 Open Access Article
Open Access ArticlePorous carbon materials for microwave absorption
Jin-Bo
Cheng
,
Hai-Gang
Shi
,
Min
Cao
,
Ting
Wang
,
Hai-Bo
Zhao
 * and
Yu-Zhong
Wang
*
* and
Yu-Zhong
Wang
*
Collaborative Innovation Center for Eco-Friendly and Fire-Safety Polymeric Materials (MoE), State Key Laboratory of Polymer Materials Engineering, National Engineering Laboratory for Eco-Friendly Polymer Materials (Sichuan), College of Chemistry, Sichuan University, Chengdu, 610064, China. E-mail: yzwang@scu.edu.cn; haibor7@163.com; Fax: +86-28-85410259; Tel: +86-28-85410755
First published on 23rd September 2020
Abstract
With the widespread use of microwaves in the civil and military fields, lots of efforts have been made to design high-performance microwave absorption (MA) materials, which should possess thin coating thickness, low density, wide absorption bandwidth, and strong absorption. Recently, porous carbon-based materials combined with the advantages of carbon and porous microstructures have demonstrated great advances in microwave absorption, due to their ultralow density, high specific surface area, strong dielectric loss, and excellent corrosion resistance. In this review, we summarize the recent progress in porous carbon-based MA materials encompassing composition and microstructure design. Representative fabrication methods, structure characterization, and properties of materials are highlighted in detail. The corresponding electromagnetic energy attenuation mechanisms, especially the effects of porous microstructures, are discussed as well. Moreover, the relative shortcomings, ongoing challenges, and perspectives at this frontier are presented.
1. Introduction
Recently, with the rapid development and expanding applications of electric technologies, electromagnetic (EM) waves have brought great convenience to people's lives.1 However, excess EM waves lead to some problems due to their harmful effects. For example, EM waves emitted by mobile phones would affect the normal work of precision electronic medical devices; EM waves can also seriously interfere with aircraft communications; besides, EM waves cause long-term harm to human body health; and, in the military aspect, the development of radar detection technology based on EM waves seriously threatens the survivability of aircraft, tanks, and ships.2–4 Therefore, developing advanced microwave absorption (MA) materials to eliminate unwanted EM energy has attracted great interest at the global scale.5–9 Usually, ideal MA materials should be thin in thickness, light in weight, wide in bandwidth, and strong in absorption. Furthermore, to increase the service life of MA materials, they need to display excellent environmental corrosion resistance (such as high temperature and humidity resistance, and thermal oxidization stability).2,10–12 Consequently, the light weight, large surface areas, excellent environmental stability, and strong EM wave attenuation abilities of carbon-based materials make them very promising in microwave absorption.Compared with traditional absorbing materials (Fe3O4, SiC, BaTiO3, magnetic metal powders, etc.), carbon-based materials exhibit lower densities and stronger EM wave absorption abilities.12–20 For example, there have been lots of studies on graphene and carbon nanotubes (CNTs) applied in MA fields due to their strong electrical conductivities, low percolation thresholds, and organized nanostructures.6,11,14,21–46 However, the cost-effective and large-scale production of graphene and CNTs remains a crucial challenge for commercial application, which seriously hinders their applications. Recently, porous carbon (PC) materials combined with carbon and porous features can be produced by a facile and simple synthesis method with low-cost raw materials, which has aroused renewed interest.
PC materials have been widely studied and applied in many fields such as catalysis, batteries, and supercapacitors due to their high specific areas, tunable pore sizes and pore structures.47–56 Meanwhile, these advantages also make PC materials expected to become MA materials: firstly, a porous material could be regarded as a composite composed of the solid component and air component, which efficiently decreases the density and increases the impedance matching.57 Secondly, the porous structure could generate more interface polarization loss, which would increase absorption of EM wave energy.58 Moreover, by utilizing different templates (aerogels, foams, metal–organic frameworks (MOFs), and biomass), PC could be prepared with controllable characteristics, i.e. pore size, pore structure, and graphitization of carbon, which directly influence the MA performance.59–64 However, pure PC materials always have the problems of a single dielectric loss mechanism and impedance mismatching. As a result, tremendous efforts have been made to exploit PC-based MA materials, containing optimized pore sizes and pore structures, adjusting the graphitization of carbon, and introducing diversified components. Therefore, it is necessary and meaningful to summarize the research progress of PC-based absorbers in recent years.
In this review, we first discuss and conclude the factors that influence the MA properties of PC-based materials. Then, according to the components and fabrication template, the research progress of various PC-based absorbing materials is comprehensively summarized. Finally, we put forward some challenges and development perspectives for future microwave absorbing materials.
2. Contribution of porous structures to microwave absorption
It is well known that porous structures can effectively reduce the densities of absorbers, which meets the lightweight requirement for absorbing materials. More importantly, recent studies have confirmed that porous structures of materials can play a positive role in microwave absorption. The main contribution factors of porous structures to MA have been summarized as follows. Firstly, porous structures can enhance the attenuation ability of electromagnetic waves by providing multiple paths for the incident EM waves and greatly increasing the contact probability between EM waves and the absorber. Thus, the EM waves can be absorbed or scattered many times in the pore channel, and further dissipated into heat, resulting in a strong attenuation property.65Secondly, porous structures can improve the impedance matching of materials for EM waves. Porous materials could be regarded as a composite composed of the solid component and air component, where Maxwell Garnett (MG) theory is a symbolic mechanism for exploring the variation of the effective permittivity.57,66,67 MG theory can be expressed as:
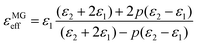 | (1) |
Lastly, the pore size and microstructure also play an important role in MA performance.63,68–70 Huang et al. prepared PC with tuneable morphologies. When changing the ratio of tertbutanol to resorcinol, the pore morphologies changed from disordered slit-shaped pores to uniform cage-like pores. Compared to the slit-shaped pores, the uniform cage-like pores in the PC contributed to a longer propagation tunnel and then caused more energy to be dissipated inside the tunnel.64 Xu et al. prepared mesoporous carbon hollow microspheres (PCHMs) with a designable mesoporous shell and interior void (Fig. 1a). The PCHMs exhibited a minimum reflection loss (RL) of −84 dB, almost four times as high as that of carbon hollow microspheres without mesopores (CHMs). The large solid–air interface in the mesoporous shell and interior void improved the impedance matching of PCHMs compared with CHMs. In addition, when the microwaves entered the PCHMs, multiple reflection and scattering would occur in the interior void and mesoporous shell. Thus, the ratio of absorption/reflection would increase since the travel path for microwave attenuation was increased63 (Fig. 1b). Cheng et al. designed mesoporous carbon hollow spheres (MCHSs) with different integrated sizes, shell sizes, hollow voids, and mesopores in the shell via a unique simultaneous hydrolyzation–polymerization process.62 The shell thickness and integrated sizes were associated with the synergistic effect of hydrolyzation and polymerization reactions, while the hollow voids and mesopores were determined by the SiO2 template morphology. Taking size-controlled MCHSs as an example, the increase in the hollow void sizes led to large ε′ and ε′′ (Fig. 1d). The expanded hollow voids would reduce the density of the MCHSs, thus increasing the filler molar quantity at the same weight, which resulted in a monotonic increase in the complex permittivity. When the filling ratio was 20 wt%, MCHSS-3 presented the best absorption capability due to the compatibility of the impedance matching and attenuation capacity.
 | ||
| Fig. 1 (a) A schematic illustration of the synthesis of mesoporous carbon hollow microspheres (PCHMs), carbon hollow microspheres (CHMs), and carbon solid microspheres (CSMs),63 and (b) schematic illustration of EM wave absorption for PCHMs.63 Reproduced with permission from ref. 63. Copyright © 2017, American Chemical Society. (c) Synthesis procedure of different mesoporous carbon hollow spheres (MCHSs): expanded hollow voids and decreased thickness with controlled integrated size.62 (d) Complex permittivity variation within 2–18 GHz for MCHS-1 (d1), MCHSS-2 (d2), MCHSS-3 (d3), MCHSS-4 (d4), and MCHSS-5 (d5).62 Reproduced with permission from ref. 62. Copyright © 2018, Elsevier. | ||
3. Research progress of porous carbon materials for microwave absorption
3.1 Pure porous carbon absorbers
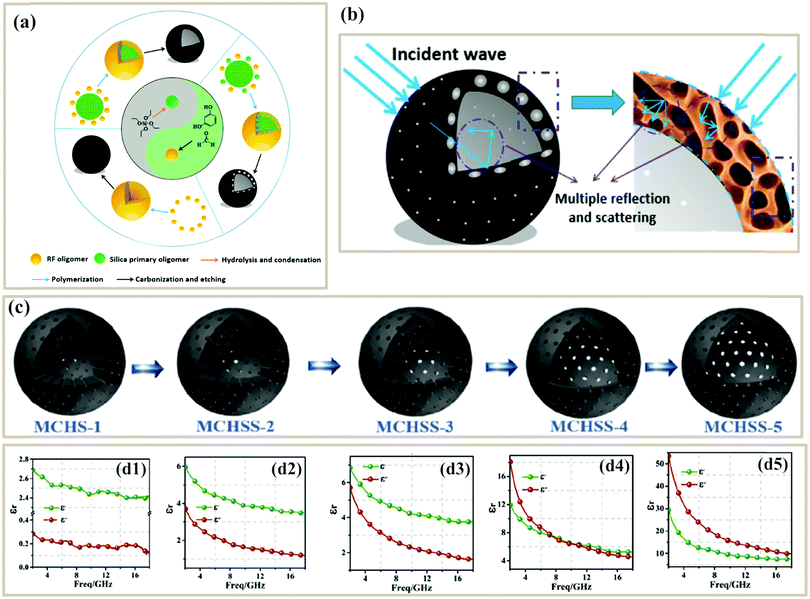
Benefitting from the above-mentioned advantages of porous structure, numerous studies have focused on the design and fabrication of PC-based microwave absorbers. Pure PC absorbers can be produced from biomass, polymer foams or aerogels, and other precursors. Among them, biomass precursors are abundant in nature and usually possess microscale porous structures, and thus a variety of biomass-derived PCs have been used as microwave absorbers. As shown in Fig. 2a, a wide range of biomass (walnut shell, spinach stems, rice husk, waste cotton, shell of pumpkin seeds, loofah sponge, jute, chiffon cake, chicken feathers, fish skin and fir wood) has been utilized to fabricate PCs through direct carbonization processes or activation methods.66,67,71–78 The resultant pore structures of the PCs are related to the texture of the biomass, carbonization conditions, or activation conditions.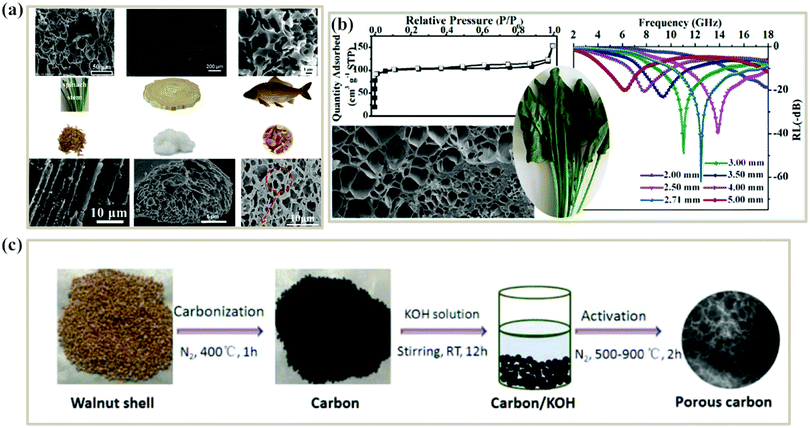 | ||
| Fig. 2 (a) Various biomass-derived PCs. Reproduced with permission from ref. 66, 71, 72 and 75–77. Copyright © 2019, Elsevier. Copyright © 2017, Elsevier. Copyright © 2018, Elsevier. Copyright © 2018, American Chemical Society. Copyright © 2017, Royal Society of Chemistry. Copyright © 2019, Springer Nature. (b) N2 adsorption–desorption isotherms, SEM, and RL curves of hierarchical porous carbons (HPCs) from spinach stems.66 Reproduced with permission from ref. 66. Copyright © 2018, American Chemical Society. (c) Schematic illustration of the fabrication of walnut shell-derived porous carbon.78 Reproduced with permission from ref. 78, Copyright © 2017, Royal Society of Chemistry. | ||
The direct carbonization process is the simplest method for preparing biomass-derived PCs. After thermal treatment, the sp3 carbons are reduced into graphitized sp2 carbons, and then free electrons can migrate along the sp2 plane and expand the conductivity loss of PCs. The carbonization temperature can adjust the graphitization degree, where the enhancement of the graphitization degree would boost the electrical conductivity (σ). According to eqn (2), the dielectric loss originates from the polarization loss (εp′′) and conductivity loss (εc′′),79
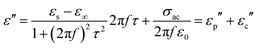 | (2) |
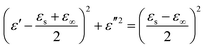 | (3) |
Wu et al. reported hierarchical porous carbons (HPCs) from spinach stems via a one-step carbonization process, where microscale pores were templated by the inherent porous tissues and nanopores were produced by in situ etching by the embedded alkaline metals (Fig. 2b). The specific surface area of the HPC carbonized at 600 °C reached 345.3 m2 g−1 and the micro-sized cavities could act as dihedral angles to promote multiple reflection. Besides, the presence of nanosized pores in the HPC could decrease the effective permittivity and optimized the impedance matching. The carbonization temperature can also adjust the chemical composition (graphitization degree) to obtain the optimal microwave absorber. Finally, the spinach-derived HPC carbonized at 600 °C exhibited a strong minimum RL of −62.2 dB and a broad effective absorption bandwidth of 7.3 GHz.66 Optimizing the carbonization conditions is vital in determining the MA performance for pure PC materials.
The activation method is used to further increase the surface areas and porosity for PC materials. Usually, biomass is carbonized into a carbon component at relatively low temperatures (<800 °C), and then the resultant carbon is activated at relatively high temperatures. Through activation, porous biomass carbon can produce numerous micropores, and increase solid–void interfaces. Particularly, the solid–void interfaces can create more interface polarization under EM fields and then generate polarization loss to attenuate microwave energy. Steam, CO2, KOH, K2CO3, H3PO4, and ZnCl2 are commonly used as activators during the activation process. Among them, KOH activation requires relatively low activation temperatures and can generate large specific surface areas. Moreover, KOH activation is facile and highly efficient, which makes it be widely used. The following chemical reactions may occur at an elevated temperature between carbon and KOH:
| 6KOH + 2C → 2K + 2K2CO3 + 3H2 | (4) |
| K2CO3 + 2C → 2K + 3CO | (5) |
| K2O + C → 2K + CO | (6) |
| K2CO3 → K2O + CO2 | (7) |
| CO2 + C → 2CO | (8) |
Qiu et al. used walnut shells to produce porous biomass carbon, where walnut shells were carbonized at 400 °C followed by KOH activation at various temperatures (Fig. 2c). The resultant PC activated at 600 °C had a specific surface area of 736.2 m2 g−1, nearly 2 times that of the non-activated sample (435.3 m2 g−1). The author claimed that the PC activated by KOH possessed a rich porous structure, which would extend the propagation path of incident microwaves to be attenuated (Fig. 2d). Finally, the minimum RL of PC-600 reached −42.4 dB with a thickness of 2 mm.78
Compared to the direct pyrolysis or activation method, PC absorbers derived from porous foam/aerogel precursors are more common, which can inherit the porous morphology of the template precursors. Zhou et al. fabricated a three-dimensional (3D) carbon foam via hydrothermal and subsequent pyrolysis processes.71 The carbon foam (CF) obtained at 650 °C (CF-650) possessed a large surface area of 1369.3 m2 g−1 and a hierarchical porous 3D structure, in which the nano-pore size was 0.644 nm and the micro-pore size was about 1 μm. The unique architecture endowed CF-650 with a broad bandwidth (RL < −10 dB) of 8.6 GHz and relatively strong RL of −33.5 dB. The 3D conductive carbon foam network made free electrons easily transport in the presence of EM fields. These electron movements would generate Joule heat to dissipate the microwave energy.
Nanoparticles such as SiO2 are also used as templates to fabricate PC. A series of mesoporous carbon hollow microspheres (MCHSs) with controlled microstructures were achieved, and different types of MCHSs demonstrated different MA properties due to the discrepancy in the volume ratio between the pores and carbon compositions.62 Interestingly, Song et al. fabricated highly ordered porous carbon (HOPC) with ordered SiO2 colloidal templates and phenol resin. The unique porous framework allowed HOPC to serve as an effective medium for sufficient electrical loss, and then the composites with 1 and 5 wt% HOPC showed an EAB of ∼2 and 4.5 GHz, respectively.68
As mentioned above, we can conclude that regulating the relationship between the microstructure and carbon composition is vital in improving the MA performance for pure PC microwave absorbers. In simple terms, pore structures usually benefit impedance matching while the carbon component contributes to the attenuation ability of microwaves, so the ideal PC absorbers often require compatibility between them. Table 1 summarizes the microwave absorption performances of pure PC-based absorbers reported in recent years. According to Table 1, pure PC-based absorbers usually possess relatively low filler loading (≤30 wt%) and strong reflection loss (usually ≤−40 dB), which originate from the light weight and strong conductive loss. Nonetheless, the high conductivity of pure PC materials always leads to unfit impedance matching, which makes most microwaves be reflected from the PC surface. Therefore, introducing other microwave absorbing components into the PC matrix to adjust the MA performance has also attracted much attention.
| Sample | Minimum RL value | Loading content (wt%) | Effective absorption bandwidth | Ref. | ||
|---|---|---|---|---|---|---|
| d (mm) | RLmin (dB) | d (mm) | f (GHz) | |||
| PBPC | 4.28 | −68.3 | — | 3.73 | 7.63 | 76 |
| PCHM | 3.9 | −84 | 20 | 1.8 | 2.7 | 63 |
| Walnut shell-derived PC | 2 | −42.4 | 30 | 1.5 | 2.24 | 78 |
| HPC-S | 2.71 | −62.2 | 30 | 2.71 | 7.3 | 66 |
| PC | 2.05 | −50.55 | 10 | 1.85 | 5.32 | 78 |
| CF | 2.6 | −52.6 | 30 | 3 | 8.6 | 71 |
3.2 Porous carbon composite absorbers
According to transmission line theory, the RL value can be calculated by the following equations: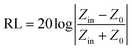 | (9) |
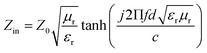 | (10) |
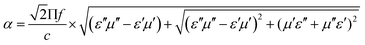 | (11) |
Although pure PC materials possess excellent dielectric loss and strong attenuation ability, their relatively poor impedance matching limits their application in microwave absorption. Hence, introducing more functional components into PC is an effective strategy to expand the MA performance.
