Open Access Article
Open Access ArticleNew findings and current controversies on oxidation of benzyl alcohol by a copper complex†
Zahra
Zand
a,
Jitendra Pal
Singh
 b,
Robabeh
Bagheri
c,
Junfeng
Cui
de,
Keun Hwa
Chae
b,
Robabeh
Bagheri
c,
Junfeng
Cui
de,
Keun Hwa
Chae
 b,
Zhenlun
Song
b,
Zhenlun
Song
 c and
Mohammad Mahdi
Najafpour
c and
Mohammad Mahdi
Najafpour
 *afg
*afg
aDepartment of Chemistry, Institute for Advanced Studies in Basic Sciences (IASBS), Zanjan, 45137-66731, Iran. E-mail: mmnajafpour@iasbs.ac.ir; Tel: +98 24 3315 3201
bAdvanced Analysis Center, Korea Institute of Science and Technology (KIST), Seoul 02792, Republic of Korea
cKey Laboratory of Marine Materials and Related Technologies, Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, Ningbo 315201, China
dKey Laboratory for Precision and Non-Traditional Machining Technology of Ministry of Education, Dalian University of Technology, Dalian 116024, China
eSurface Protection Research Group, Surface Department, Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, 519 Zhuangshi Road, Ningbo 315201, China
fCenter of Climate Change and Global Warming, Institute for Advanced Studies in Basic Sciences (IASBS), Zanjan, 45137-66731, Iran
gResearch Center for Basic Sciences & Modern Technologies (RBST), Institute for Advanced Studies in Basic Sciences (IASBS), Zanjan 45137-66731, Iran
First published on 11th May 2020
Abstract
Herein, we report new findings and details for the alcohol-oxidizing activity of a copper complex in the presence of molecular oxygen. Using scanning electron microscopy, transmission electron microscopy, UV-vis spectroscopy, Fourier transform infrared spectroscopy, X-ray absorption near edge structure, extended X-ray absorption fine structure, and X-ray diffraction, it is suggested that the true catalyst for the reaction is a copper complex mixed with potassium carbonate.
Introduction
The oxidation of alcohols to carbonyl compounds is an important reaction, which is done typically using permanganate and dichromate salts. These salts are moderately corrosive, unstable, expensive, and toxic and show no selectivity. These salts also require hazardous procedures for preparation and over-oxidation to carboxylic acids is observed in the presence of these oxidants. For the first time, Semmelhack's group used CuCl (10 mol%) and TEMPO (10 mol%) to oxidize primary alcohols in the presence of molecular oxygen at room temperature.1 In 1996, Marko's group reported an efficient copper-based catalyst that oxidized many alcohols into aldehydes and ketones under mild conditions.2 The true catalyst under Marko's conditions is suggested to be a heterogeneous and adsorbed compound on K2CO3. Such oxidation reactions have been performed using many copper complexes with different ligands.3–42Marko's group proposed that an initial hydrogen-transfer reaction within the copper-alkoxide/azo complex generates the carbonyl-bound hydrazino-copper species, which produces a binuclear copper(II) peroxide upon reaction with oxygen. After homolytic cleavage followed by a hydrogen-atom abstraction, the hydroxy Cu(I) complex is formed (Scheme 1). The rapid exchange between the OH ligand and the alcohol regenerates the catalyst (Scheme 1).
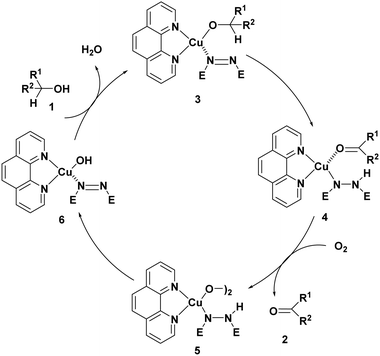 | ||
| Scheme 1 The proposed mechanism for the alcohol oxidation by Marko's complex. The role of diethylazodicarboxylate is the reduction of the copper(II) complex and the formation of the copper(I) complex. The image is modified from ref. 2. | ||
The clear role of K2CO3 or the true catalyst is not known; however, some points and questions are:
(i) HCl is formed during this reaction. K2CO3 is a base and could react with HCl.
(ii) K2CO3 is a support.
(iii) K2CO3 could act as a water scavenger.
(iv) What is the role of K2CO3?
(v) What is the true catalyst: Marko's complex, a Cu(II) complex, CuCl2, or CuCl?
