Microfluidic system for Caenorhabditis elegans culture and oxygen consumption rate measurements
Roger
Krenger
 ,
Matteo
Cornaglia
,
Thomas
Lehnert
and
Martin A. M.
Gijs
,
Matteo
Cornaglia
,
Thomas
Lehnert
and
Martin A. M.
Gijs
 *
*
Laboratory of Microsystems, Ecole Polytechnique Fédérale de Lausanne, CH-1015 Lausanne, Switzerland. E-mail: martin.gijs@epfl.ch
First published on 15th November 2019
Abstract
Mitochondrial respiration is a key signature for the assessment of mitochondrial functioning and mitochondrial dysfunction is related to many diseases including metabolic syndrome and aging-associated conditions. Here, we present a microfluidic Caenorhabditis elegans culture system with integrated luminescence-based oxygen sensing. The material used for the fabrication of the microfluidic chip is off-stoichiometry dual-cure thiol–ene–epoxy (OSTE+), which is well-suited for reliably recording on-chip oxygen consumption rates (OCR) due to its low gas permeability. With our microfluidic approach, it was possible to confine a single nematode in a culture chamber, starting from the L4 stage and studying it over a time span of up to 6 days. An automated protocol for successive worm feeding and OCR measurements during worm development was applied. We found an increase of OCR values from the L4 larval stage to adulthood, and a continuous decrease as the worm further ages. In addition, we performed a C. elegans metabolic assay in which exposure to the mitochondrial uncoupling agent FCCP increased the OCR by a factor of about two compared to basal respiration rates. Subsequent treatment with sodium azide inhibited completely mitochondrial respiration.
Introduction
Mitochondrial dysfunction is associated with many pathologies, such as diabetes mellitus, obesity and cardiovascular disease, but also neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, Huntington's disease and amyotrophic lateral sclerosis.1–7 Potential drug candidates for combatting such disorders need to be evaluated with respect to adverse effects on the mitochondria.8 Mitochondrial functioning, i.e. adenosine triphosphate (ATP) production in response to cellular energy demand, can be assayed by measuring the oxygen consumption rate (OCR) of an organism in vivo. Oxygen uptake can be considered as a direct indicator of cellular respiration indeed.9 Actually, measuring OCR to evaluate mitochondrial functioning on a whole-organism level has higher physiological relevance than looking at cell lines or even at isolated mitochondria, but the analysis of such results is more complex and leaves more room for interpretation. In this context, the nematode C. elegans is an organism of significant interest, as it is a convenient model organism for researching diseases and for whole-organism drug screening due to its genetic amenability and the availability of human disease models.10–12 Furthermore, worms are suitable for assays extending over their whole lifespan.Oxygen concentration control in on-chip applications involving worm culture is an important parameter for well-defined assay conditions, in particular for maintaining homeostasis and physiological conditions in general. Up to now, microfluidic culture and phenotyping platforms have been developed for a wide range of assays otherwise impossible with conventional methods, including measuring the lifespan of worms, monitoring embryonic and post-embryonic development, study of the sensory response or characterization of changes in the physiological state of worms upon chemical stimulation or drug exposure.13–16 These reviews present a large range of different microfluidic devices, including approaches for worm manipulation, immobilization, exposure to drugs or chemicals, imaging or other analytical techniques. Such devices are also widely used to study neuronal activity in C. elegans.17–19 Microfluidics enables performing these studies in precise environmental conditions in an automated and parallelized manner, often with high-throughput and/or single-organism resolution. A platform developed by Letizia et al., for example, allows evaluating parameters such as egg hatching rate, embryonic and larval development time, worm length, and, more importantly, the mitochondrial stress in mutant green fluorescent protein (GFP) expressing worms (hsp-6p::gfp) during their full lifespan.20 The authors used fluorescence imaging to quantify mitochondrial stress after inducing mitochondrial unfolded protein response in doxycycline-treated worms and compared the signals to untreated worms. Microchamber-based devices were also designed previously for single-worm OCR experiments, but only the approach of Huang et al. allowed versatile treatment of the worms using microfluidics.21,22 Commercial equipment for OCR sensing, e.g. state-of-the-art microplate format Seahorse XF respirometers, allow parallelized OCR measurements in microwells containing 10–25 worms and the injection of treatment compounds.23 Nevertheless, due to the lack of on-chip culturing capabilities, OCR assays to assess mitochondrial functioning of C. elegans worms extending over the whole lifespan were not possible so far in neither commercial nor customized academic approaches. The development of a microfluidic lifespan phenotyping platform with the capability of integrated oxygen sensing, while keeping the possibility of measuring the response of a single animal, could be crucial for enabling more advanced screening of the mitochondrial respiration on the whole-organism level.
The development of miniaturized optical sensors opened the way for better integration of OCR assays in microfluidic environments.24 Such sensors take advantage of a luminescence-based method, where oxygen molecules quench the intensity or lifetime of a light-emitting excited state in a specific dye molecule.25,26 The technique is contactless and fully non-invasive, easily scalable and does not intrinsically consume oxygen.27–30 For microfluidic chips, polydimethylsiloxane (PDMS) soft lithography is generally used for manufacturing due to the simplicity of replicating low feature sizes, low cost and the biocompatibility of the material.31 However, PDMS has a high gas permeability and it was shown that in PDMS devices on-chip levels of dissolved oxygen (DO) are continuously replenished and stabilized by oxygen diffusion through the bulk polymer.32–35 In general, this is an advantage for microfluidic culture devices, where on-chip oxygen supply to the living organisms is an important issue. For oxygen sensing applications, however, it is necessary to prevent fast oxygen resupply into the culture media in order to be able to measure oxygen depletion generated by the biological sample. Glass or thermoplastics are nearly impermeable to gas, but are not suited for fast prototyping of microfluidic chips. The off-stoichiometry dual-cure thiol–ene–epoxy (OSTE+) polymer was recently introduced for prototyping of rigid microfluidic devices with properties similar to thermoplastics.36 The biocompatibility of cured OSTE+ polymer is comparable to polystyrene and polycarbonate.36,37 In a recent study by Sticker et al., the polymer was shown to scavenge DO from fluids on-chip at tunable rates depending on the curing times and temperatures.38 The authors used this capability to create an on-chip anoxic environment for the culture of anaerobic bacteria. On the other hand, for culture of aerobic organisms such as C. elegans nematodes, fabrication parameters need to be adjusted to minimize oxygen scavenging, and adequate control experiments must be performed to correctly assess relevant biological OCR data.
In the present work, we describe the development of a microfluidics-assisted platform for lifetime culture of single C. elegans worms with integrated luminescence-based oxygen sensing capability. The platform, incorporating an OSTE+ polymer chip, takes advantage of automated fluidic operation, for maintaining well-defined culture conditions, washing out of metabolic waste products and progeny, and the reversible application of active compounds, respectively. Our approach thus enables OCR measurements over the worms' lifespan with single-organism resolution. Furthermore, we demonstrated the versatility of the method by performing an on-chip metabolic assay.
Materials and methods
C. elegans culture and metabolic assays
C. elegans N2 wildtype worms were obtained from the Caenorhabditis Genetics Center (CGC). Standard nematode growth medium (NGM) agar plates were provided by the EPFL Solution Preparation Facility (EPFL SV-IN). Escherichia coli OP50, S-basal medium and S-medium were prepared following standard protocols.39 An E. coli OP50 bacterial lawn was added to the center of the NGM agar plates, on which worm populations were subsequently grown at room temperature.10 Age-synchronized worm populations were obtained by a worm bleaching protocol (adapted from Stiernagle et al.,39 described in Krenger et al.40), followed by incubation of the embryos at room temperature until L1 larvae hatching. Subsequently, 500–1000 larvae were seeded on fresh NGM plates. The plates were then incubated for a duration of ∼40 h at room temperature until L4 worms were obtained and successively used for the experiments. For on-chip feeding of the worms, a suspension of tetracycline-resistant E. coli HT115 bacteria was prepared by inoculation of a bacterial glycerol stock into L-broth medium containing 10 μg mL−1 tetracycline and shaking overnight at 37 °C. Afterwards, the L-broth was removed by centrifugation, then the bacteria pellet was resuspended in freshly filtered S-medium and vortexed until a uniform suspension was obtained. Optical density measurements were used to determine the bacteria concentration and for producing bacterial suspension of 2 × 109 HT115 cells per mL for the experiments.For metabolic assays of the worms, carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP) powder, dimethyl sulfoxide (DMSO) and a 100 mmol L−1 sodium azide (NaN3) solution were purchased from Sigma Aldrich (Switzerland). S-medium containing 30 μmol L−1 FCCP was prepared by first diluting FCCP powder in DMSO to a 10 mmol L−1 stock solution, then diluting the stock in S-medium to obtain the final concentration. Sodium azide solution was directly diluted in S-medium to a concentration of 40 mmol L−1.
C. elegans culture and metabolic assays
Ostemer 322 Crystal Clear resin (OSTE+) was obtained from Mercene Labs AB (Stockholm, Sweden). 4-inch, 550 μm thick Si wafers were provided by the Center of MicroNanoTechnology (EPFL-CMi). GM3050 SU-8 negative structural resist was acquired from micro resist technology GmbH (Berlin, Germany). A single layer 80 μm thick SU-8 layer was patterned on a Si wafer to serve as a mold, after which the wafer was diced using standard methods. Subsequently, the SU-8 on Si was silanized with trimethylsilyl chloride (TMCS) in a vacuum chamber for at least 15 min. For the OSTE+ replica molding, the SU-8 on Si chip was inserted into a custom mold defining the final chip height of 2 mm, similar to what proposed in Sandström et al.41 Accurate control of the chip height is important in the molding process to guarantee uniform UV exposure of the Ostemer resin. The aluminum part of the mold guarantees that heat generated in the UV curing reaction is efficiently dissipated to prevent early initiation of a second curing step. Inlet holes for the connection of microfluidic tubing are made by inserting thin metal pins through holes in the poly(methyl methacrylate) (PMMA) cover of the mold. After mixing the two components of the OSTE+ resin according to the manufacturer's specifications (ratio 1.09![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and degassing in a vacuum chamber for 15 min, the resin was injected into the mold, where the OSTE+ was cured by UV-exposure for 35 min with a standard UV lamp. Finally, the flexible OSTE+ chip containing the microfluidic channels was unmolded.
1) and degassing in a vacuum chamber for 15 min, the resin was injected into the mold, where the OSTE+ was cured by UV-exposure for 35 min with a standard UV lamp. Finally, the flexible OSTE+ chip containing the microfluidic channels was unmolded.
The oxygen-sensitive nanoparticle dye (a polystyrene–silicone rubber composite matrix with embedded palladium(II) meso-tetra(4-fluorophenyl)tetrabenzoporphyrin) was spotted onto a standard microscopy glass slide to a diameter of ≈1 mm and a thickness of ≈2 μm using a computerized numerical control (CNC) airbrush. The spots were manufactured by the Institute of Analytical Chemistry and Food Chemistry (Prof. T. Mayr, Graz University of Technology, Austria) according to the procedure described in the work of Ehgartner et al.42 The presence of oxygen quenches luminescence of the dye by inhibition of photon re-emission by absorbing the energy of the excited state of the dye. The silicone rubber improves the adherence of the dye to glass and prevents the dye from dispersing into the surrounding fluid. The response time (t < 1 s) of the dye was qualitatively evaluated during the 2-point calibration procedure (see “Experimental” section). Changes of the dissolved oxygen concentration upon perfusion of the microchamber with calibration liquids could be measured almost instantaneously and occurred on a much faster timescale than during the experiments. More details on the sensor dye composition and preparation of the spots can be found in the work of Ehgartner et al., where the same sensor has been used in Si-glass microreactors.42 The OSTE+ chip was aligned to the glass slide and permanently bonded at 110 °C for 2 h under pressure in a metal clamp. OSTE+ release liner (Mercene Labs AB, Stockholm, Sweden) was used to prevent bonding of the OSTE+ resin to the metal clamp. Thermal bonding also induced a second curing step of the OSTE+ material, which is now fully polymerized and rigid. To make leakage free connections using microfluidic tubing to the rigid OSTE+, a slab of cured PDMS (thickness ≈3 mm) with 1.5 mm diameter punched inlet holes, corresponding to the positions of the inlet holes on the OSTE+ chip was first manufactured. A custom laser-cut PMMA mold was used to align and tightly clamp the PDMS slab onto the OSTE+ to form the final chip assembly.
Experimental
Worm culture chip
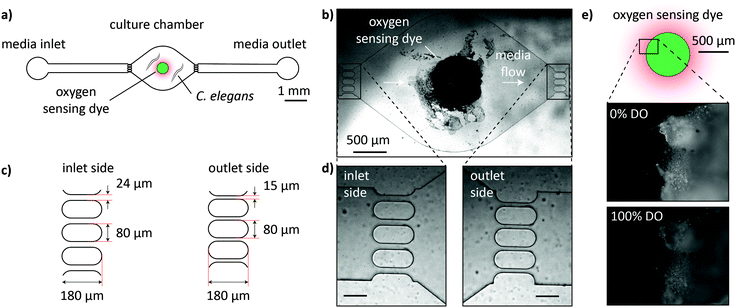
The OSTE+ microfluidic chip with integrated luminescence-based oxygen sensing capabilities for long-term culture and OCR quantification of C. elegans nematodes is shown in Fig. 1a. It comprises a single-worm chamber (height h = 80 μm) with one media inlet and one outlet. The shape of the chamber is circular (diameter d = 2 mm) and tapers towards the inlet and outlet to prevent bubble formation during perfusion. The total volume of the chamber is approximately 0.25 μL. The oxygen-sensitive dye, visible as black dot in the bright-field microscopy image in Fig. 1b, is deposited in the center of the microchamber. Filter structures for trapping of L4 worms are integrated near the inlet and outlet sides of the chamber. The filter shape with dimensions is illustrated in Fig. 1c and microscopy images are shown in Fig. 1d. A filter spacing of 24 μm near the chip inlet ensures that L4 worms may pass unharmed during worm loading, but prevents the organism from escaping by its own force in stopped-flow conditions. On the outlet side, filter spacing is 15 μm, which prevents L4 worms from passing, but allows washing away progeny and bacteria, thereby avoiding clogging of the fluidic structures. Before on-chip worm culture, microfluidic channels were debubbled, perfused with ethanol and rinsed thoroughly with S-medium. For initial loading, a single worm is manually picked from an agar plate and sucked into a piece of fluidic tubing. Subsequently the tube is connected to the inlet channel of the chip and the liquid is injected gently until the worm passes the inlet filter structures. Prior to the experiments, the oxygen-dependent luminescence of the oxygen sensing dye was qualitatively evaluated using a filter (Filter Set 50, Zeiss, Germany) mounted into our imaging system (see following section). The dye was excited at λ ≈ 620 nm and re-emitted light was collected in the near-infrared (NIR) range at λ ≈ 700 nm. In Fig. 1e, we recorded images (20× magnification) of the sensor dye with constant exposure (t = 10 s) in a 0% dissolved oxygen (DO) condition (higher image) and a 100% DO condition (lower image). We clearly see a higher emission intensity in the case of oxygen depletion, as expected.Microfluidic oxygen sensing setup
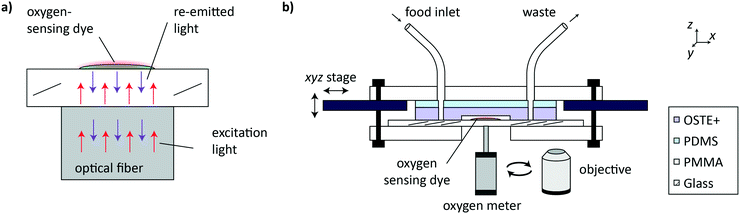
For readout of the oxygen concentration cO2 in the OSTE+ microfluidic chips, we used a commercial Piccolo2 oxygen meter (PICO2-OEM, pyroscience, Germany) combined with an optical fiber (PICFIB2, pyroscience, Germany). The Piccolo2 oxygen meter was placed in contact with the glass slide directly below the sensor spot, as shown in Fig. 2a. Light from the oxygen meter is guided through the optical fiber onto the sensor spot and re-emitted NIR light is sent back to the oxygen detector. Oxygen sensor spots were calibrated individually using a 2-point calibration procedure that is directly executed in the Pyro Oxygen Logger software (pyroscience, Germany). The 0% standard was obtained by adding 30 g L−1 Na2SO3 to DI water. The saturated 100% standard solution was obtained by vigorously shaking S-medium in a Falcon tube. The experimental setup for imaging, automated microfluidic worm culture and OCR measurements is shown in Fig. 2b. The imaging system was previously described in the work of Letizia et al.20 Syringes containing E. coli HT115 for on-chip feeding of the worms were equipped with a magnetic stirring system to prevent bacteria sedimentation.Automated on-chip C. elegans culture and OCR measurement protocols
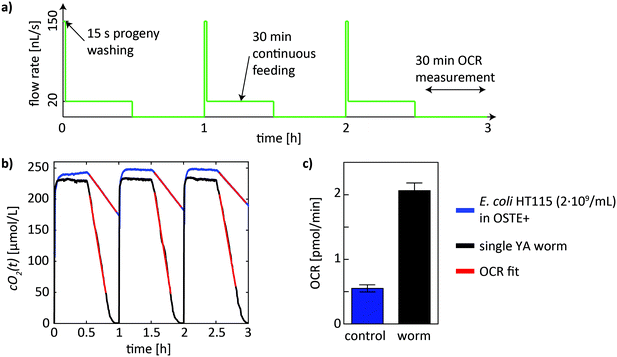
The automated fluidic protocol was initiated after loading of a single worm into the on-chip chamber. The syringe pump of the setup was programmed to continuously repeat a culture/feeding phase with a duration of 30 min, followed by an OCR measurement phase with a duration of 30 min. This cycle with a total duration of 1 h was repeated until the end of each experiment. A schematic of the flow protocol, comprising 3 full cycles of successive feeding and measuring phases in this case, is shown in Fig. 3a. Additionally, the feeding phase is preceded by a 15 s flow pulse to eject freshly hatched L1 larvae from the culture chamber. Subsequently, a slower continuous flow of bacterial food suspension (20 nL s−1) was applied for 30 min. This phase is the main feeding phase and serves also for replenishing the on-chip oxygen concentration for normal worm respiration. During the following measurement phase, flow in the microchamber was stopped for 30 min.C. elegans metabolic assay
The basal respiration rate of C. elegans L4 worms was assayed during a defined data acquisition window of the on-chip oxygen levels cO2(t), after which the DO was replenished by perfusion of the culture chamber with fresh S-medium. Subsequently, S-medium containing FCCP was injected to measure maximal respiration rates during uncoupled respiration. Finally, sodium azide was used to inhibit respiration in worms. OCR measurements for each condition were repeated 3 times. For the metabolic assay, the time-lapse of the measurement phases was reduced to 20 min and the liquid on chip was replenished manually in between repetitions of the measurements. The duration of the measurement phase could be reduced as in experiments with more than one worm in the chamber (here n = 2) cO2 reached zero in less than 20 min.Results
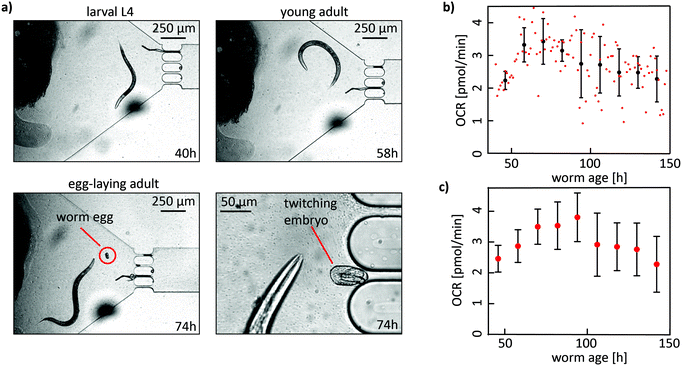
The time-dependent oxygen concentration cO2(t) in the culture chamber was measured in an automated manner during the whole fluidic protocol, i.e. during the culture phase and during the stopped-flow measurement phase of each cycle (Fig. 3a). OCR values were determined in the measurement phase of each cycle, where the DO level in the chamber was not replenished. In Fig. 3b, on-chip DO concentrations cO2(t) during 3 cycles are shown for a control (blue curve) and for a single-worm OCR measurement (black curve). For the control, the culture chamber was perfused with a concentration of 2 × 109 cells per mL of E. coli HT115 in S-medium. During perfusion, where slow flows are applied to provide a continuous influx of nutrients, cO2(t) stays constant at around 250 μmol L−1, indicating that oxygen is effectively supplied in addition to nutrients. During the measurement window (stopped flow), DO is decreasing in a linear manner due to O2 uptake by the E. coli bacteria, as well as by the OSTE+ material itself (see Discussion). To extract the corresponding OCR value from the time-dependent cO2(t) curves (baseline), a linear fit was automatically performed over all measured cycles using a custom MATLAB fitting algorithm (red curve in Fig. 3b). The OCR corresponds to the slope of the fitted line in units of μmol L−1 s−1, which was converted to units of pmol min−1 using a chamber volume of 0.25 μL for better comparison to reference data. The same protocol was applied for the on-chip OCR worm assays. In this case, the microchamber contained bacterial suspension and a worm. As for the control, OCR values were obtained by linear fitting of the measurement portion of a full cycle (red curve in Fig. 3b). Subsequently, the OCR baseline value was subtracted to obtain the OCR contribution of the sample of interest. In Fig. 3c the mean ± SD values for the control, as well as the mean ± SD OCR values of an approximately 38 h old YA nematode are shown (averaged over 3 cycles). For longer experiments, starting from L4 worms that continued for several days until the worm reached old age, up to 1 day lasting culture/measurement cycle sequences were implemented before the bacterial food source in the syringe was depleted and manually refilled. For imaging, the chip assembly was transferred to the inverted microscope.As a proof-of-concept, we performed long-term on-chip culture of single worms, combined with OCR measurements at regular intervals. In this assay, single L4 worms were trapped in the culture chambers for up to 6 days with sufficient bacterial food supply. In principle, normal on-chip worm development was observed, as illustrated by the bright-field microscopy images in Fig. 4a, showing a L4 larvae (aged 40 h) that developed to a YA worm (aged 58 h) and to an egg-laying adult (aged 74 h). A zoom on the worm pharynx and on an embryo in the late twitching stage also confirms normal morphology. However, compared to nematodes growing on standard NGM plates, on-chip worm development was delayed, likely due to lower overall mean on-chip oxygen levels (see Discussion). Results of OCR quantification during long-term worm culture are shown in Fig. 4b. The plot shows the whole data set of OCR values (red dots), obtained by fitting of the cO2(t) depletion curve by linear regression (as shown in Fig. 3b). To compute the OCR profile of the worm (Fig. 4b, black curve), single OCR data points were averaged over 12 h (n = 12 data points). The single-worm OCR profile shows a trend indicative of an increase of the mean respiration rate from larval to adult stage, and a subsequent decrease for the worm reaching old age. In this specific case, the initial mean OCR value at worm age ≈46 h was equal to 2.2 ± 0.3 pmol min−1 per worm and increased to a maximum value of 3.4 ± 0.7 pmol min−1 at worm age ≈70 h. From this point forward, an overall decrease of OCR values was observed. The lowest OCR value of 1.5 ± 0.7 pmol min−1 was measured in an old worm aged ≈166 h. An averaged OCR profile summarizing lifespan OCR results from all assays is shown in Fig. 4c. The profile evolves in a similar age-dependent way, with highest OCR values during the reproductive phase of the organism.
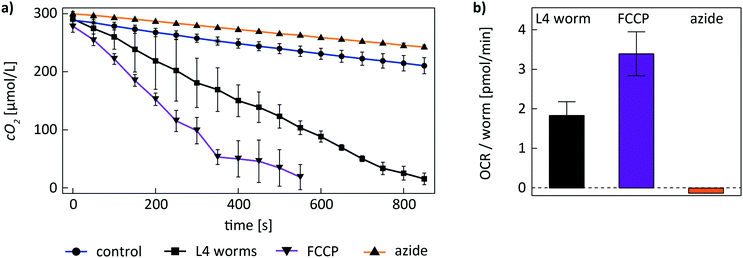
To evaluate basal and maximal respiration rates during coupled and uncoupled respiration of C. elegans worms on-chip, we recorded cO2(t) curves of worms in untreated and FCCP-treated conditions. The metabolic assay was performed according to the protocol described before. Fig. 5a shows experimental cO2(t) curves recorded with an on-chip population of 2 L4 worms in 4 different conditions (mean ± SD of n = 3 repetitions on the same worm population). OCR values were determined by the slope of a linear fit of the corresponding cO2(t) curve. In a control experiment without on-chip worm population (Fig. 5a, blue curve), the OCR contribution of the OSTE+ material and any potential contributions from the culture medium were determined. After trapping the worms in the culture chamber filled with S-medium, on-chip DO is consumed rapidly in a time span of ≈15 min (Fig. 5a, black curve), indicating that the contribution of the two worms to DO depletion is dominant. After FCCP treatment, on-chip oxygen levels decreased even faster with respect to the untreated worms (Fig. 5a, purple curve). This observation is in agreement with the fact that the worms are in a state of uncoupled respiration. Subsequently worms were exposed to a high concentration of sodium azide to inhibit respiration. The depletion rate of DO reverts approximately to control conditions, indicating that respiration has stopped (Fig. 5a, orange curve); the curve is even slightly above the control suggesting possible adverse effect of the sodium azide on the bacteria in solution too. In Fig. 5b, mean ± SD OCR values extracted by linear regression of the experimental data from Fig. 5a are shown. The data has been corrected to account for the OSTE+ oxygen scavenging rate (Fig. 5a, blue curve) by subtraction of the control OCR value of 1.3 ± 0.3 pmol min−1 obtained without worms. The resulting mean values were normalized by the worm number (n = 2). For a single untreated YA worm in basal respiration conditions, we found an OCR value of 1.8 ± 0.3 pmol min−1. The value increased by about a factor of two to 3.4 ± 0.6 pmol min−1 during uncoupled respiration after treatment with FCCP. After killing the 2 worms with sodium azide, OCR values returned to the low level of the control condition (−0.10 ± 0.04 pmol min−1).
Discussion
We developed an OSTE+ chip-based microfluidic device for C. elegans culture, on-chip luminescence-based OCR measurements of C. elegans and metabolic assays. OSTE+ is a versatile and cheap material for fast prototyping of microchips with feature sizes in the μm-range. In contrast to gas-highly-permeable PDMS chips, OSTE+ has a very low gas permeability and thus does not replenish on-chip oxygen levels during the assay. We took advantage of this material property when measuring the depletion of DO in a microfluidic chamber and extracting the OCR of the biological sample. For OCR quantification in OSTE+ microfluidic chips, the oxygen scavenging properties of this polymer have to be considered. Sticker et al. performed a series of experiments for characterizing oxygen scavenging by the material and discovered that this property strongly depends on the curing time and temperature of the resin, as well as on the surface-to-volume ratio of the chip itself, defined by the geometry.38 High curing temperatures of up to 130 °C and long curing durations of up to 6 h decreased the oxygen absorption of OSTE+, mainly because of a reduction of the number of free thiols on the chip surface, reduced bulk polymer oxidation and an increase of the polymer density. In our work, we used fabrication parameters in the higher range of these parameters to minimize the effect of oxygen absorption of OSTE+. However, the baseline drift due to OSTE+ oxygen scavenging could be removed safely from the experimental results, thus excluding any adverse effect on the reliability and reproducibility of our on-chip OCR experiments with living samples.We presented OCR measurements of C. elegans from L4 stage to late adulthood, requiring long-term on-chip culture of the worms (Fig. 4). The previously mentioned devices by Suda et al. and Huang et al., as well as the Seahorse respirometers, were not suitable for long-term culturing applications.21–23 On the other hand our device enables automated OCR measurements during worm culture experiments over a period of several days. Our microfluidic approach is capable to confine the sample close to the oxygen sensor in a well-controlled culture environment of precisely defined feeding conditions and concentrations of treatment agents. In addition, the measurement protocol allows automated washing away of metabolic waste products and worm progeny, thus further improving the accuracy of our approach. Worms apparently developed normally on-chip, however, we observed delayed adulthood with egg laying starting only at a worm age of 74 h, i.e. approximately 1 day later than is expected for worms growing on standard agar culture plates. We attribute this fact to the design of the experimental protocol, where the worms experience regular fluctuations of DO concentrations in the culture environment. Specifically, DO is replenished only during a period of 30 min in a 1 h lasting culturing/measurement cycle, whereas during the OCR measurement phase of the cycle, no oxygen is resupplied to the worm culturing chamber. A rough estimation reveals that oxygen molecules take approximately t ≈ 4 min to diffuse over a typical chamber dimension of lch = 1 mm (using 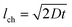 with a diffusion coefficient D = 1.9 × 109 m2 s−1 for molecular oxygen in water).43 We can therefore assume that during a typical measurement cycle of 30 min, oxygen in the whole chamber is consumed, not only in the proximity of the sensor spot. On-chip oxygen levels in the culture chamber could in principle be replenished by diffusion through the filter structures from the two adjacent microchannels. In this case, the oxygen diffusion rate Q can be estimated by Q = D × ΔcO2/lF × A, with an oxygen concentration difference ΔcO2 between the chamber and the microchannels, the length lF of the filter structures and the total open cross-sectional area A between the filter posts. Assuming cO2 = 0 in the chamber, we find Q = 1.7 × 10−3 pmol min−1 and can deduce that oxygen diffusion through the filters into the chamber can be neglected in our experiments. In our assays, complete depletion of DO in the chamber occurs after about 15 to 25 min (i.e. ≈5 to 15 min before refreshing the culture medium). Moreover, the concentration of dissolved carbon dioxide is assumed to increase simultaneously as it is a byproduct of mitochondrial respiration. It has been shown that worms growing in oxygen-deficient conditions experience a slowdown in development and an increase in lifespan.44,45 Lower motility and reduced fertility were also observed in these conditions. Similar to hypoxic conditions, hypercapnia (elevated CO2 levels) slows down development and increases lifespan.46 In principle, our observations are in line with results shown in these studies. While we believe that the culturing protocol for our proof-of-concept study is working well for single-worm assays, on-chip control of hypoxic and hypercapnic conditions might become more critical when testing more worms per chamber, which evidently are competing for the same resource. However, this issue can be easily circumvented by optimizing the assay protocol, in particular by shortening the OCR quantification phases and by extending the culture phases in fresh medium.
with a diffusion coefficient D = 1.9 × 109 m2 s−1 for molecular oxygen in water).43 We can therefore assume that during a typical measurement cycle of 30 min, oxygen in the whole chamber is consumed, not only in the proximity of the sensor spot. On-chip oxygen levels in the culture chamber could in principle be replenished by diffusion through the filter structures from the two adjacent microchannels. In this case, the oxygen diffusion rate Q can be estimated by Q = D × ΔcO2/lF × A, with an oxygen concentration difference ΔcO2 between the chamber and the microchannels, the length lF of the filter structures and the total open cross-sectional area A between the filter posts. Assuming cO2 = 0 in the chamber, we find Q = 1.7 × 10−3 pmol min−1 and can deduce that oxygen diffusion through the filters into the chamber can be neglected in our experiments. In our assays, complete depletion of DO in the chamber occurs after about 15 to 25 min (i.e. ≈5 to 15 min before refreshing the culture medium). Moreover, the concentration of dissolved carbon dioxide is assumed to increase simultaneously as it is a byproduct of mitochondrial respiration. It has been shown that worms growing in oxygen-deficient conditions experience a slowdown in development and an increase in lifespan.44,45 Lower motility and reduced fertility were also observed in these conditions. Similar to hypoxic conditions, hypercapnia (elevated CO2 levels) slows down development and increases lifespan.46 In principle, our observations are in line with results shown in these studies. While we believe that the culturing protocol for our proof-of-concept study is working well for single-worm assays, on-chip control of hypoxic and hypercapnic conditions might become more critical when testing more worms per chamber, which evidently are competing for the same resource. However, this issue can be easily circumvented by optimizing the assay protocol, in particular by shortening the OCR quantification phases and by extending the culture phases in fresh medium.
When comparing on-chip single-worm respiration rates to reference values, obtained with a commercial Seahorse XF96 respirometer, we notice that our OCR values are significantly lower, i.e. by factors ranging from about 2 to 5 depending on the worm age.23 However, we observe a comparable overall age-dependent evolution of the OCR rates in both cases, namely with a peak value during the reproduction phase of adult worms and lower respiration rates during the L4 and YA stages, and for worms at old age. It is worth mentioning that in the Seahorse study OCR measurements had to be performed separately for each development stage, whereas in our study we were able to culture worms and measure OCR rates over their whole lifespan continuously. Interestingly, using direct calorimetry, Braeckman et al. have shown that worms growing in liquid culture produce less metabolic heat (by a factor of ≈1.6) than worms growing on NGM agar plates.47 Consequently, as for the Seahorse XF96 measurements worms were always taken from agar plates and stayed in the device for only about 30 min, we also expect reduced OCR values in a microfluidic chip. This fact, in combination with partially hypoxic conditions, may explain the discrepancy in reported OCR values measured with significantly different experimental setups. Comparison of our data to Seahorse XF reference values is not meant to be a proper validation of our chip-based approach. Further going comparison with our approach (or validation) seems not to be feasible, in particular because, with the Seahorse system, separate experiments need to be perform for each development stage, whereas we demonstrate continuous and long-term experimentation and culture on-chip.
Moreover, using our microfluidic platform, we were able to quantify the variation of respiration rates of C. elegans worms upon treatment with the uncoupling agent FCCP and the metabolic inhibitor sodium azide. Similar assays have also been performed by Huang et al. in a microdevice and in the Seahorse XF96 respirometer.22,23 In these studies, coupled respiration rates increased by a factor of ≈2 upon treatment with FCCP, i.e. during the state of uncoupled respiration, where maximal cellular respiration in mitochondria occurs. After treatment with sodium azide, the authors could measure small OCR values related to residual non-mitochondrial respiration. Our on-chip assays confirmed an increased OCR of C. elegans worms after FCCP exposure (Fig. 5). Respiration rates also increased by a factor of ≈2 during uncoupled respiration. In our case, however, measured respiration values approached zero level after inhibiting respiration with sodium azide, indicating worm death possibly due to the harsh on-chip sodium azide concentrations.
Conclusions
We described a new, versatile microfluidic platform with integrated luminescence-based oxygen detection capability by using an oxygen-sensitive dye. The fabrication process of OSTE+ microfluidic chips was optimized by developing a custom molding process, allowing reliable and scalable implementation of rigid features in the μm-range for trapping and culture of C. elegans worms starting at the L4 stage. The OSTE+ polymer is particularly suitable for on-chip OCR assays thanks to its very low oxygen permeability and its biocompatibility. An automated fluidic protocol enabled successive worm feeding and OCR quantification of living worms. In a proof-of-concept study, we measured the respiration rate of a single worm trapped on-chip during its growth from the L4 stage to an old age of 7 days and compared our results to reference values obtained by a commercial Seahorse XF96 respirometer. Due to the temporarily lowered on-chip oxygen concentration caused by our OCR assay protocol, worms show slower development compared to worms cultured on microfluidic chips with constant oxygen supply and show lower respiration rates than worms developing on agar plates. Furthermore, a metabolic assay was performed, where we quantified the effect of the mitochondrial uncoupling agent FCCP on the basal respiration rate of C. elegans worms in the YA stage. With respect to the feasibility of long-term studies, our system offers several advantages over standard OCR commercial equipment, e.g. confinement of the sample in close proximity to the oxygen-sensitive dye, continuous and controlled feeding conditions, as well as removal of progeny and metabolic waste products. In future, more versatile assays can be performed by injection of drugs or treatment compounds, together with worm imaging capabilities. This promising technological approach may be combined with other analytical methods, e.g. direct calorimetry, thus opening the way to gain new insights on the coupling of anabolic and catabolic processes in living organisms.Author contributions statement
R. K. conceived the study, conducted the experiments and analyzed the data. M. C. provided molds and expertise for OSTE+ chip fabrication. R. K. and T. L. wrote the manuscript. All authors reviewed and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank Torsten Mayr (Institute of Analytical Chemistry and Food Chemistry, Graz University of Technology, 8010 Graz, Austria for providing the oxygen sensor spots. We also would like to thank Daniel Migliozzi (Microsystems Laboratory 4, École Polytechnique Fédérale de Lausanne, 1015 Lausanne, Switzerland) for help with acquiring the fluorescent images. Funding was provided by the École Polytechnique Fédérale de Lausanne.References
- P. Fernyhough, S. K. Roy Chowdhury and R. E. Schmidt, Expert Rev. Endocrinol. Metab., 2010, 5, 39–49 CrossRef CAS.
- I. L. Ferreira, R. Resende, E. Ferreiro, A. C. Rego and C. F. Pereira, Curr. Drug Targets, 2010, 11, 1193–1206 CrossRef CAS PubMed.
- H. Kawamata and G. Manfredi, Mech. Ageing Dev., 2010, 131, 517–526 CrossRef CAS.
- R. Kones, Nutr. Clin. Pract., 2010, 25, 371–389 CrossRef.
- J. Ren, L. Pulakat, A. Whaley-Connell and J. R. Sowers, J. Mol. Med., 2010, 88, 993–1001 CrossRef CAS.
- T. R. Rosenstock, A. I. Duarte and A. C. Rego, Curr. Drug Targets, 2010, 11, 1218–1236 CrossRef CAS PubMed.
- S. B. Vafai and V. K. Mootha, Nature, 2012, 491, 374–383 CrossRef CAS.
- M. Vuda and A. Kamath, Mitochondrion, 2016, 31, 63–74 CrossRef CAS.
- M. D. Brand and D. G. Nicholls, Biochem. J., 2011, 435, 297–312 CrossRef CAS PubMed.
- S. Brenner, Genetics, 1974, 77, 71–94 CAS.
- T. Kaletta and M. O. Hengartner, Nat. Rev. Drug Discovery, 2006, 5, 387–399 CrossRef CAS.
- L. P. O'Reilly, C. J. Luke, D. H. Perlmutter, G. A. Silverman and S. C. Pak, Adv. Drug Delivery Rev., 2014, 69–70, 247–253 CrossRef.
- A. San-Miguel and H. Lu, WormBook, 2013, DOI:10.1895/wormbook.1.162.1.
- N. A. Bakhtina and J. G. Korvink, RSC Adv., 2014, 4, 4691 RSC.
- M. M. Shanmugam and T. S. Santra, Molecules, 2016, 21, 1006 CrossRef.
- M. Cornaglia, T. Lehnert and M. A. M. Gijs, Lab Chip, 2017, 17, 3736–3759 RSC.
- N. Chronis and C. I. Bargmann, Nat. Methods, 2007, 4, 727–731 CrossRef CAS.
- A. Ben-Yakar, N. Chronis and H. Lu, Curr. Opin. Neurobiol., 2009, 19, 561–567 CrossRef CAS.
- J. Larsch, D. Ventimiglia, C. I. Bargmann and D. R. Albrecht, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 4266–4273 CrossRef.
- M. C. Letizia, M. Cornaglia, R. Trouillon, V. Sorrentino, L. Mouchiroud, M. S. B. Sleiman, J. Auwerx and M. A. M. Gijs, Microsyst. Nanoeng., 2018, 4, 6 CrossRef.
- H. Suda, T. Shouyama, K. Yasuda and N. Ishii, Biochem. Biophys. Res. Commun., 2005, 330, 839–843 CrossRef CAS.
- S.-H. Huang and Y.-W. Lin, Sensors, 2018, 18, 2453 CrossRef.
- M. Koopman, H. Michels, B. M. Dancy, R. Kamble, L. Mouchiroud, J. Auwerx, E. A. A. Nollen and R. H. Houtkooper, Nat. Protoc., 2016, 11, 1798–1816 CrossRef CAS.
- S. M. Grist, L. Chrostowski and K. C. Cheung, Sensors, 2010, 10, 9286–9316 CrossRef CAS.
- O. Stern and M. Volmer, Phys. Z., 1919, 20, 183–188 CAS.
- I. Bergman, Nature, 1968, 218, 396 CrossRef CAS.
- P. Hartmann, W. Ziegler, G. Holst and D. W. Lübbers, Sens. Actuators, B, 1997, 38, 110–115 CrossRef CAS.
- A. Mills, Platinum Met. Rev., 1997, 41, 115–127 CAS.
- D. B. Papkovsky, T. O'Riordan and A. Soini, Biochem. Soc. Trans., 2000, 28, 74–77 CrossRef CAS.
- S. Suresh, V. C. Srivastava and I. M. Mishra, J. Chem. Technol. Biotechnol., 2009, 84, 1091–1103 CrossRef CAS.
- Y. Xia and G. M. Whitesides, Annu. Rev. Mater. Sci., 1998, 28, 153–184 CrossRef CAS.
- H. Yasuda and K. Rosengren, J. Appl. Polym. Sci., 1970, 14, 2839–2877 CrossRef CAS.
- K. S. Houston, D. H. Weinkauf and F. F. Stewart, J. Membr. Sci., 2002, 205, 103–112 CrossRef CAS.
- Y. Amao, Microchim. Acta, 2003, 143, 1–12 CrossRef CAS.
- C. J. Ochs, J. Kasuya, A. Pavesi and R. D. Kamm, Lab Chip, 2014, 14, 459–462 RSC.
- T. Haraldsson, C. F. Carlborg and W. van der Wijngaart, in Microfluidics, BioMEMS, and Medical Microsystems XII, International Society for Optics and Photonics, 2014, vol. 8976897608 Search PubMed.
- C. Errando-Herranz, A. Vastesson, M. Zelenina, G. Pardon, W. van der Wijngaart, T. Haraldsson, H. Brismar and K. B. Gylfason, Biocompatibility of OSTE polymers studied by cell growth experiments, in Proceedings of the 17th Int. Conf. on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS 2013, Freiburg, Germany, 2013, vol. 1, pp. 143–145 Search PubMed.
- D. Sticker, M. Rothbauer, J. Ehgartner, C. Steininger, O. Liske, R. Liska, W. Neuhaus, T. Mayr, T. Haraldsson, J. P. Kutter and P. Ertl, ACS Appl. Mater. Interfaces, 2019, 11, 9730–9739 CrossRef CAS.
- T. Stiernagle, WormBook, 2006, DOI:10.1895/wormbook.1.101.1.
- R. Krenger, T. Lehnert and M. A. M. Gijs, Lab Chip, 2018, 18, 1641–1651 RSC.
- N. Sandström, R. Z. Shafagh, A. Vastesson, C. F. Carlborg, W. van der Wijngaart and T. Haraldsson, J. Micromech. Microeng., 2015, 25, 075002 CrossRef.
- J. Ehgartner, M. Strobl, J. M. Bolivar, D. Rabl, M. Rothbauer, P. Ertl, S. M. Borisov and T. Mayr, Anal. Chem., 2016, 88, 9796–9804 CrossRef CAS.
- C. R. Wilke and P. Chang, AIChE J., 1955, 1, 264–270 CrossRef CAS.
- J. A. Powell-Coffman, Trends Endocrinol. Metab., 2010, 21, 435–440 CrossRef CAS.
- S. F. Leiser, M. Fletcher, A. Begun and M. Kaeberlein, J. Gerontol., Ser. A, 2013, 68, 1135–1144 CrossRef CAS.
- K. Sharabi, A. Hurwitz, A. J. Simon, G. J. Beitel, R. I. Morimoto, G. Rechavi, J. I. Sznajder and Y. Gruenbaum, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 4024–4029 CrossRef CAS PubMed.
- B. P. Braeckman, K. Houthoofd, A. De Vreese and J. R. Vanfleteren, Mech. Ageing Dev., 2002, 123, 105–119 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |