Synthesis and biological evaluation of S-lipidated lipopeptides of a connexin 43 channel inhibitory peptide†
Abstract
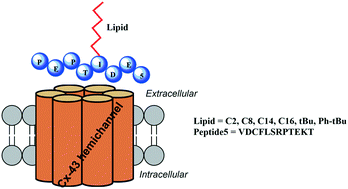
The synthesis and biological activity of 42 novel S-lipidated analogues of a connexin 43 channel inhibitory Peptide5 is described. Unmodified Peptide5 moderates hemichannels and gap junctions that are both implicated in the progression of neurological disease. Peptide5 was site-specifically modified with a cysteine residue, which then underwent thiol–ene mediated S-lipidation to afford S-lipidated Peptide5 analogues containing straight-chain, branched, or aromatic lipids. The modified peptides were assessed for their effect on hemichannel opening and the most promising candidates were evaluated in serum stability studies.


 Please wait while we load your content...
Please wait while we load your content...