(vi) What is the nature of the dark copper-based compound on K2CO3?
The finding of the true catalyst is important to design and synthesize new, stable, and efficient catalysts. In 2010, Liang et al. reported a procedure using “copper(II) chloride-cesium carbonate” to aerobically oxidize primary alcohols.43 Cs2CO3 and the solvents such as toluene and 1,2-dichloroethane play important roles to form a catalytically active intermediate containing a hydroxyl-bridged trinuclear copper moiety. From the X-ray crystal structure of trinuclear copper, an electrostatic interaction between Cl− and Cs+ was observed, which stimulated the suggestion of the roles of Cs2CO3 in this reaction as a base, and stabilizing and “solvating” intermediates in toluene and 1,2-dichloroethane.43
In this study, we report new findings for the alcohol-oxidizing activity of a Cu complex in the presence of molecular oxygen. Herein, using scanning electron microscopy (SEM), transmission electron microscopy (TEM), UV-vis spectroscopy, Fourier transform infrared spectroscopy (FTIR), X-ray absorption near edge structure (XANES), extended X-ray absorption fine structure (EXAFS), and X-ray diffraction (XRD), it is proposed that the true catalyst for the alcohol oxidation in the presence of Marko's complex is a mixture of the copper complex and potassium carbonate particles rather than nanosized copper oxide or copper carbonate.
Experimental
Materials and methods
See the ESI.†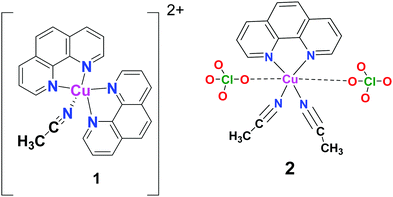 | ||
| Fig. 1 Schematic structures of 1 ([Cu(C12H8N2)2(CH3CN)](ClO4)2) and 2 ([Cu(ClO4)(C12H8N2)(CH3CN)2]ClO4).44,45 | ||
Catalytic procedure
Results and discussion
Recently, some studies have addressed the mechanism of oxidation by Marko's complex.46 However, more investigation on the true catalyst for this reaction is essential. A question is whether Cu(II) complexes with Phen ligands are active for the oxidation of benzyl alcohol to benzaldehyde under Marko's conditions or not. The oxidation of benzyl alcohol to benzaldehyde using K2CO3 as a base and 1 as the catalyst was selected as the model reaction following Marco's report in 1996.2 [Cu(Phen)2CH3CN(ClO4)2] (1) was simply obtained by the reaction of 1, Phen and Cu(ClO4)2 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, and blue-green crystals were grown in CH3CN. The crystal structure of 1 comprises two Phen ligands and one acetonitrile coordinated to CuII lying in a distorted square-pyramidal geometry (Fig. 1).44,45
1 ratio, and blue-green crystals were grown in CH3CN. The crystal structure of 1 comprises two Phen ligands and one acetonitrile coordinated to CuII lying in a distorted square-pyramidal geometry (Fig. 1).44,45
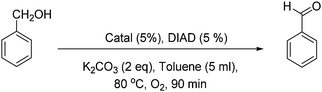
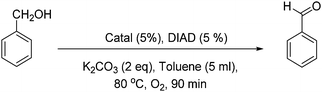
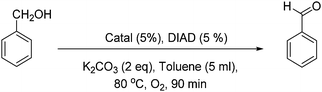
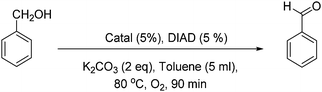
First, Marco's reaction was performed (Scheme 2a). As previously reported,2 the formation of a dark solid adsorbed on K2CO3 was observed, and alcohol oxidation was detected. 2 was inactive for the alcohol-oxidation reaction. However, 1 indicated alcohol-oxidizing activity in the presence of molecular oxygen (Scheme 2b) according to Marko's report.
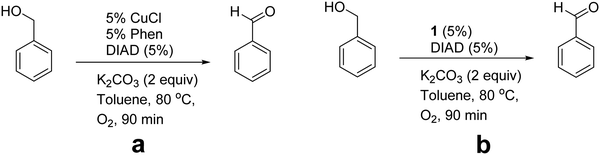 | ||
| Scheme 2 Schematic conditions of the alcohol-oxidation reaction: Marco's reaction (a) and in the presence of 1 under Marco's reaction conditions (b). | ||
First, the color of the solution of 1 in toluene is light green, but after 45 minutes under conditions similar to Marko's conditions (Scheme 2b) the color turns dark (Fig. S1, ESI†). The profile of the reaction was monitored by gas chromatography (Fig. 2). In the first 45 min, the alcohol-oxidation reaction is slow, but after the formation of a dark solid, an increase in the activity was observed. The highest yield of benzaldehyde (∼23%) was measured after 90 min. An important question in this regard is whether the dark solid is a true catalyst or a product of decomposition? The obtained dark precipitate was separated, rinsed three times with toluene, and resuspended in toluene and benzyl alcohol (BnOH) in the presence of O2 at 80 °C (Fig. 2). Immediately, benzaldehyde formation was observed, and the yield was 38% at 90 min. The result showed that this dark precipitate could perform the alcohol-oxidation reaction as a catalyst (Fig. 2). As shown in Table S1 (ESI†), CuO in the presence and absence of Phen could not oxidize benzyl alcohol.
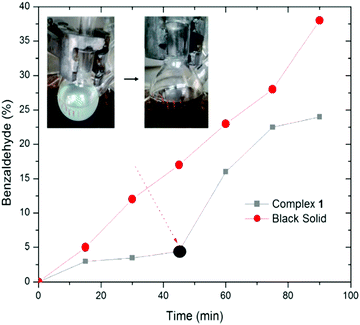 | ||
| Fig. 2 The reaction profiles of benzaldehyde formation by 1 and the centrifuged nanoparticles from the reaction of 1. The dark circle shows the time for the dark solid. | ||
As shown by Marko,2 azo compounds, such as diethyl azodicarboxylate or its analog, significantly improve the turnover for alcohol oxidation, the lifetime of the catalyst, and also the rate of the reaction. The role of DIAD is the reduction of the copper(II) complex and the formation of the copper(I) complex.
2 was not a catalyst under Marko's conditions (Table 1; for a complete table see Table S1, ESI,† entry 2), but 1 (entry 3) or the centrifuged nanoparticles from the reaction of 1 (solid 1) showed activity for the alcohol-oxidation reaction (entry 4). Replacing CuCl by Cu(ClO4)2 in Marko's reaction resulted in low activity for the alcohol-oxidation reaction (entry 5). No activity was observed in the presence of CuCl or CuI in the absence of Phen (entries 6 and 7). CuI in the presence of Phen indicated a high activity toward the alcohol-oxidation reaction (entry 8). K2Cu(CO3)2 or CuCO3 in the presence or absence of Phen could not oxidize benzyl alcohol. 1 performed the alcohol-oxidation reaction using other bases such as Na2CO3 and Cs2CO3, where the conversions of benzyl alcohol were the same. However, a poor conversion was achieved using Et3N. These results suggest that mixing the catalyst with K2CO3 plays an important role to increase the reactivity of the copper complex in alcohol oxidation.
Afterward, the dark solid obtained under the alcohol-oxidation reaction by 1 was analyzed by SEM, TEM, EDX, and XRD analyses.
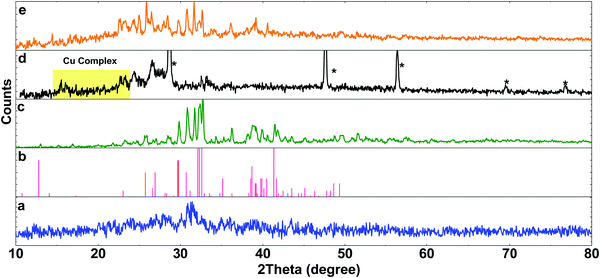
The SEM images of the obtained dark solid (solid 1) displayed nanoparticles without any special morphology in an average size of 30–200 nm (Fig. 3a and Fig. S3, ESI†). Both crystalline and amorphous particles could be detected in the SEM images. These two types of particles were significantly aggregated. According to the XRD patterns, the crystalline particles could be both K2CO3·1.5 H2O and 1 (Fig. 3b). Based on the EDX data, in addition to O and C, the obtained solid contained Cu and Cl (Fig. 3b and c).