Generally, the method of fabricating biomass-based PC composites could be divided into two types: one is to modify functional compounds on the surface of biomass carbides; another is directly pyrolyzing the biomass containing functional precursor. Wang et al. first acquired porous jute biomass carbon (PJBC) through carbonization and activation processes, and then embellished PJBC with Fe3O4 nanoparticles via a chemical coprecipitation method at a low temperature of 60 °C (Fig. 3a).73 Based on the different contents of PJBC, the Fe3O4/PJBC samples were labeled as S1, S2, and S3, of which the saturation magnetization (MS) was 72.7 emu g−1, 65.3 emu g−1, and 29.6 emu g−1, respectively (Fig. 3b). For ferromagnetic microwave absorbers, high initial permeability (μi) usually predicts strong magnetic loss, and high MS is beneficial to improve the initial permeability (μi) according to eqn (12):
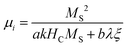 | (12) |
 | ||
| Fig. 3 (a) Schematic illustration of the fabrication of Fe3O4/PJBC composites.73 (b) Magnetic hysteresis loops for pure Fe3O4 and Fe3O4/PJBC composites at room temperature.73 (c) Eddy current loss (denoted by μ′′(μ′)−2f−1versus f) of pure Fe3O4 and Fe3O4/PJBC composites.73 Reproduced with permission from ref. 73, Copyright © 2019, Elsevier. (d) Schematic illustration of preparing a hierarchically porous C/Fe3C composite; and the SEM image, nitrogen adsorption–desorption isotherm, and RL curve of the porous C/Fe3C composite.99 Reproduced with permission from ref. 99, Copyright © 2018, Elsevier. | ||
Besides, many studies also focus on the method of directly pyrolyzing the biomass containing functional precursor. Huang et al. prepared hierarchical porous C/Fe3C composites via drying the gels of gelatin/iron nitrate and subsequent carbonization processes99 (Fig. 3d). Micropores (observed from the SEM image) were formed from the self-foaming of the gel while nanopores (observed from the nitrogen adsorption–desorption isotherm) were generated in the carbonization process. Eventually, a minimum RL of −57.1 dB and a broad effective absorption bandwidth of 8.16 GHz at a thickness of 2.5 mm were achieved in the hierarchically porous C/Fe3C composites. Consequently, direct pyrolysis of biomass containing functional precursors can better maintain the pore morphology, while the loading of the functional absorbing component is less controllable.
To sum up, biomass-based PC composite absorbers possess the advantages of low cost (including cheap raw materials and convenient preparation routes), environment friendliness (no toxic solvents) and adjustable MA performance, which give them the prospect of industrial applications in the future. But the irregular pore structures and poor repeatability of biomass remain an urgent problem to be solved. Hence, many researchers designed a matrix with a regular pore structure (metal–organic frameworks, foams, aerogels, etc.) to synthesize regular PC composite absorbers.
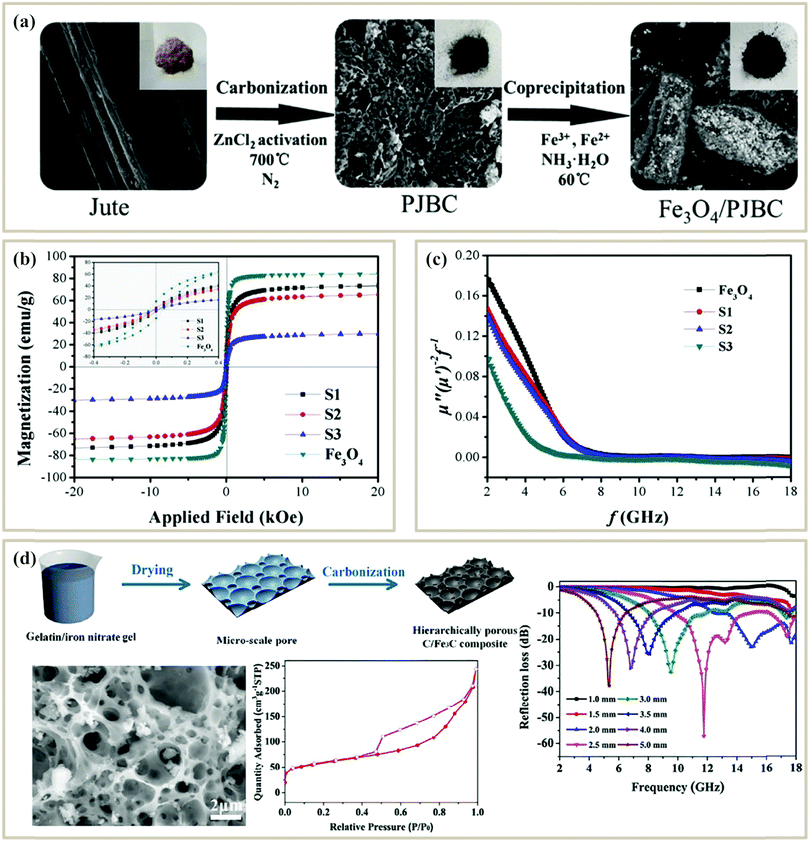
Direct pyrolysis of MOFs is the simplest and fastest method to fabricate MOF-based PC absorbers. After pyrolysis, the organic ligand is pyrolyzed into conductive carbon, while the metal ions can be transferred into a metal or metal oxide. Yin et al. carbonized Co-based zeolitic framework ZIF-67 in a reducing atmosphere (H2/Ar) to fabricate a porous carbon/Co composite107 (Fig. 4a). H2 could reduce Co2+ into metallic Co nanoparticles and then catalyzed the growth of CNTs. Controlling the pyrolysis conditions could tune the morphology and microstructure of the composite. Lower temperature led to well-dispersed Co nanoparticles and short CNT clusters, while higher temperatures or longer heating time led to the aggregation of Co nanoparticles. Finally, the porous CNT/Co composite calcined at 900 °C for 3.5 h displayed a strong RL of −60.4 dB at a matching thickness of 1.81 mm. The excellent MA performance originated from the special microstructure and the synergistic effect between conductive CNTs and magnetic Co nanoparticles.
 | ||
| Fig. 4 (a) Synthetic process of a CNT/Co composite from a ZIF-67 precursor, and the RL curve and TEM images of CNT/Co.107 Reproduced with permission from ref. 107, Copyright © 2016, American Chemical Society. (b) Synthetic process of Co@NPC@TiO2.116 Reproduced with permission from ref. 116, Copyright © 2016, Royal Society of Chemistry. (c) Synthetic process of CuO@NPC.109 Reproduced with permission from ref. 109, Copyright © 2016, Royal Society of Chemistry. (d) Synthetic process of ZnO/NPC@Co/NPC.113 Reproduced with permission from ref. 113, Copyright © 2016, Royal Society of Chemistry. (e) Synthetic process of Co-doped NPCs/CNTs.111 Reproduced with permission from ref. 111, Copyright © 2019, Elsevier. | ||
To further tune the impedance matching of PC composites from direct pyrolysis, some researchers tried to mix microwave absorbers with sintered PC composites for a secondary calcination process. Zhang et al. first pyrolyzed ZIF-67 to get Co@nanoporous carbon (Co@NPC), and then coated a TiO2 shell by n-butyl titanate hydrolysis and secondary pyrolysis116 (Fig. 4b). The obtained Co/NPC@TiO2 possessed a high surface area of 232.84 m2 g−1 and an optimal RL of −31.7 dB with an absorbent thickness of 1.5 mm. Ma et al. etched the Co of Co@NPC and then coated CuO nanoparticles at the NPC surface109 (Fig. 4c). The hollow CuO/carbon composite exhibited an optimal RL of −57.5 dB with a thickness of 1.55 mm. The introduction of TiO2 and CuO could decrease the complex permittivity and improve the impedance matching to get enhanced absorption. However, this method needs a secondary calcination, which increases the cost.
It is necessary to develop a more cost-effective route to reduce the pyrolysis process. One efficient method is by compounding MOFs with different materials to prepare the pyrolysis precursors, while another is introducing other materials during the process of preparing the MOF. Liang et al. grew ZIF-67 on the surface of ZIF-8 via a seed-mediated growth process. After thermal treatment, a core–shell structured ZnO/NPC@Co/NPC composite was obtained113 (Fig. 4d). The electromagnetic parameters and the shell thickness could be tuned through varying the mole ratios of Co2+/Zn2+. The resultant composite can be endowed with multiple loss mechanisms to improve the attenuation ability. Besides, Liu et al. combined a MOF with glucose and fabricated porous Co/C composites, and the attenuation ability and impedance matching condition could be improved through the construction of interfacial structures between inner cobalt and surface carbon. Finally, a minimum RL of −20 dB could be achieved at a low frequency (2–8 GHz).117 However, when the compounding conditions are relatively harsh (such as strong acid, strong alkali, and high-temperature environments), the microstructure and the components of the MOF may be destroyed.
