 ameliorates cardiac microvascular endothelial cell injury and inflammatory reaction by suppressing the NLRP3 signaling pathway.16 miR-129-3p/Smad3 can be regulated by tetrahydroxy stilbene glycoside to alleviate palmitic acid-induced cardiomyocyte inflammation and apoptosis.17 As the main signal transduction pathway, the p38 and NF-κB signal transduction pathways are two important pathways that mediate inflammatory response.18 In LPS-stimulated hDPCs, the NF-κB signaling pathway was negatively regulated by miR-21-5p by way of downregulation of TRAF6 and PDCD4 expression, which led to decreased expression of inflammatory factors iNOS, PTGE, COX-2, etc.19 In addition, miR-210 can attenuate inflammation induced by activation of the LPS-induced NF-κB and p38MAPK pathways.20 T cells can be divided into Th1 cells characterized by secretion of interferon-γ (IFN-γ) and Th2 cells characterized by secretion of interleukin-4 (IL-4) and interleukin-6 (IL-6),21 and their functions are in a state of dynamic balance. When the body is attacked by heterogeneous antigens, the function of one subgroup of Th1 and Th2 cells increases, while the function of the other subgroup decreases, called Th1/Th2 drift. A study has shown that the stimulation of exogenous proinflammatory substance H2S caused Th1/Th2 imbalance characterized by increased expression of TNF-α, IL-4 and IL-6 and decreased expression of IFN-γ, while TNF-α could activate the NF-κB signal transduction pathway and lead to inflammation.22,23
At present, the protective effect of selenium on inflammatory injury of chicken myocardium and the involvement mechanism of miR-128-3p are still unclear. To this end, we have established a Se-LPS co-processing model. We detected the expression of miR-128-3p and inflammation-related genes by qRT-PCR and Western blot. We attempted to reveal that selenomethionine inhibits the activation of p38MAPK by miR-128-3p, thereby inhibiting the activation of NF-κB and ultimately achieving the goal of antagonizing LPS-induced myocardial inflammation in chickens.
2. Materials and methods
2.1 Treatment of experimental animals
All experiments were performed in compliance with the relevant laws of China and Chinese guidelines for animal welfare and experimental protocols, and all procedures used in this study were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University (SRM-11). Eighty 4-month-old laying hens (a hatchery in Harbin of China) were randomly divided into two groups of 40 birds each. One group served as a control group to feed a commercial granulated diet (control group, 0.2 mg kg−1 Se in the diet). Another group was fed selenomethionine diet (selenomethionine group, 0.5 mg kg−1 Se in the diet). All chickens were fed ad libitum, and supplied with drinking water and kept in a standardized mode. When grown to 8-month-old, they were randomly divided into control group, LPS group, 0.5 g kg−1 Se group and 0.5 g kg−1 Se + LPS group (intravenous injection of LPS at a dose of 200 μg kg−1 body weight). Broilers were euthanized after 5 hours, and myocardial samples were extracted, dispensed and marked. Tissues were frozen immediately with liquid nitrogen and stored at −80 °C until required.242.2 Observation of myocardial morphological changes
Myocardial tissue (size, 5 mm × 5 mm × 3 mm) was fixed in 10% formalin phosphate buffer for at last 24 h, dehydrated with different concentrations of alcohol, and embedded in paraffin. After sectioning, the samples were dewaxed with xylene and stained with hematoxylin and eosin (H&E). The stained sections were dehydrated in alcohol and xylene and sealed with neutral resin. After drying, they were observed under a microscope.2.3 Real-time quantitative PCR analysis on miRNA and mRNA levels
In order to quantitatively detect miR-128-3p and the mRNA of other related genes by qRT-PCR, total RNA was isolated from extracted myocardial tissue. According to the manufacturer's instructions, miRNA and mRNA were performed using a miRcute microRNA First-Strand cDNA Synthesis Kit (Tiangen Biotech Co. Ltd, Beijing) and BioRT Master HiSensi cDNA First Strand Synthesis Kit (Bioer Technology Co. Ltd, Hangzhou), respectively. Primers for detecting miR-128-3p and mRNA of various genes in qRT-PCR are shown in Table 1. GAPDH or U6 is used as an internal reference. qRT-PCR was performed using the Light Cy-cler®480 System (Roche, Basel, Switzerland) and Fast Universal SYBR Green Master Mix (Roche, Basel, Switzerland). Only one peak for each PCR product was shown in the melting curve analysis. The relative abundance of mRNA was calculated according to the 2-ΔΔCt method, accounting for gene-specific efficiencies and was normalized to the mean expression of the abovementioned index.| Target gene | Primer sequence(5′ → 3′) |
|---|---|
| miR-128-3p | Forward: cgcggTCACAGTGAACCGGTCTCTTT |
| U6 | Forward: CACGCAAATTCGTGAAGCGTTCCA |
| GAPDH | Forward: GGCACTGTCAAGGCTGAGAA |
| Reverse: CACCTGCATCTGCCCATTTG | |
| p38MAPK | Forward: AGGGCCTGAGCTTTTGAAGAA |
| Reverse: CCACTGGTTCGTCATCTGGG | |
| IL-4 | Forward: AGCCAGCACTGCCACAAGAAC |
| Reverse: GTGGAAGAAGGTACGTAGGTCTGC | |
| P65 | Forward: CACCACCACCACCACCACAAC |
| Reverse: CAACTCAGCGGCGTCGATGG | |
| IκB-α | Forward: TTCTCCACTTGGCGATCATTCACG |
| Reverse: GTCTGGCTGAGGTTGTTCTGGAAG | |
| TNF-α | Forward: CAGGACAGCCTATGCCAACA |