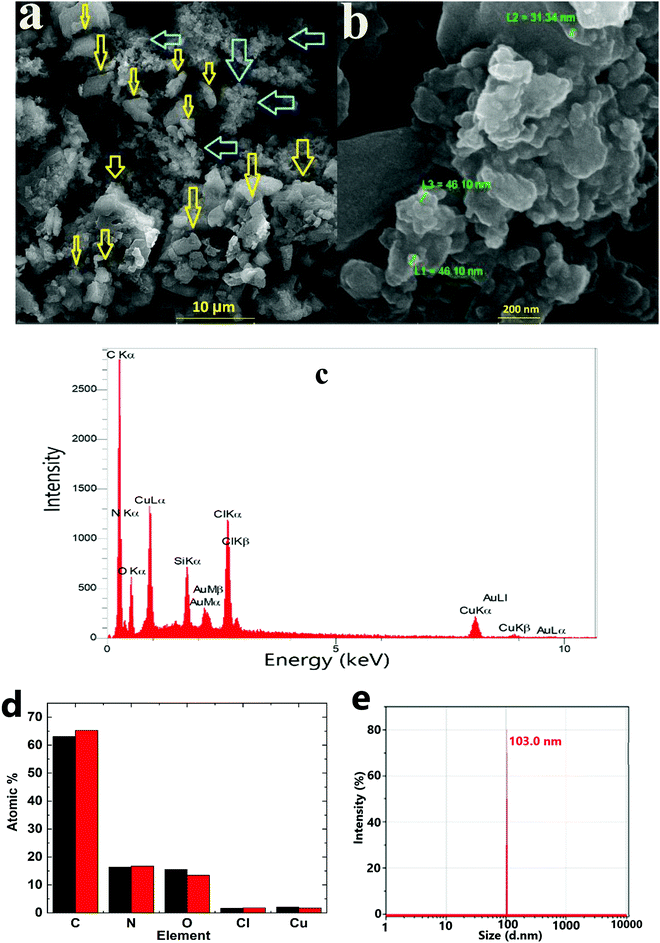 | ||
| Fig. 3 SEM images (a and b), EDX spectrum (c) and EDX results for two points (d). DLS (e) of solid 1 under Marko's conditions. Yellow and blue arrows show two different morphologies. | ||
90 min after the reaction of 1 with K2CO3, the formed particles were separated and investigated by dynamic light scattering (DLS). DLS in water indicated a diameter ∼100 nm for the nanoparticles. Compared with particles in SEM or TEM images, different sized particles were observed in DLS analysis (Fig. 3e). K2CO3 is soluble in water, and thus only Cu-containing particles could be investigated by DLS.
TEM of solid 1 showed two different particles: K-containing particles (light particles) and Cu-containing particles (dark particles) (Fig. 4a). As shown by the SEM images, these two types of particles were significantly aggregated. In Marko's report, it was suggested that the active catalyst is a heterogeneous catalyst supported on K2CO3.2 The TEM images confirmed the presence of nanoparticles in the size range 50–200 nm (Fig. 4a). As can be seen, dark nanoparticles (ca. 100 nm) are dispersed among big light particles (Fig. 4b and Fig. S4–S7, ESI†). Thus, TEM images similar to the SEM images showed a mixture of Cu and K-containing particles for solid 1 under Marko's conditions.
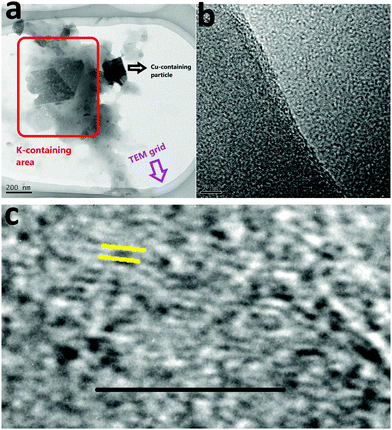 | ||
| Fig. 4 TEM (a) and (HR)TEM (b and c) of solid 1 under Marko's conditions. The scale bar in image (c) is 5 nm. | ||
The HRTEM image of solid 1 showed non-crystallinity of the nanoparticles (Fig. 4b), but in a few areas patterns with a d-spacing of 0.27 nm (Fig. 4c) corresponding to planes in the range 2θ: 30–33° for 1 or K2CO3 and in agreement with the powder X-ray diffraction (XRD) pattern were observed.