Directly introducing different components during MOF preparation is more controllable. Wu et al. synthesized a porous rambutan-like carbon composite using Co-doped zeolitic imidazolate framework-8 (ZIF-8) as a self-sacrificing precursor111 (Fig. 4e). The in situ generated Co nanoparticles were uniformly inserted into the NPC matrix, which also catalyzed the formation of graphitized CNTs. When the carbonization temperature was 700 °C, the metal-doped NPC/CNTs achieved a minimum RL of −32.0 dB. The introduction of nanoparticles could give better dispersion and isolation of the MOF metal center, thus leading to more evenly dispersed ZnCo nanoparticles. Besides, the additive content of the nanoparticles could tune the impedance matching to improve the MA ability.
As discussed above, MOF-based PC composite absorbers have the superiorities of adjustable components, ordered pore structures, excellent repeatability, and strong absorption. However, the relatively heavy nanoparticle composites usually resulted in higher filler loading. In addition, the fabrication process of MOFs usually requires the use of toxic solvents and harsh fabrication conditions. Thus, developing a simple and efficient process for MOF-based PC composite absorber industrial production to reduce the energy and toxic substances in the synthetic route is necessary. On the other hand, in order to maintain a regular pore structure while reducing toxic substances, foam/aerogel-based PC composite absorbers have been extensively studied.
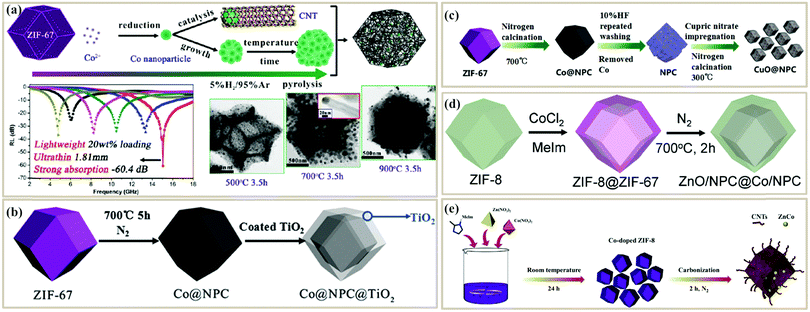
The post-treatment method is commonly used for fabricating PC absorbers from melamine foams (MF) due to their high porosities and interconnected conductive network structures after pyrolysis.61,119,124 The post-treatment method can be further divided into two types. One is to obtain a PC foam by pyrolysis of the foam precursor, and then modify the PC foam.125 Ye et al. first got a porous carbon foam (PCF) from a commercial MF, and then deposited an SiC film on its surface through chemical vapor deposition technology61 (Fig. 5a). SiC could effectively decrease ε′ and ε′′ of the PCF, which greatly improved the impedance matching ratio (Zr = Z/Z0 = (μr/εr)1/2) (Fig. 5b). The minimum RL of the PCF/SiC composite reached −25.93 dB when the thickness was only 1.05 mm. The other post-treatment method is to decorate the foam skeleton with functional compounds and then pyrolyze them. For example, 3D CoNi alloy particles embedded in an N-doped porous carbon (CoNi@PRM-NC) foam were prepared by using a MF as a sacrificial scaffold and supporting template by Yan et al.120 The MF was first coated with Co2+/Ni2+ and phenolic resol, and then was carbonized at high temperatures (700–1000 °C) (Fig. 5c). Finally, the 3D CoNi@PRM-NC foam displayed a strong RL of −56 dB at 17.8 GHz when the thickness was 1.7 mm (Fig. 5d). The outstanding MA performance was attributed to the multiple scatterings induced by the conductive foam structures and large attenuation as well as the balance of impedance matching between the dielectric loss and magnetic loss.
 | ||
| Fig. 5 (a) Three-step preparation process of the SiC/PCF composite.61 (b) Impedance matching ratio and attenuation constant of PCF and SiC/PCF.61 Reproduced with permission from ref. 61, Copyright © 2019, Elsevier. (c) Schematic illustration of CoNi@PRM-NC.120 (d) Reflection loss curves of CoNi@PRM-NC.120 Reproduced with permission from ref. 120, Copyright © 2019, Elsevier. | ||
Directly integrating other materials into one foam or aerogel during their fabrication process is also an important way to improve the MA performance of pure PC-based foam/aerogel absorbers.60,118,121–123 Zhao et al. fabricated Ni2+/alginate foams as precursors and then pyrolyzed them to get lightweight porous Ni/carbon foams (∼0.1 g cm−3)123 (Fig. 6a). In the pyrolysis process, alginates underwent thermal decomposition to form graphite carbons, while Ni nanoparticles were reduced simultaneously. Consequently, Ni nanoparticles were uniformly dispersed in the foam without aggregation due to the coordination-covalent bonds between Ni2+ and alginate. The resultant foam also showed a high BET surface area of 451 m2 g−1 and a moderate conductivity (6 S m−1). Benefiting from these features, the minimum RL of the Ni/carbon foam reached −45 dB with an effective absorption bandwidth of −4.5 GHz at a thickness of 2 mm when the filler loading was only 10 wt%. The conductive carbon network, nano-porosity and uniform Ni nanoparticles contributed to the great microwave absorption. Similarly, Cheng et al. used cotton fibers as 3D scaffolds, and then sequentially coated with Co2+ and dicyandiamide (DCDA).121 After thermal pyrolysis, the metal Co generated as a catalyst could promote the growth of CNTs on the carbon fiber (Fig. 6b). The resultant composite aerogel was elastic and could return to its original shape after compression (Fig. 6b), due to the unique 3D aerogel framework built from the “1D–1D” structure. An RL of −42 dB and a broad effective absorption band of 5.08 GHz were achieved at the same matching thickness of 1.6 mm. The outstanding MA performance could be explained in Fig. 6c. Firstly, the conductive carbon fiber network made it possible for electrons to move; in addition, the migrating and hopping of electrons in CNTs would consume EM energy; besides, dipolar polarization would be generated due to the defects of CNTs while interface polarization occurred among different interfaces; and, lastly, magnetic loss including natural resonance and exchange resonance caused by metal Co could improve the impedance matching. Porous banana leaflike carbon-doped MoS2 aerogels with low densities (∼0.12 g cm−3) were also manufactured by Cheng et al. via self-assembly and pyrolysis processes. The hybrid aerogel achieved a minimum RL of −43 dB at 5.4 GHz with a low filler loading of 30 wt% (Fig. 6d).89 In conclusion, foam/aerogel PC composite absorbers usually can show strong microwave absorption with relatively low filler loading due to their low densities, outstanding conductive network, and uniformly distributed functional components. It is noteworthy that reduced graphene oxide (rGO) has also been designed into aerogels to make use of these advantages. For example, Zhao et al. developed a novel CoNi/rGO aerogel with an ultralow density of 7 mg cm−3via a facile in situ solvothermal and carbonization process. A minimum RL value of 53.3 dB was achieved with an ultrathin thickness of 0.8 mm at a low filler loading of 7 wt% in the paraffin template, and the effective absorption bandwidth was >3.5 GHz (Fig. 6e), which provided a potential strategy to prepare excellent microwave absorbers with ultrathin thickness and ultralow density.131
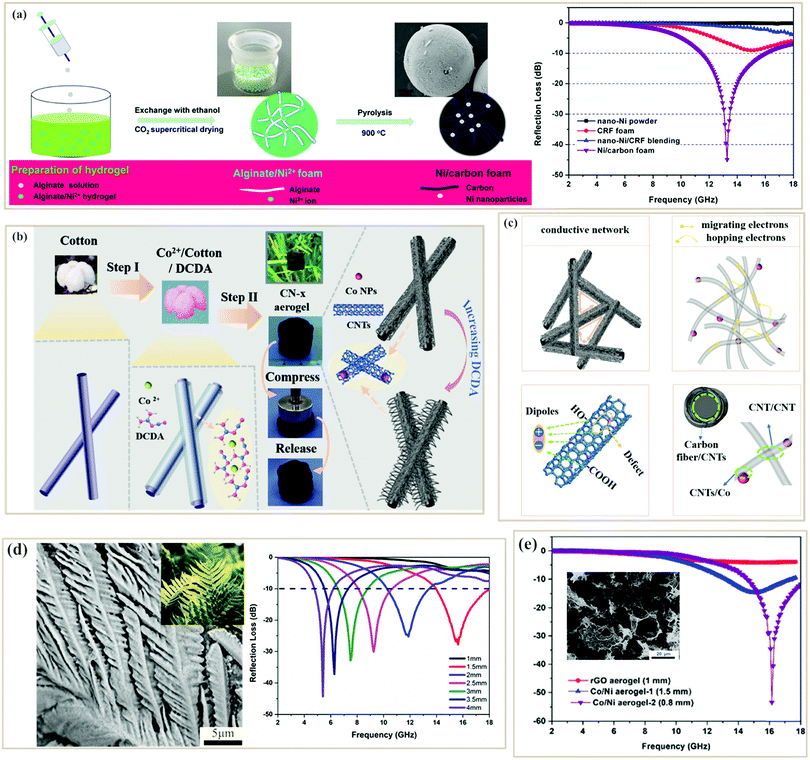 | ||
| Fig. 6 (a) Schematic illustration, SEM image, and reflection loss curve of the Ni/carbon foam.123 Reproduced with permission from ref. 123, Copyright © 2016, American Chemical Society. (b) Schematic graph of the formation process of CN-x composites.121 (c) Schematic description of possible microwave absorption mechanisms of the CN-2.5 aerogel.121 Reproduced with permission from ref. 121, Copyright © 2019, Wiley. (d) SEM image and reflection loss curves of carbon-doped MoS2 aerogels.89 Reproduced with permission from ref. 89, Copyright © 2020, American Chemical Society. (e) SEM image and reflection loss curves of CoNi/rGO aerogels.131 Reproduced with permission from ref. 131, Copyright © 2018, Royal Society of Chemistry. | ||
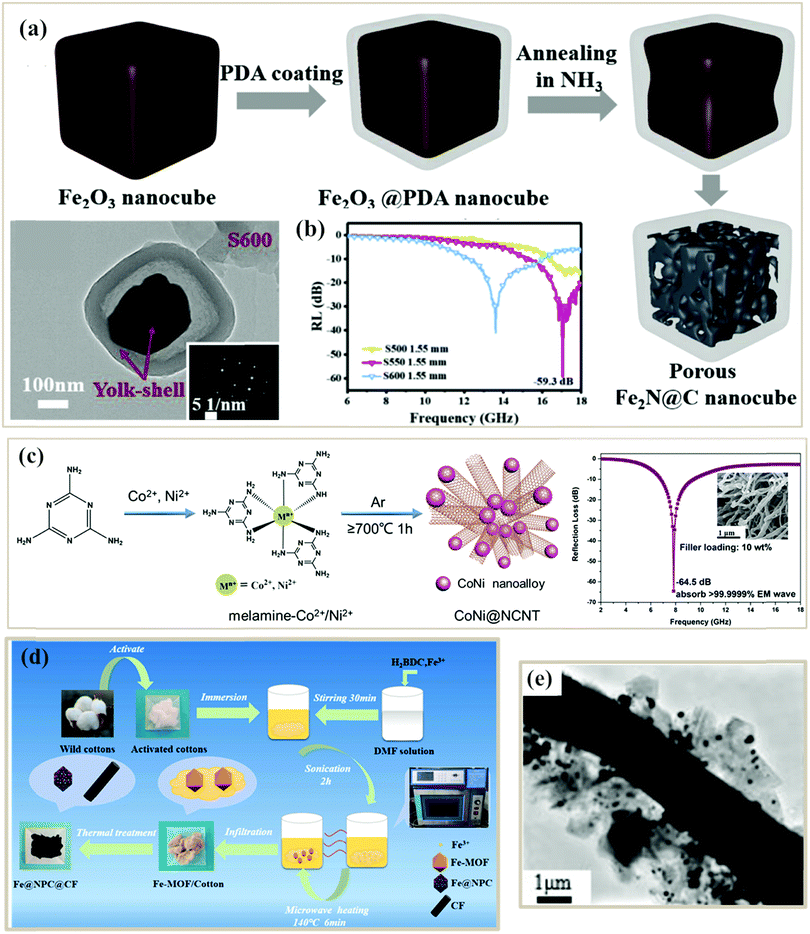 | ||
| Fig. 7 (a) Schematic illustration of the formation process and TEM image of porous Fe2N@N-doped C nanocubes.82 (b) RL curve of Fe2N@C composites.82 Reproduced with permission from ref. 82, Copyright © 2019, Elsevier. (c) Schematic illustration of the formation process, SEM image, and RL curve of porous CoNi@N-doped carbon nanotube clusters.132 Reproduced with permission from ref. 132, Copyright © 2020, Royal Society of Chemistry. (d) Formation process of Fe@NPC@CF composites.115 (e) TEM image of an Fe@NPC@CF composite (S550).115 Reproduced with permission from ref. 115, Copyright © 2019, Elsevier. | ||