| Reverse: ACAGCCAAGTCAACGCTCCT | |
| Il-6 | Forward: GCAGGACGAGATGTGCAAGA |
| Reverse: ATTTCTCCTCGTCGAAGCCG | |
| IFN-γ | Forward: ACCTTCCTGATGGCGTGAAG |
| Reverse: GCGCTGGATTCTCAAGTCGT | |
| NF-κB | Forward: ATTATTCGCCAGGCAGCAGT |
| Reverse: ACAGGATCAAGCCTCCGTGT | |
| COX-2 | Forward: TGTCCTTTCACTGCTTTCCAT |
| Reverse: TGTCCTTTCACTGCTTTCCAT | |
| iNOS | Forward: CCTGGAGGTCCTGGAAGAGT |
| Reverse: CCTGGGTTTCAGAAGTGGC | |
| IL-1 | Forward: AGCAGCCTCAGCGAAGAGACC |
| Reverse: GTCCACTGTGGTGTGCTCAGAATC | |
| PTGE | Forward: TTGCCATCATCACGGGACAA |
| Reverse: GCGTCCCACACAGAAGATGA |
2.4 Western blot analysis
After extracting the total protein, an appropriate amount of protein sample was added to a 12% SDS-polyacrylamide gel to separate the total protein under reduced conditions and then was transferred to a nitrocellulose filter in Tris-glycine buffer containing 20% methanol. Then the nitrocellulose membrane was blocked in 5% non-fat milk at 37 °C for 2 hours, and incubated overnight with the following antibodies: GAPDH(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000), NF-κB(1
5000), NF-κB(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500), IKB-α(1
500), IKB-α(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500), p38(1
500), p38(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), HSP60(1
1000), HSP60(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), HSP70(1
1000), HSP70(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) and HSP90(1
1000) and HSP90(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) at 4 °C. Then incubated with peroxidase-conjugated secondary antibody against rabbit IgG (1
1000) at 4 °C. Then incubated with peroxidase-conjugated secondary antibody against rabbit IgG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000, Santa Cruz, CA, USA) for 1 hour at room temperature. Analysis of the GAPDH content as an internal reference was performed using rabbit polyclonal antibody (Sigma, USA). Western blot strips were quantified by an ECL colorimeter.
5000, Santa Cruz, CA, USA) for 1 hour at room temperature. Analysis of the GAPDH content as an internal reference was performed using rabbit polyclonal antibody (Sigma, USA). Western blot strips were quantified by an ECL colorimeter.
2.5 Detection of oxidative stress
Myocardial cells were ground, dissolved with physiological saline and centrifuged to collect the supernatant. The contents of hydrogen peroxide (H2O2), glutathione (GSH), malondialdehyde (MDA), catalase (CAT), glutathione peroxidase (GPX), superoxide dismutase (SOD) and total antioxidant capacity (T-AOC) were determined by using the detection kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) under the guidance of the manual.2.6 Statistical analysis
Statistical analysis of all data was performed using GraphPad Prism Version 5.0 software (GraphPad software, San Diego, CA) with one-way ANOVA. All values are expressed in the form of “mean ± SD”. The asterisk (*) represents a statistically significant difference (P < 0.05).3. Results
3.1 Selenomethionine alleviates lipopolysaccharide-induced inflammation in myocardial tissue
In order to prove that selenomethionine can antagonize LPS-induced myocardial inflammation, the myocardial tissues stained with hematoxylin and eosin (H&E) were observed under light microscopy. The histopathological changes of chicken myocardial tissue are shown in Fig. 1. There was no significant change in the control group and the 0.5 g kg−1 Se group, and they all showed normal morphology. However, the myocardial tissue of the LPS group had obvious inflammatory damage, including myocardial fiber swelling and rupture, the fiber bundle was blurred, and there was a large amount of inflammatory cell infiltration between the fiber bundles. In the 0.5 g kg−1 Se + LPS group, the inflammation was significantly improved, the muscle fibers were clear, and only a small amount of inflammatory cells infiltrated. All of the above observations confirmed that selenomethionine can effectively alleviate LPS-induced myocardial inflammation. | ||
| Fig. 1 HE staining of myocardial tissue. Black arrows indicate inflammatory cell infiltration; red arrows indicate myocardial fiber breaks. | ||
3.2 Selenomethionine alleviates oxidative damage of myocardium induced by LPS in chickens
To find out whether oxidative stress is involved in the antagonistic effect of selenium on LPS-induced inflammation, we examined the activities of antioxidant enzymes SOD, GPX, and CAT and the expression levels of GSH, MDA and H2O2 in chicken myocardial tissue from different treatment groups, as shown in Fig. 2. It can be seen from the results that the activities of antioxidant enzymes SOD, GPX, and CAT and GSH levels were significantly inhibited by LPS, while the levels of MDA and H2O2 were significantly increased (p < 0.05). The addition of Se significantly reversed these changes (p < 0.05). Se treatment alone increased the activities of SOD, GPX and CAT, and GSH was significantly increased (p < 0.05). For MDA and H2O2, their expression levels were inhibited in the Se treatment alone. These results indicate that oxidative stress induced by LPS can be significantly inhibited by selenomethionine. | ||
| Fig. 2 Effect of selenomethionine on oxidative stress in chicken myocardial tissue. Data are expressed as mean ± SD (n = 6). The asterisk (*) represents a significant difference (p < 0.05). | ||