In FTIR spectra for 1 and solid 1, some peaks at 1400–1600 cm−1 were observed that are attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N/C
N/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, suggesting the intact structure of 1 (Fig. 5). The FTIR spectrum of phenanroline showed the stretching vibration peaks of C
C, suggesting the intact structure of 1 (Fig. 5). The FTIR spectrum of phenanroline showed the stretching vibration peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds at 1643 cm−1 and 1583 cm−1 and the skeleton vibration peak at 1555 cm−1, and the out-plane bending vibration peak of C–H bonds appears at 734 cm−1. Compared with the spectrum of phenanroline, the stretching vibration peaks for 1 were observed at 1626 (C
C double bonds at 1643 cm−1 and 1583 cm−1 and the skeleton vibration peak at 1555 cm−1, and the out-plane bending vibration peak of C–H bonds appears at 734 cm−1. Compared with the spectrum of phenanroline, the stretching vibration peaks for 1 were observed at 1626 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) and 1584 (C
N) and 1584 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) cm−1. The out-of-plane bending vibration peak of C–H bonds also shifts from 735 cm−1 in Phen to 720 cm−1 in the complex. After the reaction, the FTIR spectrum of solid 1 showed related peaks at 1620 and 1583 cm−1 attributed to C
C) cm−1. The out-of-plane bending vibration peak of C–H bonds also shifts from 735 cm−1 in Phen to 720 cm−1 in the complex. After the reaction, the FTIR spectrum of solid 1 showed related peaks at 1620 and 1583 cm−1 attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds in the metal complex. The out-of-plane bending vibration peak of C–H bonds was also observed at 720 cm−1.47 Thus, the FTIR experiment showed that the copper complex in solid 1 was intact.
C double bonds in the metal complex. The out-of-plane bending vibration peak of C–H bonds was also observed at 720 cm−1.47 Thus, the FTIR experiment showed that the copper complex in solid 1 was intact.
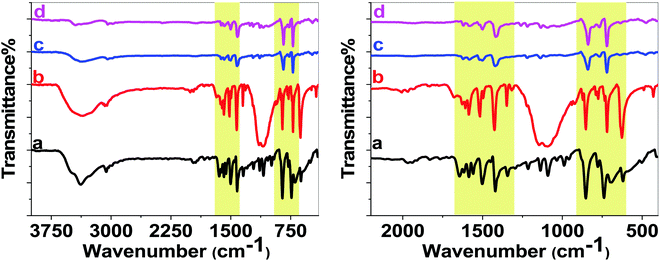 | ||
| Fig. 5 IR spectra of Phen (a), 1 (b) and solid 1 after a few seconds (c) and after 90 minutes (d) under Marko's conditions in different ranges. | ||
Marko's group also reported that the solution obtained from filtration of the heterogeneous mixture was devoid of any oxidizing ability. Conversely, the obtained precipitate indicated activity for the oxidation reaction.
The XRD patterns also showed the presence of a Cu(Phen) based-material on K2CO3 (Fig. 6d and e). It seems that Marko's conditions highly depend on forming Cu(Phene) in the presence of K2CO3. When K2CO3 was added after Cu(Phen) forming, the conversion of benzyl alcohol was achieved in lower yield (35% in 90 min).
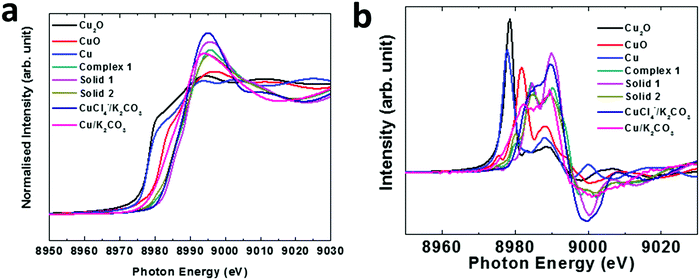
To further characterize the solid, XANES spectra were used. Fig. 7 illustrates the normalized XANES spectra along with derivative spectra (Fig. 7b) of various compounds.
The main edges of the dark compounds obtained in the reaction of complex 1 and 2 and solid 1 and 2, respectively, along with those of various materials, appear at higher energy than CuO. This reveals that the valence state of Cu ions is higher than that of 2. However, this shift is also associated with the presence of the ligand in complex organic systems.48,49 Thus, the ligand effect may be one of the reasons for the higher energy shift in these complex systems. The first derivative curve also exhibits a similar effect (Fig. 7b). To get information on bond distances for these materials, these spectra were simulated using a quick shell parameter fit. The fitted inverse Fourier transforms of the EXAFS spectra are shown in Fig. 8a. The values of the coordination number, N, and radial distance, R, are shown in Fig. 8b.