Besides, combining different templates can also be used to fabricate PC composites.125 Li et al. designed an Fe@nanoporous carbon@carbon fiber (Fe@NPC@CF) composite from MOF and biomass cotton double templates (Fig. 7d), where Fe@NPC was evenly dispersed on the carbon fiber115 (Fig. 7e). The porous structure of the composite not only facilitated multiple reflections and scatterings of the incident microwaves but also increased the dipole strength, which further strengthened the interface polarization. Liang et al. designed a ZnO@nanoporous carbon/3D porous carbon network (3DPCN) using a MOF and organic glucose as a carbon source, where the 3DPCN structure acted as the electronic pathway and the nucleation locus for ZIF-8 particles.127 Besides, a melamine foam compound with MOF double templates has also been designed by Gu et al. as a multifunctional microwave absorber. Remarkably, the resultant carbon-based foam presented strong microwave absorbing properties with an effective microwave bandwidth of 5.64 GHz at a thin layer of 2.1 mm. The high porosity, three-dimensional structure and combined effects of multiple components of the hybrid foam contributed to a superior heat insulating feature and thermal stealth character.128 Despite many benefits, the complex preparation process still seriously limited their applications.
Especially, it should be noted that some interesting technologies such as arc-discharge plasma technology, electrospinning technology, and microwave-assisted methods are also utilized for fabricating PC, which provides a new strategy for the development of microwave absorbers.126,129,130 For example, Li et al. synthetized a tremella-like porous NiCo/C composite through a microwave-assisted method, and the fabrication time was greatly decreased.126
Table 2 summarizes the MA properties of recent PC-based composite absorbers. Accordingly, PC-based composites have been widely used in MA, and some of them could meet the demand of “thin thickness, light weight, wide frequency bandwidth, and strong absorption”. However, many studies paid attention to designing new nanostructures to obtain satisfactory MA performance, and the relevant absorption mechanism remains to be studied. Also, current absorbing materials mainly focus on the absorption of EM waves in the band of 2–18 GHz. Whereas, with the development of meter-wave and millimeter-wave detection technology, higher requirements are put forward for multi-band absorption of absorbing materials.
| Sample | Minimum RL value | Loading content (wt%) | Effective absorption bandwidth | Ref. | ||
|---|---|---|---|---|---|---|
| d (mm) | RLmin (dB) | d (mm) | f (GHz) | |||
| RHPC/Fe | 1.4 | −21.8 | 25 | 1.4 | 5.6 | 77 |
| RHPC/Co | 1.8 | −40.1 | 25 | 1.8 | 2.7 | 77 |
| Fe3O4/PJBC | 4 | −39.5 | 50 | 1.6 | 6.4 | 73 |
| SiC/PCF | 1.05 | −25.93 | — | 1.15 | 3.24 | 119 |
| CoNi@PRM-NC | 1.7 | −56 | 35 | 2 | 4.78 | 120 |
| CN-2.5 | 4.5 | −53 | — | 1.6 | 5.08 | 121 |
| Ni/C foam | 2 | −45 | 10 | 1.5 | 4.5 | 123 |
| NCM | 2.5 | −50.57 | 15 | 2.5 | 7.44 | 118 |
| CuO@NPC | 1.55 | −57.5 | 50 | 1.55 | 4.7 | 109 |
| FeCo@C-PCF | 1.85 | −56 | 30 | 2.5 | 8.3 | 93 |
| Fe@NPC@CF | 2.5 | −46.2 | 25 | 2.5 | 5.2 | 115 |
| BDC/MnO NR | 1.65 | −58.5 | 30 | 1.42 | 4.5 | 94 |
| Porous C/Fe3C | 2.5 | −57.1 | 50 | 2.5 | 8.16 | 99 |
| Fe2N@C | 1.9 | −59.3 | 50 | 1.9 | 4.32 | 82 |
4. Conclusions and perspectives
Based on the above discussion, it can be concluded that PC-based absorbers are potential microwave absorption materials due to their facial synthesis strategies, low densities, strong attenuation abilities, adjustable EM parameters, simple modification methods, and abundant raw materials. In this review, we discussed the influence of porous carbon materials on MA performances. That is, the porous structure will contribute to light weight and better impedance matching while the complex permittivity can be tuned via the graphitization of carbon. Then we systematically summarized the latest research progress on PC-based absorbers, according to the precursor types, and put forward promising approaches for improving the MA performance of PC-based materials. For pure PC absorbers, the direct pyrolysis method is the simplest, while the activation method would further increase the pore volumes and the template method could better control the pore size. But pure PC absorbers are sometimes limited by weak impedance matching due to their high conductivity and strong dielectric loss. To solve this problem, regulating the relationship between the microstructure and carbon composition can effectively improve the impedance matching, thereby ensuring application prospects in MA. Besides, hybridization with other functional materials can introduce more interface polarization loss and magnetic loss, which would also tune the impedance matching and improve the MA performance of PC absorbers. For PC-based composites, on one hand, the precursors are abundant, including polymer foams, aerogels, MOFs, biomass, and organic materials. On the other hand, various modification methods are effective strategies in tailoring the microstructures and introducing different compositions. In summary, controlling the pyrolysis temperature, tuning the composition and designing the microstructure are the important factors to achieve excellent MA performance of PC-based materials.As shown in Tables 1 and 2, PC-based materials have made prominent achievements in MA. Even so, it should be emphasized that current research is still restricted by many aspects. Firstly, it has been proved that the porous structure would improve the impedance matching and enhance the MA properties of PC-based materials. However, the influences of the pore size and pore distribution on microwave absorption are still unclear. Secondly, plenty of achievements have been made regarding PC-based composite absorbers. Nevertheless, many studies paid attention to designing new nanostructures to obtain satisfactory MA properties, and it remains essential to study the relevant absorption mechanism. For example, it has been widely recognized that conductivity loss and polarization (CaP) are two of the dominant factors in determining the complex permittivity. However, few studies have been reported on the quantitative relationship between CaP and permittivity, as well as the preferred dissipation mechanism in different types of dielectrics (e.g., semiconductors/graphitized carbon, graphene, and so on).80 Thirdly, the construction of high-performance absorbing materials with “low density, thin coating thickness, wide absorption bandwidth and strong absorption” is still a major concern in the field of absorbing materials. But current absorbing materials mainly focus on the absorption of EM waves in the band of 2–18 GHz. Whereas, with the development of meter-wave and millimeter-wave detection technology, higher requirements are put forward for multi-band absorption of absorbing materials. Future absorbing materials should cover multi-band absorption including infrared light, microwaves, etc. Finally, considering wide applications, the combination of MA with other functions (such as higher temperature resistance, corrosion resistance, superhydrophobicity, etc.) is also an important direction for the future development of PC-based absorbers. Ideal absorbing materials should adapt to different harsh environments. The development of multifunctional and multiband absorbing materials will be a future research trend.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Sichuan Science and Technology Program (2019YJ014), Fundamental Research Funds for the Central Universities, and Young Elite Scientists Sponsorship Program by CAST.References
- B. Quan, X. Liang, G. Ji, Y. Cheng, W. Liu, J. Ma, Y. Zhang, D. Li and G. Xu, J. Alloys Compd., 2017, 728, 1065–1075 CrossRef CAS.