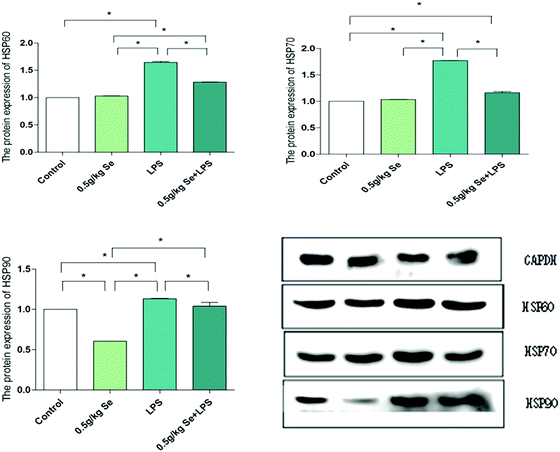
3.3 Selenomethionine improved the abnormally high expression of HSP60, HSP70 and HSP90 in chicken myocardium
The results of detection of protein expression of HSP60, HSP70 and HSP90 are shown in Fig. 3. The protein expression levels of HSP60, HSP70 and HSP90 were significantly increased in the LPS-stimulated group, and this increase was significantly inhibited in the Se and LPS co-treatment group (p < 0.05). The protein levels of HSP60 and HSP90 in the Se-treated groups were significantly decreased compared with the control group (p < 0.05), but there was no significant change in the level of HSP70 (p > 0.05).3.4 Selenomethionine alleviates the abnormal expression of miR-128-3p and p38MAPK induced by LPS
We first examined the relationship between miR-128-3p and p38MAPK and the results are shown in Fig. 4. From the results of qPCR, we can see that the expression of miR-128-3p was the least in the LPS-stimulated group, and Se supplementation significantly inhibited this decrease (p < 0.05). In the detection of p38MAPK, we obtained the opposite result to miR-128-3p. The expression level of the LPS group was the highest at both mRNA and protein levels, and Se supplementation significantly reduced this increase (p < 0.05). To further confirm the correlation between the two, we used the Bio-predictive website (http://www.targetscan.org/vert_72/) TargetScan, which shows that miR-128-3p can specifically bind to the specific 3'UTR sites of p38MAPK to restrict it. Therefore, the above results indicate that there is a targeting relationship between miR-128-3p and p38MAPK, and it is regulated by Se.3.5 Selenomethine reverses the changes in inflammatory protein and gene levels regulated by LPS
To explore whether selenomethionine can alleviate LPS-induced myocardial inflammation in chickens and its underlying mechanism, we examined the expression of NF-κB pathway-related factors and the Th1/Th2 balance, as shown in Fig. 5. The expression of IκB-α and NF-κB at the mRNA level is shown in Fig. 5A. For IκB-α, LPS stimulation significantly reduced the expression of its mRNA level (p < 0.05), and the addition of Se restored it to a state that was not statistically different from the control group. The mRNA expression of NF-κB is just opposite to IκB-α. Fig. 5B shows the protein expression of IκB-α and NF-κB, with the overall trend being the same as their mRNA expression levels. The mRNA changes of the NF-κB pathway-associated pro-inflammatory factors are shown in Fig. 5C. There was no significant difference in the expression of all pro-inflammatory factors between the Se-treated group and control group (p > 0.05). For pro-inflammatory factors IL-1, TNF-alpha, COX-2, PTGE and iNOS, the expression level of the LPS-treated group was the highest among the four treatment groups, and the Se and LPS co-treatment group significantly inhibited this increase (p < 0.05). There was no significant difference in IFN-γ secreted by Th1 cells between the control group and the selenomethionine group, but it decreased significantly in the LPS group (p < 0.05), and increased in the LPS + selenomethionine group, although still lower than the control group. The expression of IL-4 and IL-6 secreted by Th2 cells was contrary to that of IFN-γ (Fig. 5D).4. Discussion
A large number of related studies have shown that Se has protective effects on inflammatory damage of various tissues and organs such as the liver,25 trachea,26 heart27 and olfactory bulb.28 Se can significantly inhibit the high expression of inflammation-related genes TNF-α, NF-κB, and iNOS in chicken breast muscle caused by cadmium.29 Selenized corn grain has the ability to regulate the AA pathway to inhibit the inflammatory response in RAW264.7 macrophages stimulated by LPS.30 The protective effect of Se on various tissues and organs is closely related to miRNA. In a rat model of lead injury, Se nanoparticles alleviate functional damage of the thyroid by maintaining the function of selenoprotein and inhibiting the expression of miR-224.31 Sodium selenite utilizes miR-328 to target Atp2a2 to regulate intracellular Ca2+ concentration to attenuate H2O2-induced H9c2 cell apoptosis.32 In the study of miRNA and inflammatory responses, miR-146a was found to be a potent regulator of inflammation that attenuates inflammatory responses in chicken umbilical vein endothelial cells induced by IL-1β.33 Similarly, miR-21 inhibits LPS-induced overexpression of inflammatory cytokines in teleosts through IRAK4-mediated NF-κB signaling.34 In our study, we found significant inflammatory lesions in HE-stained sections of LPS-stimulated chicken myocardial tissue. LPS stimulation reduced the expression of miR-128-3p and increased the expression of its target gene p38MAPK, which induced activation of the NF-κB pathway and Th1/Th2 imbalance. Additionally, oxidative stress occurred, and the expression of heat shock protein was increased. Supplementation with selenomethionine can alleviate LPS-induced myocardial inflammatory lesions, and alleviate oxidative stress, Th1/Th2 imbalance, heat shock protein expression and miR-128-3p targeting p38MAPK-induced NF-κB pathway activation in myocardial tissue.Multiple miRNAs play a role in heart disease and inflammation.35,36 For example, miR-155-5p could be loaded into NPs, potentially increasing the endogenous cell protection response and reducing damage in infracted hearts.37 The miR-34 family and its target LGR4 can promote the inflammatory response in wounds through the NF-κB pathway.38 LPS can regulate myocardial injury by miRNAs. For example, LPS can cause excessive apoptosis of cardiomyocytes by decreasing the expression of miR-23b,39 and miR-874 plays an important role in LPS-induced sepsis and myocardial dysfunction.40 Similarly, our study showed that the expression of miR-128-3p was decreased with LPS stimulation. We used the TargetScan website to predict that miR-128-3p targets p38 MAPK in chickens, and that miR-128-3p binds to the 3'UTR site of p38 MAPK in mammals such as rats, mice and humans. In contrast, we found that chickens and other species of animals have the same binding sites. It has also been reported that p38MAPK is an important target of miR-128. Increased miR-128 attenuates the TNF-α response via the p38 signaling pathway, thereby reducing inflammatory lesions in periodontitis.41 miR-128-3p prevents neuronal death during cerebral ischemia in mice by reducing the activity of p38α MAPK.42 Furthermore, miR-128 enhances dendritic cell-mediated anti-tumor immunity by targeting p38 and blocking its translation process.43 In our study, LPS resulted in decreased expression of miR-128-3p and increased expression of p38MAPK, indicating that LPS altered the regulation pattern of miR-128-3p on p38MAPK. LPS, as an exogenous inflammatory agent, can undoubtedly cause oxidative stress. LPS can increase the levels of MDA and H2O2 in intestinal epithelial cells, but decrease the activities of antioxidant enzymes SOD, CAT and GPX, resulting in oxidative stress.44 After intraperitoneal injection of LPS, NO and MDA increased significantly, and SOD/GPX and HO-1 decreased significantly in the aorta of rats.45 Oxidative stress is closely related to inflammatory response. On the one hand, oxidative stress can increase the expression of NF-κB and TNF-α.46 On the other hand, oxidative stress can induce damage-related molecular patterns including HSPs,47 which subsequently lead to inflammation.48 Similar to the above results, our results showed that LPS led to an increase in oxidative stress products MDA and H2O2, and decreased SOD, GPX and CAT activity and GSH content in our experiments. At the same time, the expression of HSP60, HSP70 and HSP90 increased. These results indicate that LPS caused a decrease of the activity of antioxidant enzymes and impaired the antioxidant system, resulting in oxidative stress and increased expression of heat shock proteins in myocardial tissue. It can’t be ignored that the protein expression of HSP90 in the Se treatment group was significantly decreased compared with the control group. Shi et al. also found that the expression of HSP90 in the Se group was significantly lower than that in the control group in chicken trachea.49 Huang et al. also showed that the mRNA expression of HSP90 in chicken hearts decreased at 90 days after selenium treatment, although there was no significant difference from the control group. And the protein level of HSP70 in the Se treatment group was significantly reduced.50 This change in HSPs is yet to be further explored. Studies have confirmed that there is a correlation between the two classical pathways of p38MAPK protein kinase and NF-κB. Nick et al. found that LPS stimulated neutrophils led to MKK3 activating p38αMAPK, which ultimately regulated the activation of NF-κB, enhanced the gene expression of TNF-α and regulated the synthesis of TNF-α,51 and P38 and the extracellular signal-regulated kinase mitogen-activated protein kinase pathway are necessary for transactivation of NF-kB p65 mediated by tumor necrosis factor.52 Activation of the NF-κB pathway can lead to much inflammation, which is mainly achieved by promoting the expression of pro-inflammatory factors.18 In normal organisms, NF-κB binds to IκB-α in the form of a dimer and exists in inactive form. When the body is subjected to adverse stimulation, IκB-α is degraded by IKK leading to activation of NF-κB,53 followed by increased expression of pro-inflammatory factors IL-1, TNF-α, iNOS, COX-2, and PTGE.54,55 Interestingly, these inflammatory factors can in turn promote the activation of NF-κB, forming an inflammatory cascade amplification reaction.56 In addition, Th1/Th2 imbalance is another pathway that causes inflammation regulated by the NF-κB pathway. Studies have shown that activation of the NF-κB pathway during H2S stimulation can cause Th1/Th2 imbalance and lead to thymitis in broilers. In our study, we showed consistent results with the above studies: NF-κB inhibitor IκB-α was significantly reduced, and the expression of NF-κB and related pro-inflammatory factors IL-1, TNF-α, COX-2, iNOS, and PTGE increased strongly. In addition, the expression of IL-4 and IL-6 was also strongly increased, IFN-γ was significantly decreased, and Th1/Th2 was imbalanced. In conclusion, we can confirm that LPS can cause miR-128-3p-p38 MAPK axis changes and oxidative stress, leading to activation of the NF-κB pathway and overexpression of heat shock protein. Activated NF-κB can promote the expression of a variety of pro-inflammatory factors and Th1/Th2 imbalance, leading to inflammation.
Se has been shown to reduce oxidative damage and inflammatory responses. Se has anti-oxidative effects on oxidative stress and MeHg-induced oxidative stress in the lymphoblastoid cell line of Alzheimer's disease.57,58 Se has a protective effect on inflammatory damage of chicken cardiomyocytes induced by reactive oxygen species free radicals.59 Se can reduce the high expression of HSP60, HSP70 and HSP90 in peripheral blood neutrophils induced by lead,60 and also reduce the mRNA levels of HSP70 and HSP90 in a heat stress model.61 Our experiments showed similar results. After adding Se, oxidative stress products MDA and H2O2 decreased, SOD, GPX, and CAT activity and GSH content increased, while HSP60, HSP70 and HSP90 expression decreased. Yang's research shows that Se deficiency can target RNF11 by promoting the expression of miR-200a-5p and eventually lead to programmed myocardial necrosis.62 Liu et al. also showed that miR-2954 can inhibit the PI3K signaling pathway and induce autophagy and cell apoptosis in Se-deficient myocardia.63 Similarly, in our experiments, the addition of Se increased the expression of miR-128-3p. For p38MAPK, although its expression of the Se + LPS group was higher than those of the control group and Se group, there was still a significant decrease compared with the LPS group. In addition, Se reduced the activation of NF-κB, and decreased the expression of pro-inflammatory factors, and the Th1/Th2 imbalance was alleviated. The morphological observation was consistent with this result, and the inflammatory damage was significantly improved after Se supplementation. In other words, Se can alleviate the abnormality of the miR-128-3p-p38MAPK axis caused by LPS, as well as the occurrence of oxidative stress and the overexpression of heat shock proteins, and then inhibit the activation of the NF-κB pathway to relieve the inflammatory response.
In conclusion, our study revealed that LPS could induce oxidative stress and high expression of heat shock proteins, and led to the imbalance of the miR-128-3p-p38MAPK axis, which could induce inflammatory damage of chicken myocardium through the NF-κB pathway, and Se could alleviate this damage. This provides a new basis for elucidating the anti-inflammatory mechanism of Se.
Abbreviations
| Se | Selenium |
| LPS | Lipopolysaccharide |
| NF-κB | Nuclear factor kappa-B |
| p38MAPK | p38 mitogen-activated protein kinase |
| Th1/Th2 | Helper T cell 1/helper T cell 2 |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| TNF-α | Tumor necrosis factor-α |
| IL-1 | Interleukin-1 |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| PTGEH | Prostaglandin E synthase |
| COX-2 | Cyclooxygenase-2 |
| iNOS | Inducible nitric oxide synthase |
| IFN-γ | Interferon-γ |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
| GSH | Glutathione |
| GPX | Glutathione peroxidase |
| CAT | Catalase |
| HSP60 | Heat shock protein 60 |
| HSP70 | Heat shock protein 70 |
| HSP90 | Heat shock protein 90 |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the International (Regional) Cooperation and Exchange Projects of the National Natural Science Foundation of China (31772814). China Agriculture Research System-41-17.References
- R. Zeng, Y. Liang and M. U. Farooq, et al., Alterations in transcriptome and antioxidant activity of naturally aged mice exposed to selenium-rich rice, Environ. Sci. Pollut. Res., 2019, 26, 17834–17844 CrossRef CAS PubMed.
- L. Jiang, L.-L. Peng and Y.-Y. Cao, et al., Effect of Dietary Selenium Supplementation on Growth and Reproduction of Silkworm Bombyx mori L, Biol. Trace Elem. Res., 2019 DOI:10.1007/s12011-019-01690-x.
- X. Wang, Z. Shen and C. Wang, et al., Dietary supplementation of selenium yeast enhances the antioxidant capacity and immune response of juvenile Eriocheir Sinensis under nitrite stress, Fish Shellfish Immunol., 2019, 87, 22–31 CrossRef CAS.
- X. Li, M. Chen and Z. Yang, et al., Selenoprotein S silencing triggers mouse hepatoma cells apoptosis and necrosis involving in intracellular calcium imbalance and ROS-mPTP-ATP, Biochim. Biophys. Acta, Gen. Subj., 1862, 2018, 2113–2123 Search PubMed.
- Q. Chi, Y. Luan and Y. Zhang, et al., The regulatory effects of miR-138-5p on selenium deficiency-induced chondrocyte apoptosis are mediated by targeting SelM, Metallomics, 2019, 11, 845–857 RSC.
- H. Huang, Y. Wang and Y. An, et al., Selenium alleviates oxidative stress and autophagy in lead-treated chicken testes, Theriogenology, 2019, 131, 146–152 CrossRef CAS.
- H. Yao, Q. Wu and Z. Zhang, et al., Gene Expression of Endoplasmic Reticulum Resident Selenoproteins Correlates with Apoptosis in Various Muscles of Se-Deficient Chicks, J. Nutr., 2013, 143, 613–619 CrossRef CAS.
- Gao X-j, B. Tang and H-h Liang, et al., Selenium deficiency induced an inflammatory response by the HSP60-TLR2-MAPKs signalling pathway in the liver of carp, Fish Shellfish Immunol., 2019, 87, 688–694 CrossRef.
- X. Gao, B. Tang and H. Liang, et al., Selenium deficiency inhibits micRNA-146a to promote ROS-induced inflammation via regulation of the MAPK pathway in the head kidney of carp, Fish Shellfish Immunol., 2019, 91, 284–292 CrossRef CAS.
- X. Jin, T. Jia and R. Liu, et al., The antagonistic effect of selenium on cadmium-induced apoptosis via PPAR-γ/PI3K/Akt pathway in chicken pancreas, J. Hazard. Mater., 2018, S0304389418304412 Search PubMed.
- A. M. Casaril, M. Domingues and S. R. Bampi, et al., The selenium-containing compound 3-((4-chlorophenyl)selanyl)-1-methyl-1H-indole reverses depressive-like behavior induced by acute restraint stress in mice: modulation of oxido-nitrosative stress and inflammatory pathway, Psychopharmacology, 2019, 236(10) DOI:10.1007/s00213-018-5151-x.
- Y. Wang, K. Wang and H. Huang, et al., Alleviative effect of selenium on inflammatory damage caused by lead via inhibiting inflammatory factors and heat shock proteins in chicken testes, Environ. Sci. Pollut. Res., 2017, 24, 13405–13413 CrossRef CAS.
- R. Zhang, Y. Liu and L. Xing, et al., The protective role of selenium against cadmium-induced hepatotoxicity in laying hens: Expression of Hsps and inflammation-related genes and modulation of elements homeostasis, Ecotoxicol. Environ. Saf., 2018, 159, 205–212 CrossRef CAS.
- Z. Su, Z. Yang and Y. Xu, et al., MicroRNAs in apoptosis, autophagy and necroptosis, Oncotarget, 2015, 6, 8474–8490 Search PubMed.
- Y. Yang, J. Yang and X. Liu, et al., Down-Regulation of miR-327 Alleviates Ischemia/Reperfusion-Induced Myocardial Damage by Targeting RP105, Cell. Physiol. Biochem., 2018, 49, 1090–1104 CAS.
- T. Zhou, D. Xiang and S. Li, et al., MicroRNA-495 Ameliorates Cardiac Microvascular Endothelial Cell Injury and Inflammatory Reaction by Suppressing the NLRP3 Inflammasome Signaling Pathway, Cell. Physiol. Biochem., 2018, 49, 798–815 CrossRef CAS.
- Y. Zou and M. Kong, Tetrahydroxy stilbene glucoside alleviates palmitic acid-induced inflammation and apoptosis in cardiomyocytes by regulating miR-129-3p/Smad3 signaling, Cell. Mol. Biol. Lett., 2019, 24 DOI:10.1186/s11658-018-0125-x.
- X. Hu, Q. Chi and Q. Liu, et al., Atmospheric H2S triggers immune damage by activating the TLR-7/MyD88/NF-κB pathway and NLRP3 inflammasome in broiler thymus, Chemosphere, 2019, 237, 124427 CrossRef CAS.
- K. Nara, N. Kawashima and S. Noda, et al., Anti-inflammatory roles of microRNA 21 in lipopolysaccharide-stimulated human dental pulp cells, J. Cell. Physiol., 2019, 234, 21331–21341 CrossRef CAS.
- Y. Chen and H. Li, Alkannin protects human renal proximal tubular epithelial cells from LPS-induced inflammatory injury by regulation of microRNA-210, Biomed. Pharmacother., 2018, 108, 1679–1685 CrossRef CAS.
- O. Hyunju and G. Sankar, NF-κB: roles and regulation in different CD4(+) T-cell subsets, Immunol. Rev., 2013, 252, 41–51 CrossRef.
- W. Wang, M. Chen and X. Jin, et al., H 2 S induces Th1/Th2 imbalance with triggered NF-κB pathway to exacerbate LPS-induce chicken pneumonia response, Chemosphere, 2018, 208, 241 CrossRef CAS.
- M. S. Hayden and S. Ghosh, Signaling to NF-kappaB, Genes Dev., 2004, 18, 2195 CrossRef CAS.
- S. Wang, Q. Chi and X. Hu, et al., Hydrogen sulfide-induced oxidative stress leads to excessive mitochondrial fission to activate apoptosis in broiler myocardia, Ecotoxicol. Environ. Saf., 2019, 183, 109578 CrossRef CAS.
- J. Qu, W. Wang and Q. Zhang, et al., Inhibition of Lipopolysaccharide-Induced Inflammation of Chicken Liver Tissue by Selenomethionine via TLR4-NF-κB-NLRP3 Signaling Pathway, Biol. Trace Elem. Res., 2019 DOI:10.1007/s12011-019-01841-0.
- X. Shi, W. Wang and S. Zheng, et al., Selenomethionine relieves inflammation in the chicken trachea caused by LPS though inhibiting the NF-κB pathway, Biol. Trace Elem. Res., 2019 DOI:10.1007/s12011-019-01789-1.
- C. Cao, X. Li and Q. Fu, et al., Selenium-Rich-Yeast Protects Against Aluminum-Induced Activating Nuclear Xenobiotic Receptors and Triggering Inflammation and Cytochromes P450 Systems in Mice Heart, Biol. Trace Elem. Res., 2019 DOI:10.1007/s12011-019-01763-x.
- R. Zheng, Z. Zhang and Y. Zhao, et al., Transcriptomic Insights into the Response of the Olfactory Bulb to Selenium Treatment in a Mouse Model of Alzheimer's Disease, Int. J. Mol. Sci., 2019, 20 DOI:10.3390/ijms20122998.
- K. Tang, H. Li and K. Qu, et al., Selenium alleviates cadmium-induced inflammation and meat quality degradation via antioxidant and anti-inflammation in chicken breast muscles, 2019 Search PubMed.
- N. I. Dhanjal, S. Sharma and A. V. Skalny, et al., Selenium-rich maize modulates the expression of prostaglandin genes in lipopolysaccharide-stimulated RAW264.7 macrophages, Food Funct., 2019, 10, 2839–2846 RSC.
- H. H. Atteia, M. H. Arafa and K. Prabahar, Selenium nanoparticles prevents lead acetate-induced hypothyroidism and oxidative damage of thyroid tissues in male rats through modulation of selenoenzymes and suppression of miR-224, Biomed. Pharmacother., 2018, 99, 486–491 CrossRef CAS.
- X. Zheng, X. Hu and T. Ge, et al., MicroRNA-328 is involved in the effect of selenium on hydrogen peroxide-induced injury in H9c2 cells, J. Biochem. Mol. Toxicol., 2017, 31, e21920 CrossRef.
- R. Waters, S. Subham and S. Pacelli, et al., Development of MicroRNA-146a-Enriched Stem Cell Secretome for Wound-Healing Applications, Mol. Pharmaceutics, 2019, 16, 4302–4312 CrossRef CAS.
- Q. Chu, X. Yan and L. Liu, et al., The Inducible microRNA-21 Negatively Modulates the Inflammatory Response in Teleost Fish via Targeting IRAK4, Front. Immunol., 2019, 10 DOI:10.3389/fimmu.2019.01623.
- I. Sadakatsu and W. T. Pu, Expression and function of microRNAs in heart disease, Curr. Drug Targets, 2010, 11, 913–925 CrossRef.
- B. Balci-Peynircioglu, Y. Z. Akkaya-Ulum and T. H. Akbaba, et al., Potential of miRNAs to predict and treat inflammation from the perspective of Familial Mediterranean Fever, 2019 Search PubMed.
- J. C. Antunes, L. Benarroch and F. C. Moraes, et al., Core-Shell Polymer-Based Nanoparticles Deliver miR-155-5p to Endothelial Cells, Molecular therapy, Nucleic Acids, 2019, 17, 210–222 CrossRef CAS.
- J. Wu, X. Li and D. Li, et al., MicroRNA-34 family enhances wound inflammation by targeting LGR4, J. Invest. Dermatol., 2019 DOI:10.1016/j.jid.2019.07.694.
- C. Cao, Y. Zhang and Y. Chai, et al., Attenuation of Sepsis-Induced Cardiomyopathy by Regulation of MicroRNA-23b Is Mediated Through Targeting of MyD88-Mediated NF-κB Activation, Inflammation, 2019, 42, 973–986 CrossRef CAS.
- Y. Fang, J. Hu and Z. Wang, et al., LncRNA H19 functions as an Aquaporin 1 competitive endogenous RNA to regulate microRNA-874 expression in LPS sepsis, Biomed. Pharmacother., 2018, 105, 1183–1191 CrossRef CAS.
- H. S. Na, M. H. Park and Y. R. Song, et al., Elevated miR-128 in Periodontitis Mitigates Tumor Necrosis Factor-Alpha Response via P38 Signaling Pathway in Macrophages, J. Periodontol., 2016, 87, 1–18 CrossRef.
- G. Mao, P. Ren and G. Wang, et al., MicroRNA-128-3p Protects Mouse Against Cerebral Ischemia Through Reducing p38α Mitogen-Activated Protein Kinase Activity, J. Mol. Neurosci., 2017, 61, 1–7 CrossRef.
- X. Liang, W. Shangguan and M. Zhang, et al., miR-128 enhances dendritic cell-mediated anti-tumor immunity via targeting of p38, Mol. Med. Rep., 2017, 16, 1307–1313 CrossRef CAS.