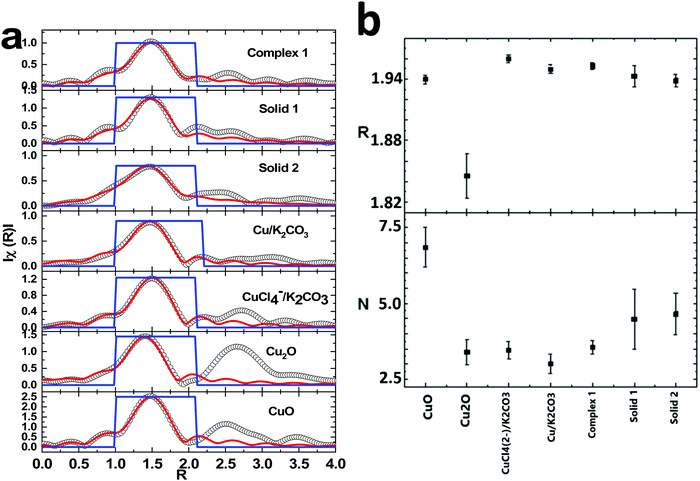 | ||
| Fig. 8 Inverse Fourier transform spectra of various materials along with oxides (a); coordination number and radial distances for different materials (b). | ||
Both the experimental and fitted data points are in agreement in the fitting range. The values of the coordination number and radial distances for the Cu–O shell for oxides like CuO and Cu2O are similar to reported values. In the case of solids 1 and 2, the values of the radial distances are 1.94 ± 0.01 and 1.938 ± 0.006 Å, respectively, with the coordination numbers being 4.5 ± 1.0 and 4.7 ± 0.7, respectively. However, compared to previous reports, the Cu–O bonds occur at 2.433 Å with a coordination number of 2 for solid 2, and Cu ions are surrounded by 4 N ion bonds having an average bond distance ∼1.98 Å, which are comparable to the values estimated for solids 1 and 2 (Fig. 8b). Thus, these materials contain Cu–N bonds. Based on the above observations, we infer that the true catalyst is the Cu complex mixed with K2CO3.
Finally, a question posed of Marko's catalyst is why such a system in the presence of a strong base is not efficient for alcohol oxidation. A simple experiment indicated that copper complexes with one Phen ligand such as 2 were simply decomposed or dimerized in the presence of KOH or NaOH (Fig. S4, ESI†).50–53 As we showed that a molecular structure is an active species in Marko's system, it is not surprising that strong bases such as KOH or NaOH are not appreciated under Marko's conditions. However, the alcohol-oxidation reaction is usually very complicated and other factors could also be important.54,55
Conclusions
Using SEM, TEM, UV-vis spectroscopy, FTIR, XANES, EXAFS, and XRD analyses, we conclude that a Cu molecule-based complex is the true catalyst for the alcohol-oxidation reaction under Marko's conditions in the presence of molecular oxygen. In the presence of K2CO3 and 1, a solid was observed. Characterization of the solid showed that it is a Cu(II) complex mixed with K2CO3. It seems that heterogeneous catalysts are more complicated than we thought before.According to our experiments:
(i) Cu(I) rather than Cu(II) is critical for the reaction.
(ii) Phen is important for the reaction.
(iii) The stable salts such as CuCO3, CuO and K2Cu(CO3)2 showed no activity.
(iv) The active catalyst was mixed with K2CO3.
(v) In Marko's conditions, a Cu(I) complex in the presence of oxygen is oxidized to Cu(II). It is suggested that the adduct is produced by the reaction of oxygen and the Cu(I) complex for the alcohol-oxidation reaction because a Cu(II) complex such as 1 or 2 is not highly active under Marko's conditions.
(vi) Nanosized K2CO3 significantly mixed with the complex.
Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
The authors are grateful to the Institute for Advanced Studies in Basic Sciences and the National Elite Foundation for financial support.References
- M. F. Semmelhack, C. R. Schmid, D. A. Cortes and C. S. Chou, J. Am. Chem. Soc., 1984, 106, 3374–3376 CrossRef CAS.
- I. E. Marko, P. R. Giles, M. Tsukazaki, S. M. Brown and C. J. Urch, Science, 1996, 274, 2044–2046 CrossRef CAS PubMed.
- D. S. Sigman, A. Mazumder and D. M. Perrin, Chem. Rev., 1993, 93, 2295–2316 CrossRef CAS.
- I. E. Marko, P. R. Giles, M. Tsukazaki, I. Chelle-Regnaut, A. Gautier, R. Dumeunier, F. Philippart, K. Doda, J.-L. Mutonkole and S. M. Brown, Adv. Inorg. Chem., 2004, 56, 211–240 CrossRef CAS.
- S. E. Allen, R. R. Walvoord, R. Padilla-Salinas and M. C. Kozlowski, Chem. Rev., 2013, 113, 6234–6458 CrossRef CAS PubMed.
- Q. Cao, L. M. Dornan, L. Rogan, N. L. Hughes and M. J. Muldoon, Chem. Commun., 2014, 50, 4524–4543 RSC.
- I. E. Markó, M. Tsukazaki, P. R. Giles, S. M. Brown and C. J. Urch, Angew. Chem., Int. Ed. Engl., 1997, 36, 2208–2210 CrossRef.
- I. E. Marko, A. Gautier, R. Dumeunier, K. Doda, F. Philippart, S. M. Brown and C. J. Urch, Angew. Chem., Int. Ed., 2004, 43, 1588–1591 CrossRef CAS PubMed.
- P. Gamez, I. W. Arends, R. A. Sheldon and J. Reedijk, Adv. Synth. Catal., 2004, 346, 805–811 CrossRef CAS.
- E. T. Kumpulainen and A. M. Koskinen, Chem. – Eur. J., 2009, 15, 10901–10911 CrossRef CAS PubMed.
- J. E. Steves and S. S. Stahl, J. Am. Chem. Soc., 2013, 135, 15742–15745 CrossRef CAS PubMed.
- M. T. Musser, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KgaA, Weinheim, Germany, 6th edn, 2011, vol. 11, p. 49 Search PubMed.
- D. H. Barton, S. D. Bévière and D. R. Hill, Tetrahedron, 1994, 50, 2665–2670 CrossRef CAS.
- U. Schuchardt, R. Pereira and M. C. Rufo, J. Mol. Catal. A: Chem., 1998, 135, 257–262 CrossRef CAS.
- S.-I. Murahashi, Y. Oda, T. Naota and N. Komiya, J. Chem. Soc., Chem. Commun., 1993, 139–140 RSC.
- G. Glatz, T. Schmalz, T. Kraus, F. Haarmann, G. Motz and R. Kempe, Chem. – Eur. J., 2010, 16, 4231–4238 CrossRef CAS PubMed.
- C. Evangelisti, G. Vitulli, E. Schiavi, M. Vitulli, S. Bertozzi, P. Salvadori, L. Bertinetti and G. Martra, Catal. Lett., 2007, 116, 57–62 CrossRef CAS.
- T. Balandina, T. Y. Larina, N. Kuznetsova and B. Bal’zhinimaev, Kinet. Catal., 2008, 49, 499–505 CrossRef CAS.
- G. B. Shul’pin, M. M. Bochkova and G. V. Nizova, J. Chem. Soc., Perkin Trans. 2, 1995, 1465–1469 RSC.
- K. Takaki, J. Yamamoto, K. Komeyama, T. Kawabata and K. Takehira, Bull. Chem. Soc. Jpn., 2004, 77, 2251–2255 CrossRef CAS.
- Y. Li, M. Wu, W. Liu, Z. Yi and J. Zhang, Catal. Lett., 2008, 123, 123–128 CrossRef CAS.
- I. E. Markó, A. Gautier, I. Chellé-Regnaut, P. R. Giles, M. Tsukazaki, C. J. Urch and S. M. Brown, J. Org. Chem., 1998, 63, 7576–7577 CrossRef.
- Y. Kurusu and D. Neckers, J. Org. Chem., 1991, 56, 1981–1983 CrossRef CAS.
- T. Tsuritani, N. A. Strotman, Y. Yamamoto, M. Kawasaki, N. Yasuda and T. Mase, Org. Lett., 2008, 10, 1653–1655 CrossRef CAS PubMed.
- Y. Li, L. X. Gao and F. S. Han, Chem. – Eur. J., 2010, 16, 7969–7972 CrossRef CAS PubMed.
- X. Q. Yu, Y. Yamamoto and N. Miyaura, Chem. – Asian J., 2008, 3, 1517–1522 CrossRef CAS PubMed.
- K. Nishiura, Y. Urawa and S. Soda, Adv. Synth. Catal., 2004, 346, 1679–1684 CrossRef CAS.
- A. Deagostino, C. Prandi, C. Zavattaro and P. Venturello, Eur. J. Org. Chem., 2007, 1318–1323 CrossRef CAS.
- T. D. Quach and R. A. Batey, Org. Lett., 2003, 5, 4397–4400 CrossRef CAS PubMed.
- G. W. Kabalka and L.-L. Zhou, Lett. Org. Chem., 2006, 3, 320–323 CrossRef CAS.
- N. Joubert, E. Baslé, M. Vaultier and M. Pucheault, Tetrahedron Lett., 2010, 51, 2994–2997 CrossRef CAS.
- Y. Bolshan and R. A. Batey, Angew. Chem., Int. Ed., 2008, 47, 2109–2112 CrossRef CAS PubMed.
- Y. Bolshan and R. A. Batey, Tetrahedron, 2010, 66, 5283–5294 CrossRef CAS.
- A. Y. Fedorov and J.-P. Finet, Tetrahedron Lett., 1999, 40, 2747–2748 CrossRef CAS.
- R.-J. Song, C.-L. Deng, Y.-X. Xie and J.-H. Li, Tetrahedron Lett., 2007, 48, 7845–7848 CrossRef CAS.
- R. Keder, H. Dvořáková and D. Dvořák, Eur. J. Org. Chem., 2009, 1522–1531 CrossRef CAS.
- A. K. Bakkestuen and L.-L. Gundersen, Tetrahedron Lett., 2003, 44, 3359–3362 CrossRef CAS.
- S. Benard, L. Neuville and J. Zhu, Chem. Commun., 2010, 46, 3393–3395 RSC.
- P. Y. Lam, D. Bonne, G. Vincent, C. G. Clark and A. P. Combs, Tetrahedron Lett., 2003, 44, 1691–1694 CrossRef CAS.
- S. Yu, J. Saenz and J. K. Srirangam, J. Org. Chem., 2002, 67, 1699–1702 CrossRef CAS PubMed.
- W. Li, Y. Fan, Y. Xia, P. Rocchi, R. Zhu, F. Qu, J. Neyts, J. L. Iovanna and L. Peng, Helv. Chim. Acta, 2009, 92, 1503–1513 CrossRef CAS.
- Y. Hari, Y. Shoji and T. Aoyama, Tetrahedron Lett., 2005, 46, 3771–3774 CrossRef CAS.
- L. Liang, G. Rao, H.-L. Sun and J.-L. Zhang, Adv. Synth. Catal., 2010, 352, 2371–2377 CrossRef CAS.
- M.-D. Şerb, B. Calmuschi-Cula, F. Dumitru, T. Dols, U. Englert and C. Guran, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2007, 63, m1292–m1293 CrossRef.
- M. D. Şerb, C. Guran, F. Dumitru, U. Englert and B. Calmuschi-Cula, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2007, 63, m1484–m1486 CrossRef.
- S. D. McCann and S. S. Stahl, J. Am. Chem. Soc., 2016, 138, 199–206 CrossRef CAS PubMed.
- X. Wang, G. Jia, Y. Yu, Y. Gao, W. Zhang, H. Wang, Z. Cao and J. Liu, Quim. Nova, 2015, 38, 298–302 CAS.
- T. L. Daulton and B. J. Little, Ultramicroscopy, 2006, 106, 561–573 CrossRef CAS.
- M. Tromp, J. Moulin and G. Reid, J. Evans, In AIP Conference Proceedings, 2007, 882, 699–701.
- B. N. Figgis, R. Mason, A. R. Smith, J. N. Varghese and G. A. Williams, J. Chem. Soc., Dalton Trans., 1983, 703–711 RSC.
- C. Harris and E. McKenzie, J. Inorg. Nucl. Chem., 1967, 29, 1047–1068 CrossRef CAS.
- D. Perrin and V. Sharma, J. Inorg. Nucl. Chem., 1966, 28, 1271–1278 CrossRef CAS.
- S. Mahadevan and M. Palaniandavar, Inorg. Chem., 1998, 37, 3927–3934 CrossRef CAS PubMed.
- M. Wang, F. Wang, J. Ma, M. Li, Z. Zhang, Y. Wang, X. Zhang and J. Xu, Chem. Commun., 2014, 50, 292–294 RSC.
- A. Abad, P. Concepción, A. Corma and H. García, Angew. Chem., Int. Ed., 2005, 44, 4066–4069 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ma00222d |
| This journal is © The Royal Society of Chemistry 2020 |