- L. Huang, C. Chen, Z. Li, Y. Zhang, H. Zhang, J. Lu, S. Ruan and Y. J. Zeng, Nanotechnology, 2020, 31, 162001 CAS.
- G. He, Y. Duan and H. Pang, Nano-Micro Lett., 2020, 12, 57 CrossRef CAS.
- B. Deng, Z. Xiang, J. Xiong, Z. Liu, L. Yu and W. Lu, Nano-Micro Lett., 2020, 12, 55 CrossRef CAS.
- Z. Xiang, J. Xiong, B. Deng, E. Cui, L. Yu, Q. Zeng, K. Pei, R. Che and W. Lu, J. Mater. Chem. C, 2020, 8, 2123–2134 RSC.
- X. Wang, Y. Lu, T. Zhu, S. Chang and W. Wang, Chem. Eng. J., 2020, 388, 124317 CrossRef CAS.
- X. Shi, Z. Liu, W. You, X. Zhao and R. Che, J. Mater. Chem. C, 2018, 6, 7790–7796 RSC.
- Y. Jiang, X. Xie, Y. Chen, Y. Liu, R. Yang and G. Sui, J. Mater. Chem. C, 2018, 6, 8679–8687 RSC.
- Y. Duan, Z. Xiao, X. Yan, Z. Gao, Y. Tang, L. Hou, Q. Li, G. Ning and Y. Li, ACS Appl. Mater. Interfaces, 2018, 10, 40078–40087 CrossRef CAS.
- J.-C. Shu, X.-Y. Yang, X.-R. Zhang, X.-Y. Huang, M.-S. Cao, L. Li, H.-J. Yang and W.-Q. Cao, Carbon, 2020, 162, 157–171 CrossRef CAS.
- F. Meng, H. Wang, F. Huang, Y. Guo, Z. Wang, D. Hui and Z. Zhou, Composites, Part B, 2018, 137, 260–277 CrossRef CAS.
- X. Liang, W. Liu, Y. Cheng, J. Lv, S. Dai, D. Tang, B. Zhang and G. Ji, J. Alloys Compd., 2018, 749, 887–899 CrossRef CAS.
- H. Zhao, Y. Cheng, W. Liu, L. Yang, B. Zhang, L. P. Wang, G. Ji and Z. J. Xu, Nano-Micro Lett., 2019, 11, 24 CrossRef CAS.
- N. Li, G. W. Huang, Y. Q. Li, H. M. Xiao, Q. P. Feng, N. Hu and S. Y. Fu, ACS Appl. Mater. Interfaces, 2017, 9, 2973–2983 CrossRef CAS.
- S. K. Singh, M. J. Akhtar and K. K. Kar, ACS Appl. Mater. Interfaces, 2018, 10, 24816–24828 CrossRef CAS.
- L. Wang, X. Li, Q. Li, Y. Zhao and R. Che, ACS Appl. Mater. Interfaces, 2018, 10, 22602–22610 CrossRef CAS.
- Y. Wang, X. Gao, X. Wu, W. Zhang, C. Luo and P. Liu, Chem. Eng. J., 2019, 375, 121942 CrossRef CAS.
- Y. Wang, X. Di, X. Gao, X. Wu and P. Wang, J. Alloys Compd., 2020, 843, 156031 CrossRef CAS.
- Y. Wang, X. Di, X. Wu and X. Li, J. Alloys Compd., 2020, 846, 156215 CrossRef CAS.
- Y. Wang, X. Gao, Y. Fu, X. Wu, Q. Wang, W. Zhang and C. Luo, Composites, Part B, 2019, 169, 221–228 CrossRef CAS.
- A. Chaudhary, S. Kumari, R. Kumar, S. Teotia, B. P. Singh, A. P. Singh, S. K. Dhawan and S. R. Dhakate, ACS Appl. Mater. Interfaces, 2016, 8, 10600–10608 CrossRef CAS.
- M.-M. Lu, W.-Q. Cao, H.-L. Shi, X.-Y. Fang, J. Yang, Z.-L. Hou, H.-B. Jin, W.-Z. Wang, J. Yuan and M.-S. Cao, J. Mater. Chem. A, 2014, 2, 10540 RSC.
- F. Wen, F. Zhang and Z. Liu, J. Phys. Chem. C, 2011, 115, 14025–14030 CrossRef CAS.
- M.-S. Cao, W.-L. Song, Z.-L. Hou, B. Wen and J. Yuan, Carbon, 2010, 48, 788–796 CrossRef CAS.
- W.-l. Song, M.-s. Cao, Z.-l. Hou, J. Yuan and X.-y. Fang, Scr. Mater., 2009, 61, 201–204 CrossRef CAS.
- Y. Liu, Z. Chen, Y. Zhang, R. Feng, X. Chen, C. Xiong and L. Dong, ACS Appl. Mater. Interfaces, 2018, 10, 13860–13868 CrossRef CAS.
- Y. Jiang, Y. Chen, Y.-J. Liu and G.-X. Sui, Chem. Eng. J., 2018, 337, 522–531 CrossRef CAS.
- Y. Zheng, X. Wang, S. Wei, B. Zhang, M. Yu, W. Zhao and J. Liu, Composites, Part A, 2017, 95, 237–247 CrossRef CAS.
- W. Feng, Y. Wang, J. Chen, L. Wang, L. Guo, J. Ouyang, D. Jia and Y. Zhou, Carbon, 2016, 108, 52–60 CrossRef CAS.
- Y. Zhang, Y. Huang, T. Zhang, H. Chang, P. Xiao, H. Chen, Z. Huang and Y. Chen, Adv. Mater., 2015, 27, 2049–2053 CrossRef CAS.
- M. Zhang, X. Fang, Y. Zhang, J. Guo, C. Gong, D. Estevez, F. Qin and J. Zhang, Nanotechnology, 2020, 31, 275707 CrossRef CAS.
- X. Xu, F. Ran, Z. Fan, Z. Cheng, T. Lv, L. Shao and Y. Liu, ACS Appl. Mater. Interfaces, 2020, 12, 17870–17880 CrossRef CAS.
- S. Xiao, C. Zhou, X. Ye, Z. Lian, N. Zhang, J. Yang, W. Chen and H. Li, ACS Appl. Mater. Interfaces, 2020, 12, 32604–32614 CrossRef CAS.
- C. Wu, Z. Chen, M. Wang, X. Cao, Y. Zhang, P. Song, T. Zhang, X. Ye, Y. Yang, W. Gu, J. Zhou and Y. Huang, Small, 2020, 16, 2001686 CrossRef CAS.
- M. Ma, W. Li, Z. Tong, Y. Yang, Y. Ma, Z. Cui, R. Wang, P. Lyu and W. Huang, Mater. Des., 2020, 188, 108462 CrossRef CAS.
- J. Hu, Y. Shen, L. Xu and Y. Liu, Chem. Phys. Lett., 2020, 739, 136953 CrossRef CAS.
- H. Duan, H. Zhu, J. Gao, D.-X. Yan, K. Dai, Y. Yang, G. Zhao, Y. Liu and Z.-M. Li, J. Mater. Chem. A, 2020, 8, 9146–9159 RSC.
- S. Dong, X. Zhang, X. Li, J. Chen, P. Hu and J. Han, Chem. Eng. J., 2020, 392, 123817 CrossRef CAS.
- Y. Chen, P. Potschke, J. Pionteck, B. Voit and H. Qi, ACS Appl. Mater. Interfaces, 2020, 12, 22088–22098 CrossRef CAS.
- Z. Zhang, Q. Lv, Y. Chen, H. Yu, H. Liu, G. Cui, X. Sun and L. Li, Nanomaterials, 2019, 9, 833 CrossRef CAS.
- D. Zhang, T. Liu, J. Cheng, Q. Cao, G. Zheng, S. Liang, H. Wang and M.-S. Cao, Nano-Micro Lett., 2019, 11, 38 CrossRef.
- H. Wang and H. Ma, Nanotechnology, 2019, 31, 095711 CrossRef.
- J. Ma, J. Shu, W. Cao, M. Zhang, X. Wang, J. Yuan and M. Cao, Composites, Part B, 2019, 166, 187–195 CrossRef CAS.
- J. Luo, Y. Hu, L. Xiao, G. Zhang, H. Guo, G. Hao and W. Jiang, Nanotechnology, 2019, 31, 085708 CrossRef.
- J. Ma, X. Wang, W. Cao, C. Han, H. Yang, J. Yuan and M. Cao, Chem. Eng. J., 2018, 339, 487–498 CrossRef CAS.
- J. Li, Y. Xie, W. Lu and T.-W. Chou, Carbon, 2018, 129, 76–84 CrossRef CAS.
- P. Zhou, J. Wan, X. Wang, K. Xu, Y. Gong and L. Chen, J. Colloid Interface Sci., 2020, 575, 96–107 CrossRef CAS.
- H. Y. Zhou, Z. Y. Sui, F. L. Zhao, Y. N. Sun, H. Y. Wang and B. H. Han, Nanotechnology, 2020, 31, 315601 CrossRef CAS.
- W. Zhao, Y. Shen, H. Zhang, Y. Wang, Y. Wu, H. Wu, M. Zou, Q. Wang, Y. Li and A. Cao, ACS Appl. Mater. Interfaces, 2020, 12, 27045–27054 CrossRef CAS.
- L. Tong, L. L. Zhang, Y. C. Wang, L. Y. Wan, Q. Q. Yan, C. Hua, C. J. Jiao, Z. Y. Zhou, Y. W. Ding, B. Liu and H. W. Liang, ACS Appl. Mater. Interfaces, 2020, 12, 25211–25220 CrossRef CAS.
- J. Li, K. Han, D. Wang, Z. Teng, Y. Cao, J. Qi, M. Li and M. Wang, Carbon, 2020, 164, 42–50 CrossRef CAS.
- G.-Y. Hou, Z.-Y. Lyu, Y.-P. Tang, H.-Z. Cao and G.-Q. Zheng, Int. J. Hydrogen Energy, 2020, 45, 16049–16059 CrossRef CAS.
- N. Guo, W. Luo, R. Guo, D. Qiu, Z. Zhao, L. Wang, D. Jia and J. Guo, J. Alloys Compd., 2020, 834, 155115 CrossRef CAS.
- Y. Zhai, J. Wang, Q. Gao, Y. Fan, C. Hou, Y. Hou, H. Liu, Q. Shao, S. Wu, L. Zhao, T. Ding, F. Dang and Z. Guo, J. Catal., 2019, 377, 534–542 CrossRef CAS.
- L. Miao, X. Qian, D. Zhu, T. Chen, G. Ping, Y. Lv, W. Xiong, Y. Liu, L. Gan and M. Liu, Chin. Chem. Lett., 2019, 30, 1445–1449 CrossRef CAS.
- M. Culebras, H. Geaney, A. Beaucamp, P. Upadhyaya, E. Dalton, K. M. Ryan and M. N. Collins, ChemSusChem, 2019, 12, 4516–4521 CrossRef CAS.
- Y. Cheng, J. Cao, Y. Li, Z. Li, H. Zhao, G. Ji and Y. Du, ACS Sustainable Chem. Eng., 2017, 6, 1427–1435 CrossRef.
- C. Liang and Z. Wang, Chem. Eng. J., 2019, 373, 598–605 CrossRef CAS.
- R. Wang, M. He, Y. Zhou, S. Nie, Y. Wang, W. Liu, Q. He, W. Wu, X. Bu and X. Yang, Carbon, 2020, 156, 378–388 CrossRef CAS.
- H.-B. Zhao, J.-B. Cheng and Y.-Z. Wang, J. Alloys Compd., 2018, 736, 71–79 CrossRef CAS.
- X. Ye, Z. Chen, S. Ai, B. Hou, J. Zhang, X. Liang, Q. Zhou, H. Liu and S. Cui, J. Alloys Compd., 2019, 791, 883–891 CrossRef CAS.
- Y. Cheng, H. Zhao, Y. Zhao, J. Cao, J. Zheng and G. Ji, Carbon, 2020, 161, 870–879 CrossRef CAS.
- H. Xu, X. Yin, M. Zhu, M. Han, Z. Hou, X. Li, L. Zhang and L. Cheng, ACS Appl. Mater. Interfaces, 2017, 9, 6332–6341 CrossRef CAS.
- Y. Huang, Y. Wang, Z. Li, Z. Yang, C. Shen and C. He, J. Phys. Chem. C, 2014, 118, 26027–26032 CrossRef CAS.
- D. Li, H. Liao, H. Kikuchi and T. Liu, ACS Appl. Mater. Interfaces, 2017, 9, 44704–44714 CrossRef CAS.
- Z. Wu, K. Tian, T. Huang, W. Hu, F. Xie, J. Wang, M. Su and L. Li, ACS Appl. Mater. Interfaces, 2018, 10, 11108–11115 CrossRef CAS.
- L. Wang, P. Zhou, Y. Guo, J. Zhang, X. Qiu, Y. Guan, M. Yu, H. Zhu and Q. Zhang, RSC Adv., 2019, 9, 9718–9728 RSC.
- W.-L. Song, M.-S. Cao, L.-Z. Fan, M.-M. Lu, Y. Li, C.-Y. Wang and H.-F. Ju, Carbon, 2014, 77, 130–142 CrossRef CAS.
- J. Chen, X. Liang, W. Liu, W. Gu, B. Zhang and G. Ji, Dalton Trans., 2019, 48, 10145–10150 RSC.
- Y. Cheng, Z. Li, Y. Li, S. Dai, G. Ji, H. Zhao, J. Cao and Y. Du, Carbon, 2018, 127, 643–652 CrossRef CAS.
- X. Zhou, Z. Jia, A. Feng, X. Wang, J. Liu, M. Zhang, H. Cao and G. Wu, Carbon, 2019, 152, 827–836 CrossRef CAS.
- Z. Zhang, H. Zhao, W. Gu, L. Yang and B. Zhang, Sci. Rep., 2019, 9, 18617 CrossRef CAS.
- L. Wang, H. Guan, J. Hu, Q. Huang, C. Dong, W. Qian and Y. Wang, J. Alloys Compd., 2019, 803, 1119–1126 CrossRef CAS.
- Y. Jiang, X. Fu, Z. Zhang, G. Fan, Y. Liu, W. Du, X. Wang, C. Cheng and R. Fan, J. Mater. Sci.: Mater. Electron., 2019, 30, 19173–19181 CrossRef CAS.
- Y. Wei, H. Liu, S. Liu, M. Zhang, Y. Shi, J. Zhang, L. Zhang and C. Gong, Compos. Commun., 2018, 9, 70–75 CrossRef.
- J. Xi, E. Zhou, Y. Liu, W. Gao, J. Ying, Z. Chen and C. Gao, Carbon, 2017, 124, 492–498 CrossRef CAS.
- J. Fang, Y. Shang, Z. Chen, W. Wei, Y. Hu, X. Yue and Z. Jiang, J. Mater. Chem. C, 2017, 5, 4695–4705 RSC.
- X. Qiu, L. Wang, H. Zhu, Y. Guan and Q. Zhang, Nanoscale, 2017, 9, 7408–7418 RSC.
- M. S. Cao, X. X. Wang, M. Zhang, J. C. Shu, W. Q. Cao, H. J. Yang, X. Y. Fang and J. Yuan, Adv. Funct. Mater., 2019, 29, 1807398 CrossRef.
- B. Quan, W. Shi, S. J. H. Ong, X. Lu, P. L. Wang, G. Ji, Y. Guo, L. Zheng and Z. J. Xu, Adv. Funct. Mater., 2019, 29, 1901236 CrossRef.
- X. Zhang, J. Qiao, J. Zhao, D. Xu, F. Wang, C. Liu, Y. Jiang, L. Wu, P. Cui, L. Lv, Q. Wang, W. Liu, Z. Wang and J. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 35959–35968 CrossRef CAS.
- X. Cui, X. Liang, J. Chen, W. Gu, G. Ji and Y. Du, Carbon, 2020, 156, 49–57 CrossRef CAS.
- X. Cui, X. Liang, W. Liu, W. Gu, G. Ji and Y. Du, Chem. Eng. J., 2020, 381, 122589 CrossRef CAS.
- A. Bhatnagar, W. Hogland, M. Marques and M. Sillanpää, Chem. Eng. J., 2013, 219, 499–511 CrossRef CAS.
- D. Liu, R. Qiang, Y. Du, Y. Wang, C. Tian and X. Han, J. Colloid Interface Sci., 2018, 514, 10–20 CrossRef CAS.
- D. Ding, Y. Wang, X. Li, R. Qiang, P. Xu, W. Chu, X. Han and Y. Du, Carbon, 2017, 111, 722–732 CrossRef CAS.
- Y. Wang, Y. Du, R. Qiang, C. Tian, P. Xu and X. Han, Adv. Mater. Interfaces, 2016, 3, 1500684 CrossRef.
- R. Qiang, Y. Du, Y. Wang, N. Wang, C. Tian, J. Ma, P. Xu and X. Han, Carbon, 2016, 98, 599–606 CrossRef CAS.
- J. B. Cheng, H. B. Zhao, M. Cao, M. E. Li, A. N. Zhang, S. L. Li and Y. Z. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 26301–26312 CrossRef CAS.
- T. Zhang, D. Zhao, L. Wang, R. Meng, H. Zhao, P. Zhou, L. Xia, B. Zhong, H. Wang and G. Wen, J. Alloys Compd., 2020, 819, 153269 CrossRef CAS.
- P. Zhou, X. Wang, L. Wang, J. Zhang, Z. Song, X. Qiu, M. Yu and Q. Zhang, J. Alloys Compd., 2019, 805, 1071–1080 CrossRef CAS.
- H. Wang, Y. Zhang, Q. Wang, C. Jia, P. Cai, G. Chen, C. Dong and H. Guan, RSC Adv., 2019, 9, 9126–9135 RSC.
- Z. Song, X. Liu, X. Sun, Y. Li, X. Nie, W. Tang, R. Yu and J. Shui, Carbon, 2019, 151, 36–45 CrossRef CAS.
- P. Hu, S. Dong, X. Li, J. Chen, X. Zhang, P. Hu and S. Zhang, J. Mater. Chem. C, 2019, 7, 9219–9228 RSC.
- H. Guan, H. Wang, Y. Zhang, C. Dong, G. Chen, Y. Wang and J. Xie, Appl. Surf. Sci., 2018, 447, 261–268 CrossRef CAS.
- X. Zhang, J. Xu, X. Liu, S. Zhang, H. Yuan, C. Zhu, X. Zhang and Y. Chen, Carbon, 2019, 155, 233–242 CrossRef CAS.
- L. Wang, Y. Guan, X. Qiu, H. Zhu, S. Pan, M. Yu and Q. Zhang, Chem. Eng. J., 2017, 326, 945–955 CrossRef CAS.
- Y. Cheng, J. Z. Y. Seow, H. Zhao, Z. J. Xu and G. Ji, Nano-Micro Lett., 2020, 12, 102 CrossRef.
- T. Huang, Z. Wu, Q. Yu, D. Tan and L. Li, Chem. Eng. J., 2019, 359, 69–78 CrossRef CAS.
- X. Liang, Z. Man, B. Quan, J. Zheng, W. Gu, Z. Zhang and G. Ji, Nano-Micro Lett., 2020, 12, 125 CrossRef.
- Z. Zhang, Y. Lv, X. Chen, Z. Wu, Y. He, L. Zhang and Y. Zou, J. Magn. Magn. Mater., 2019, 487, 165334 CrossRef CAS.
- X. Xu, F. Ran, H. Lai, Z. Cheng, T. Lv, L. Shao and Y. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 35999–36009 CrossRef CAS.
- J. Ouyang, Z. He, Y. Zhang, H. Yang and Q. Zhao, ACS Appl. Mater. Interfaces, 2019, 11, 39304–39314 CrossRef CAS.
- W. Liu, L. Liu, Z. Yang, J. Xu, Y. Hou and G. Ji, ACS Appl. Mater. Interfaces, 2018, 10, 8965–8975 CrossRef CAS.
- W. Liu, Q. Shao, G. Ji, X. Liang, Y. Cheng, B. Quan and Y. Du, Chem. Eng. J., 2017, 313, 734–744 CrossRef CAS.
- W. Liu, L. Liu, G. Ji, D. Li, Y. Zhang, J. Ma and Y. Du, ACS Sustainable Chem. Eng., 2017, 5, 7961–7971 CrossRef CAS.
- Y. Yin, X. Liu, X. Wei, R. Yu and J. Shui, ACS Appl. Mater. Interfaces, 2016, 8, 34686–34698 CrossRef CAS.
- R. Qiang, Y. Du, D. Chen, W. Ma, Y. Wang, P. Xu, J. Ma, H. Zhao and X. Han, J. Alloys Compd., 2016, 681, 384–393 CrossRef CAS.
- J. Ma, X. Zhang, W. Liu and G. Ji, J. Mater. Chem. C, 2016, 4, 11419–11426 RSC.
- X. Zhang, G. Ji, W. Liu, B. Quan, X. Liang, C. Shang, Y. Cheng and Y. Du, Nanoscale, 2015, 7, 12932–12942 RSC.
- Q. Wu, J. Wang, H. Jin, T. Yan, G. Yi, X. Su, W. Dai and X. Wang, Mater. Lett., 2019, 244, 138–141 CrossRef CAS.
- X. Liang, B. Quan, Y. Sun, G. Ji, Y. Zhang, J. Ma, D. Li, B. Zhang and Y. Du, Part. Part. Syst. Charact., 2017, 34, 1700006 CrossRef.
- X. Liang, B. Quan, G. Ji, W. Liu, Y. Cheng, B. Zhang and Y. Du, Inorg. Chem. Front., 2016, 3, 1516–1526 RSC.
- L. Wang, X. Yu, X. Li, J. Zhang, M. Wang and R. Che, Chem. Eng. J., 2020, 383, 123099 CrossRef CAS.
- X. Li, E. Cui, Z. Xiang, L. Yu, J. Xiong, F. Pan and W. Lu, J. Alloys Compd., 2020, 819, 152952 CrossRef CAS.
- X. Zhang, G. Ji, W. Liu, X. Zhang, Q. Gao, Y. Li and Y. Du, J. Mater. Chem. C, 2016, 4, 1860–1870 RSC.
- W. Liu, S. Tan, Z. Yang and G. Ji, ACS Appl. Mater. Interfaces, 2018, 10, 31610–31622 CrossRef CAS.
- L. Guo, Q.-D. An, Z.-Y. Xiao, S.-R. Zhai, W.-J. Cai, H.-S. Wang and Z.-C. Li, J. Alloys Compd., 2020, 812, 152167 CrossRef CAS.
- X. Ye, Z. Chen, S. Ai, B. Hou, J. Zhang, X. Liang, Q. Zhou, H. Liu and S. Cui, J. Adv. Ceram., 2019, 8, 479–488 CrossRef CAS.
- J. Yan, Y. Huang, C. Chen, X. Liu and H. Liu, Carbon, 2019, 152, 545–555 CrossRef CAS.
- Y. Cheng, H. Zhao, H. Lv, T. Shi, G. Ji and Y. Hou, Adv. Electron. Mater., 2019, 6, 1900796 CrossRef.
- P. Xie, H. Li, B. He, F. Dang, J. Lin, R. Fan, C. Hou, H. Liu, J. Zhang, Y. Ma and Z. Guo, J. Mater. Chem. C, 2018, 6, 8812–8822 RSC.
- H. B. Zhao, Z. B. Fu, H. B. Chen, M. L. Zhong and C. Y. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 1468–1477 CrossRef CAS.
- C. Liu, Y. Duan, J. Cai, X. Li, D. Zhang, J. Gao and Y. Che, Mater. Res. Express, 2019, 6, 106114 CrossRef CAS.
- N. Yang, Z. X. Luo, G. R. Zhu, S. C. Chen, X. L. Wang, G. Wu and Y. Z. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 35987–35998 CrossRef CAS.
- C. Li, J. Sui, Z. Zhang, X. Jiang, Z. Zhang and L. Yu, Chem. Eng. J., 2019, 375, 122017 CrossRef CAS.
- X. Liang, B. Quan, Z. Man, B. Cao, N. Li, C. Wang, G. Ji and T. Yu, ACS Appl. Mater. Interfaces, 2019, 11, 30228–30233 CrossRef CAS.
- W. Gu, J. Tan, J. Chen, Z. Zhang, Y. Zhao, J. Yu and G. Ji, ACS Appl. Mater. Interfaces, 2020, 12, 28727–28737 CrossRef CAS.
- J. Lv, X. Liang, G. Ji, B. Quan, W. Liu and Y. Du, ACS Sustainable Chem. Eng., 2018, 6, 7239–7249 CrossRef CAS.
- X. Liang, B. Quan, J. Chen, W. Gu, B. Zhang and G. Ji, ACS Appl. Nano Mater., 2018, 1, 5712–5721 CrossRef CAS.
- H.-B. Zhao, J.-B. Cheng, J.-Y. Zhu and Y.-Z. Wang, J. Mater. Chem. C, 2019, 7, 441–448 RSC.
- J.-B. Cheng, W.-J. Yuan, A.-N. Zhang, H.-B. Zhao and Y.-Z. Wang, J. Mater. Chem. C, 2020 10.1039/d0tc03377d.
| This journal is © The Royal Society of Chemistry 2020 |
