Placement of tyrosine residues as a design element for tuning the phase transition of elastin-peptide-containing conjugates: experiments and simulations†
Abstract
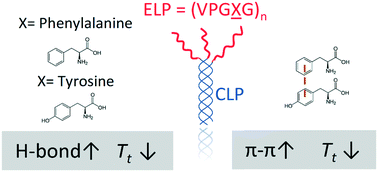
Elastin-like polypeptides (ELP) have been widely used in the biomaterials community due to their controllable, thermoresponsive properties and biocompatibility. Motivated by our previous work on the effect of tryptophan (W) substitutions on the LCST-like transitions of short ELPs, we studied a series of short ELPs containing tyrosine (Y) and/or phenylalanine (F) guest residues with only 5 or 6 pentapeptide repeat units. A combination of experiments and molecular dynamics (MD) simulations illustrated that the substitution of F with Y guest residues impacted the transition temperature (Tt) of short ELPs when conjugated to collagen-like-peptides (CLP), with a reduction in the transition temperature observed only after substitution of at least two residues. Placement of the Y residues near the N-terminal end of the ELP, away from the tethering point to the CLP, resulted in a lower Tt than that observed for peptides with the Y residues near the tethering point. Atomistic and coarse-grained MD simulations indicated an increase in intra- and inter-peptide hydrogen bonds in systems containing Y guest residues that are suggested to enhance the ability of the peptides to coacervate, with a concomitantly lower Tt. Simulations also revealed that the placement of Y-containing pentads near the N-terminus (i.e., away from the CLP tethering point) versus C-terminus of the ELP led to more π–π stacking interactions at low temperatures, in agreement with our experimental observations of a lower Tt. Overall, this study provides mechanistic insights into the driving forces for the LCST-like transitions of ELPs and offers additional means for tuning the Tt of short ELPs for biomedical applications such as on-demand drug delivery and tissue engineering.


 Please wait while we load your content...
Please wait while we load your content...