- W. Xiong, H. Ma and Z. Zhang, et al., The protective effect of icariin and phosphorylated icariin against LPS-induced intestinal epithelial cells injury, Biomed. Pharmacother., 2019, 118 DOI:10.1016/j.biopha.2019.109246.
- Y. Bai, D. Yan and H. Zhou, et al., Betulinic acid attenuates lipopolysaccharide-induced vascular hyporeactivity in the rat aorta by modulating Nrf2 antioxidative function, Inflammopharmacology, 2019 DOI:10.1007/s10787-019-00622-4.
- S. Zheng, J. Zhao and H. Xing, et al., Oxidative stress, inflammation, and glycometabolism disorder-induced erythrocyte hemolysis in selenium-deficient exudative diathesis broilers, J. Cell. Physiol., 2019, 234, 16328–16337 CrossRef CAS.
- X. Hu, Q. Chi and D. Wang, et al., Hydrogen sulfide inhalation-induced immune damage is involved in oxidative stress, inflammation, apoptosis and the Th1/Th2 imbalance in broiler bursa of Fabricius, Ecotoxicol. Environ. Saf., 2018, 164, 201–209 CrossRef CAS.
- Q. Shi, W. Wang and M. Chen, et al., Ammonia induces Treg/Th1 imbalance with triggered NF-κB pathway leading to chicken respiratory inflammation response, Sci. Total Environ., 2019, 659, 354–362 CrossRef CAS.
- X. Shi, W. Wang and S. Zheng, et al., Selenomethionine relieves inflammation in the chicken trachea caused by LPS though inhibiting the NF-κB pathway, Biol. Trace Elem. Res., 2019 DOI:10.1007/s12011-019-01789-1.
- H. Huang, X. Jiao and Y. Xu, et al., Dietary selenium supplementation alleviates immune toxicity in the hearts of chickens with lead-added drinking water, Avian Pathol., 2019, 48, 230–237 CrossRef CAS.
- J. A. Nick, N. J. Avdi and S. K. Young, et al., Selective activation and functional significance of p38α mitogen-activated protein kinase in lipopolysaccharide-stimulated neutrophils, J. Clin. Invest., 1999, 103, 851–858 CrossRef CAS.
- W. Vanden Berghe, S. Plaisance and E. Boone, et al., p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor, J. Biol. Chem., 1998, 273, 3285–3290 CrossRef CAS.
- K. Kuno, Y. Ishikawa and M. K. Ernst, et al., Identification of an IκBα-associated Protein Kinase in a Human Monocytic Cell Line and Determination of Its Phosphorylation Sites on IκBα, J. Biol. Chem., 1995, 270, 27914–27919 CrossRef CAS.
- B. B. Aggarwal, S. Shishodia and S. K. Sandur, et al., Inflammation and cancer: How hot is the link?, Biochem. Pharmacol., 2006, 72, 1605–1621 CrossRef CAS.
- S. Zheng, X. Jin and M. Chen, et al., Hydrogen sulfide exposure induces jejunum injury via CYP450s/ROS pathway in broilers, Chemosphere, 2019, 214, 25–34 CrossRef CAS.
- R. Li, X. Kou and J. Tian, et al., Effect of sulfur dioxide on inflammatory and immune regulation in asthmatic rats, Chemosphere, 2014, 112, 296–304 CrossRef CAS.
- M. Cosin-Tomas, J. Senserrich and M. Arumi-Planas, et al., Role of Resveratrol and Selenium on Oxidative Stress and Expression of Antioxidant and Anti-Aging Genes in Immortalized Lymphocytes from Alzheimer's Disease Patients, Nutrients, 2019, 11 DOI:10.3390/nu11081764.
- P. Cabezas-Sanchez, S. Rainieri and N. Conlledo, et al., Impact of selenium co-administration on methylmercury exposed eleutheroembryos and adult zebrafish (Danio rerio): changes in bioaccumulation and gene expression, Chemosphere, 2019, 236, 124295 CrossRef CAS.
- W. Liu, H. Yao and W. Zhao, et al., Selenoprotein W was Correlated with the Protective Effect of Selenium on Chicken Myocardial Cells from Oxidative Damage, Biol. Trace Elem. Res., 2015, 171, 419–426 CrossRef.
- M. Xing, X. Jin and J. Wang, et al., The Antagonistic Effect of Selenium on Lead-Induced Immune Dysfunction via Recovery of Cytokine and Heat Shock Protein Expression in Chicken Neutrophils, Biol. Trace Elem. Res., 2018, 185, 162–169 CrossRef CAS PubMed.
- S. Kumbhar, A. Z. Khan and F. Parveen, et al., Impacts of selenium and vitamin E supplementation on mRNA of heat shock proteins, selenoproteins and antioxidants in broilers exposed to high temperature, AMB Express, 2018, 8 DOI:10.1186/s13568-018-0641-0.
- T. Yang, C. Cao and Y. Jie, et al., miR-200a-5p regulates myocardial necroptosis induced by Se deficiency via targeting RNF11, Redox Biol., 2017, 15, 159–169 CrossRef.
- Q. Liu, J. Cai and Y. Gao, et al., miR-2954 Inhibits PI3K Signaling and Induces Autophagy and Apoptosis in Myocardium Selenium Deficiency, Cell. Physiol. Biochem., 2018, 51, 778–792 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |