Focusing of sub-micrometer particles in microfluidic devices
Tianlong
Zhang
ab,
Zhen-Yi
Hong
a,
Shi-Yang
Tang
 c,
Weihua
Li
c,
Weihua
Li
 c,
David W.
Inglis
c,
David W.
Inglis
 b,
Yoichiroh
Hosokawa
a,
Yaxiaer
Yalikun
*a and
Ming
Li
b,
Yoichiroh
Hosokawa
a,
Yaxiaer
Yalikun
*a and
Ming
Li
 *b
*b
aDivision of Materials Science, Nara Institute of Science and Technology, Nara 630-0192, Japan. E-mail: yaxiaer@ms.naist.jp
bSchool of Engineering, Macquarie University, Sydney 2122, Australia. E-mail: ming.li@mq.edu.au
cSchool of Mechanical, Materials, Mechatronic and Biomedical Engineering, University of Wollongong, Wollongong 2522, Australia
First published on 7th November 2019
Abstract
Sub-micrometer particles (0.10–1.0 μm) are of great significance to study, e.g., microvesicles and protein aggregates are targets for therapeutic intervention, and sub-micrometer fluorescent polystyrene (PS) particles are used as probes for diagnostic imaging. Focusing of sub-micrometer particles – precisely control over the position of sub-micrometer particles in a tightly focused stream – has a wide range of applications in the field of biology, chemistry and environment, by acting as a prerequisite step for downstream detection, manipulation and quantification. Microfluidic devices have been attracting great attention as desirable tools for sub-micrometer particle focusing, due to their small size, low reagent consumption, fast analysis and low cost. Recent advancements in fundamental knowledge and fabrication technologies have enabled microfluidic focusing of particles at sub-micrometer scale in a continuous, label-free and high-throughput manner. Microfluidic methods for the focusing of sub-micrometer particles can be classified into two main groups depending on whether an external field is applied: 1) passive methods, which utilize intrinsic fluidic properties without the need of external actuation, such as inertial, deterministic lateral displacement (DLD), viscoelastic and hydrophoretic focusing; and 2) active methods, where external fields are used, such as dielectrophoretic, thermophoretic, acoustophoretic and optical focusing. This article mainly reviews the studies on the focusing of sub-micrometer particles in microfluidic devices over the past 10 years. It aims to bridge the gap between the focusing of micrometer and nanometer scale (1.0–100 nm) particles and to improve the understanding of development progress, current advances and future prospects in microfluidic focusing techniques.
1. Introduction
Particle focusing, conventionally defined as either two-dimensional (2D) focusing, where particles are horizontally focused at the center-plane of a microchannel, or three-dimensional (3D) focusing, in which particles are focused not only horizontally but also vertically, always serves as a prerequisite step for downstream processing, such as detection, separation and manipulation of target particles.1,2 Moreover, the ability to position particles (both biological and synthetic) in a tightly focused stream is important for flow cytometry, imaging flow cytometry3–5 and deformability cytometry.6,7 The position of focused particles is not necessarily limited to a single position in the channel center, and multiple focal positions may also exist. For example, multiple streamline particle focusing occurs when using inertial forces to direct particles towards sidewalls of a square microchannel.8 Microfluidic devices feature low reagent consumption, small size, fast analysis and low cost,9,10 allowing them to act as ideal platforms for particle focusing, concentration and separation in a wide range of fields, such as biology, chemistry and medical science.11–15Sub-micrometer particles with specific physical and biochemical properties, such as superconducting particles, normal mitochondria, infectious airborne viruses, etc., are of great significance to study.16–21 In this review, sub-micrometer and nanometer particles are defined as the particles with size ranging from 0.10 to 1.0 μm and 1.0 to 100 nm, respectively, to avoid confusion.22–25 Due to the biomedical, environmental and industrial significance of sub-micrometer particles, precisely control over their positions in one or multiple focal streams has diverse applications, e.g., clinical diagnosis, environmental monitoring, food analysis and drug delivery.26–28
Microvesicles (0.10–1.0 μm), a type of extracellular vesicles generated by budding or blebbing of the plasma membrane, have essential regulatory roles in the progress of rheumatoid arthritis and oncogenic processes, such as tumor proliferation and invasion.29–32 Moreover, microvesicles can be used for macromolecular drug delivery.29 Protein aggregates (e.g., immune complexes) with a similar size to microvesicles30,33 play a vital role in neurodegenerative disease progressions, such as Alzheimer's disease and prion diseases, making them as targets for therapeutic treatment.34,35 Geometrically encoded fluorescent barcodes self-assembled from DNA (0.40–0.80 μm) are used as in situ single-molecule probes, e.g., the tags for labeling proteins on yeast cell surface.36 Some bacteria in sub-micrometer range can be either harmful or beneficial to humans and the environment. For example, Staphylococcus aureus (S. aureus) is one of the major causes of skin, soft-tissue, bone, joint and endovascular disorders, while Staphylococcus epidermidis (S. epidermidis) is beneficial by balancing epithelial microflora with immune tolerance.37,38Prochlorococcus (0.50–0.70 μm), the most abundant photosynthetic organism on the planet, is of global significance due to its crucial role in the marine carbon cycle.39–44 Sub-micrometer fluorescent polystyrene (PS) particles are used as fluorescent probes for diagnosis, imaging and optical tracking,45 as PS is hardly biodegradable and does not show any short-term cytotoxicity in cellular environment.46 A study by Rogach et al. showed that sub-micrometer PS particles can be coated with luminescent crystals, indicating their potentials for telecommunication applications.47
A variety of techniques for the focusing of sub-micrometer particles in microfluidic devices have been developed. These techniques were previously classified as either 1) sheath flow focusing which employs sheath fluids to pinch suspending particles to the center of the channel or 2) sheathless focusing, where a force driving particles laterally to their equilibrium positions is applied.2,48 To elucidate the various focusing approaches and their mechanisms, in this review, we categorize microfluidic techniques for sub-micrometer particle focusing into two main groups: active and passive focusing. The active techniques require an external field such as electric, acoustic and thermal field to provide a driving force to focus particles, in contrast, the passive techniques only rely on the intrinsic properties of fluids within channels.
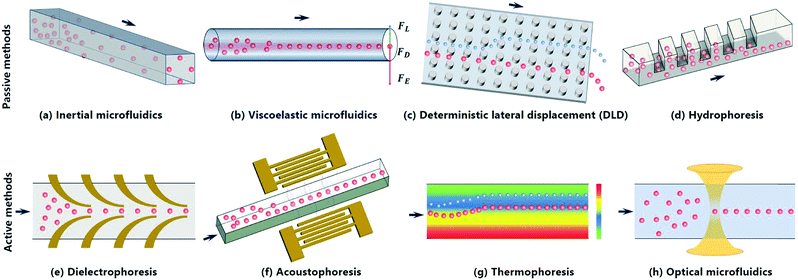
With a focus on the studies over the past 10 years, this review mainly summarizes two main groups of microfluidic techniques for the focusing of sub-micrometer particles: 1) passive focusing (e.g., inertial, DLD, viscoelastic and hydrophoretic focusing) and 2) active focusing techniques (e.g., dielectrophoresis, DEP, acoustophoresis, thermophoresis and optical tweezers). Fig. 1 schematically illustrates these techniques and their focusing performance. For each technique, we provide a definition and briefly introduce its theoretical fundamentals. Representative studies on sub-micrometer particle focusing in microfluidic devices using this technique are described and typical figures are presented to facilitate the understanding of focusing mechanisms, dynamic processes and applications. The advantages and disadvantages of each focusing technique are discussed. We also talk about main challenges in sub-micrometer particle focusing and provide design guidelines of each technique for researchers in both academia and industry. A table summarizing key characteristics of each technique, e.g., focusing criteria, methods, typical samples, merits and demerits, is presented at the end. Given the importance of sub-micrometer particles for various biological, biomedical and environmental applications, we aim to promote the understanding of the development process, current advances and future prospects of microfluidic techniques for the focusing of particles at sub-micrometer scale.
2. Passive methods
Passive microfluidic techniques depend on the intrinsic properties of fluids to achieve particle focusing, and they do not rely on external fields to provide driving forces. In this section, three types of passive microfluidic techniques for the focusing of sub-micrometer particles are discussed, namely inertial microfluidics, viscoelastic microfluidics and DLD, which mainly rely on inertial lift forces, viscoelastic forces and pillar-induced hydrodynamic forces to achieve the focusing.2.1 Inertial microfluidics
Inertial focusing uses the inertial lift forces (e.g., wall lift force and shear lift force) to drive particles to one or multiple equilibrium positions in a microchannel (Fig. 1a). In general, the wall lift force directs the particle away from the wall and decays with the distance from the wall, while the shear lift force drives the particle towards the wall and is zero at the channel centerline.49 In a straight microchannel, the net lift force (FL) on a particle can be expressed as below,50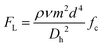 | (1) |
Inertial focusing has been widely used for the manipulation of micrometer particles (e.g., mammalian cells and microalgae cells),51,52 but the focusing of particles at sub-micrometer scale encounters challenges as the required minimum length of the microchannel for effective focusing increases dramatically with the decrease of the particle size.49,53 Only in recent years, inertial focusing devices see the advances of focusing resolution from micrometer to sub-micrometer scale. After providing a theoretical approach for the design of a curved microchannel, Cruz et al. experimentally demonstrated label-free, continuous and high-throughput inertial focusing of 1.0 μm PS particles and sub-micrometer Escherichia coli (E. coli) at a flow rate of 50 μL min−1.54 In this work, the authors firstly analyzed the correlations between scale factors, such as particle size, hydraulic diameter, fluid speed and focus length, secondly scaled down the size of the particles by adjusting these scale factors, and lastly provided experimental validations. Further optimization of channel geometry for focusing is important in inertial microfluidics due to its features such as simplicity of use and high-throughput performance.
In 2017, Wang et al. demonstrated that 0.92 μm PS particles can be focused to equilibrium positions at a flow rate of 500 μL min−1 in a serpentine microchannel.50 This channel consists of five functional components (Fig. 2a), namely 1) an inlet to introduce homogeneous suspensions, 2) a filter region to prevent channel clogging by trapping larger particles, 3) an asymmetric serpentine channel to focus particles, 4) a separation region to isolate particles of different sizes, and 5) outlets to collect fractions of samples. By reducing the dimension of the channel cross section to 10 μm × 5.0 μm, the focusing phenomenon was observed for 0.92 μm PS particles (Fig. 2b). This study is significant as it enables the investigation of individual microorganisms and subcellular organelles at the downstream.
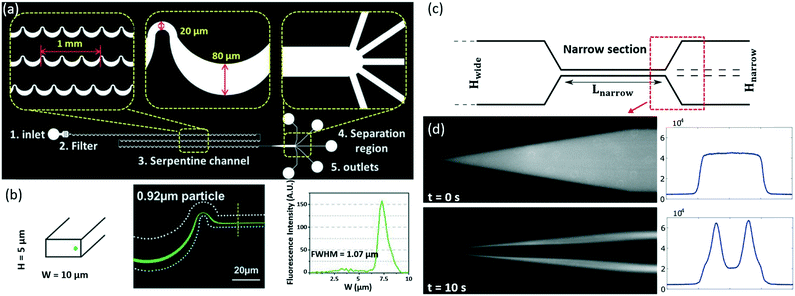 | ||
| Fig. 2 Inertial focusing of sub-micrometer PS particles.50,55 (a) Images showing the design of a serpentine microfluidic channel. Reproduced from ref. 50 with permission under open license CC BY 4.0. (b) The focusing of 0.92 μm PS particles (Green). Reproduced from ref. 50 with permission under open license CC BY 4.0. (c) A schematic of the bone-shaped microchannel (Hnarrow = 10 μm, Lnarrow = 250 μm, Hwide = 150 μm). Reproduced from ref. 55 with permission under open license CC BY-NC-ND 4.0. (d) Oscillatory inertial focusing of 0.50 μm PS particles. Both fluorescent streak images (left) and full-width half-maximum (FWHM) profiles (right) are presented to show the focusing performance. Reproduced from ref. 55 with permission under open license CC BY-NC-ND 4.0. | ||
Besides a waved microchannel, a straight bone-like microchannel (Fig. 2c) was used by Mutlu et al. to focus PS particles as small as 0.50 μm (Fig. 2d) and round-shaped bacteria S. aureus with a nominal size of 0.80 μm.55 In this design, the oscillatory microchannel switches the direction of the flow at a high frequency, but the force acting on the particles preserves its direction due to the symmetry of the velocity field along the flow axis. This allows indefinite extension of focusing length in theory, even though the channel has a short and fixed length. More recently, Cruz et al. investigated the mechanism of inertial focusing in curved channels for small (∼1.0 μm) particles, and achieved the focusing of 0.50 μm PS particles.56 This channel also realized size-based separation of sub-micrometer bacteria, e.g., Salmonella typhimurium (S. typhimurium) and Klebsiella pneumoniae (K. pneumoniae) based on their difference in lateral equilibrium positions.
Using inertial microfluidics for the focusing of sub-micrometer particles is still challenging now, as it requires the increase in channel length and improvement in theory for effective focusing.57 Inertial focusing shows the merits including high throughput, the simple sample preparation process, easy operation, no need for sheath flow, etc., but it has its demerits, e.g., the difficulty in improving focusing resolution.
2.2 Viscoelastic microfluidics
Viscoelastic focusing (Fig. 1b) takes advantage of three main hydrodynamic forces including inertial lift force (FL, eqn (2)), elastic force (FE, eqn (5)) and viscous drag force (FD, eqn (6)) to focus particles to equilibrium positions in viscoelastic fluids, e.g., dilute polymer solutions.58 Inertial lift force, which is determined by the combination of shear gradient lift force and wall lift force, can be expressed as follows,49,59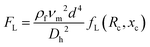 | (2) |
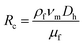 | (3) |
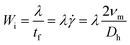 | (4) |
![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) represent fluid relaxation time, characteristic time of the channel flow, and average (characteristic) shear rate, respectively. Wi denotes the ratio of elastic force, FE, to viscous force.61 When Wi is very small, FE can be formulated as follows,62
represent fluid relaxation time, characteristic time of the channel flow, and average (characteristic) shear rate, respectively. Wi denotes the ratio of elastic force, FE, to viscous force.61 When Wi is very small, FE can be formulated as follows,62FE = −2CeLd3ηpλ∇![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) 2 2 | (5) |
| FD = 3πμfd(νf − νp) | (6) |
In viscoelastic fluids, the migration of particles can be described in viscoelasticity-dominant flow or elasto-inertial flow based on the relative importance of elastic and inertial effects.62 Please refer to the review by Squires et al. to get an in-depth understanding of the dimensionless parameters such as Rc, Wi and Peclet (Pe).64
In 2012, Kim et al. found that PS particles as small as 0.20 μm can be focused at a flow rate of 15 μL h−1 under the effect of viscoelastic flows.65 Besides, the focusing of flexible DNA molecules, including λ-DNA and T4-DNA with radii of gyration of around 0.69 μm and 1.50 μm, respectively, were observed to be enhanced using viscoelastic flows. This study lays the foundation for future development of viscoelastic focusing devices by demonstrating the importance of viscoelastic effects and the relative dimension of particle size to channel height. However, scaling the resolution down from micrometer to nanometer scale does not simply correspond to a scaling of dimensions, because new physics such as diffusion and particle–polymer interaction begin to dominate and need to be considered.66
By employing extensive computer simulations, Nikoubashman et al. revealed the focusing mechanism of colloidal particles in a viscoelastic fluid under Poiseuille flow and further exploited the mechanism to separate and capture sub-micrometer particles in simple microfluidic devices.67 In 2014, Santo et al. developed a theory that can be applied to viscoelastic focusing of sub-micrometer particles, which was confirmed experimentally by focusing 0.20 μm PS particles.68 This work links the particle trapping force caused by the viscoelasticity of the suspending fluid to a dimensionless parameter comparing viscoelasticity normal forces and Brownian forces. This theory can be used to downscale flow cytometers, and to guide the design of microfluidic devices for counting, coding and separating sub-micrometer and even nanometer particles. In addition to PS particles and DNA molecules, sub-micrometer E. coli was shown to be focused into a tight streamline by viscoelastic effects in straight microchannels.69
In 2016, Liu et al. achieved viscoelastic focusing of λ-DNA molecules, 0.10 μm PS particles and nanometer DNA origamis in a double spiral microchannel by systematically studying the effects of molecular weight, concentration of polymer, flow speed and particle size on the focusing performance.70 In this study, aqueous solutions of polyethylene oxide (PEO) with low molecule weight (Mw of 0.60 × 106 g mol−1) and low concentration (c of 0.60 wt%) were shown to be optimal for focusing 0.10 μm PS particles (Fig. 3a). Additionally, the focusing of DNA origami having three different shapes including spheres (0.04 μm in diameter), short tubes (0.03 μm in diameter and 0.04 μm in length) and long tubes (0.01 μm in diameter and 0.30 μm in length) were demonstrated (Fig. 3b). Liu et al. also demonstrated the selectively focusing and separation of a binary mixture of 0.10 and 0.50 μm PS particles by viscoelastic microfluidics in a straight microchannel,71 where 0.50 μm PS particles were focused into the center of the microchannel, while 0.10 μm PS particles were deflected towards the sidewalls. Both of the particles were collected at the outlets with a recovery rate of over 90%. The same design was applied to separate exosomes ranging from 0.03 to 0.20 μm using PEO solutions of different concentrations.
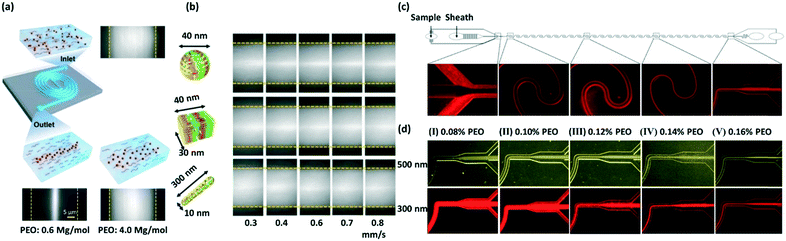 | ||
| Fig. 3 Viscoelastic focusing of DNA origami structures,70 large extracellular vesicles and sub-micrometer PS particles.73 (a) Viscoelastic focusing of 0.10 μm PS particles in a double spiral microchannel using PEO solutions (not to scale). Reproduced from ref. 70. Copyright 2016, American Chemical Society. (b) Viscoelastic focusing of DNA origami with three different structures, i.e., sphere, short and long tube in a waved microchannel using a PEO solution of Mw = 0.60 × 106 g mol−1 and c = 1.80 wt%. Reproduced from ref. 70. Copyright 2016, American Chemical Society. (c) A schematic for the focusing of large extracellular vesicles ranging from 0.20 to 1.0 μm using viscoelastic fluids. Zoom-in experimental figures showing the lateral migration of large extracellular vesicles from sidewalls to the channel center. Reproduced from ref. 73. Copyright 2019, American Chemical Society. (d) The focusing of 0.30 and 0.50 μm PS particles at the trifurcated outlets using PEO solutions with five different concentrations of 0.08%, 0.10%, 0.12%, 0.14% and 0.16%. Reproduced from ref. 73. Copyright 2019, American Chemical Society. | ||
More recently, Liu et al. reported a λ-DNA- and aptamer-mediated approach for the separation and detection of subpopulations of extracellular vesicles, including exosomes (0.03 to 0.2 μm), microvesicles (0.20 to 1.0 μm) and apoptotic bodies (>1.0 μm).72 The focusing behaviors of PS particles with different sizes (i.e., 0.10, 0.20, 0.30, 0.50 and 2.0 μm) and size-based particle separation were visualized, confirming the feasibility of separating extracellular vesicles subpopulations. Moreover, Zhou et al. realized the focusing of large extracellular vesicles ranging from 0.20 to 1.0 μm by a wavy channel using viscoelastic fluids (Fig. 3c). The focusing of 0.30 and 0.50 μm PS particles at different PEO concentrations (e.g., 0.08%, 0.10%, 0.12%, 0.14% and 0.16%) was investigated (Fig. 3d) before running experiments with biological samples, which provides a foundation for the focusing and size-based sorting of large extracellular vesicles.73
With the improvement in theory and the optimization in experimental operating conditions, the resolution of viscoelastic particle focusing has advanced from micrometer to sub-micrometer and even nanometer scale. Viscoelastic focusing method is label-free, size-dependent and easy to operate, but the particle–particle interaction directly impairs the focusing performance. Thus, it is necessary to establish theoretical models and perform experiments to fully evaluate the effects of particle–particle interaction on viscoelastic microfluidics to improve the focusing performance.62
2.3 Deterministic lateral displacement (DLD)
DLD uses pillar arrays tilted with respect to the average flow direction to generate unique flow streamlines, which can be used for focusing particles in a size-dependent manner (Fig. 1c). For example, particles larger than the critical diameter (Dc, eqn (7)), usually considered to be the width of the first streamline, move forward in a bumping mode, while particles smaller than Dc go laterally in a zig-zag mode through the pillars. The bumping and zig-zag trajectories are considered as focusing streamlines. An equation expressing Dc of the DLD device is shown below,74| Dc = 1.4Gε0.48 | (7) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = ε), in which θ is the angle of the pillar array gradient.
θ = ε), in which θ is the angle of the pillar array gradient.
In 2004, Huang et al. demonstrated that 0.80, 0.90 and 1.03 μm fluorescent particles can be focused and sorted continuously to different streamlines based on size at a flow rate of ∼400 μm s−1.75 In this device, the Dc at the inlet was smaller (i.e., <0.80 μm), so that all particles follow the displacement mode, but the gaps increased at the downstream, causing smaller particles to follow the zig-zag mode. This work showed that 0.80 μm particles quickly move in a zig-zag mode, while the 0.90 and 1.03 μm particles change their flow behaviors in the fourth and eighth sections, respectively. Further, the focusing and sorting of two bacterial artificial chromosome DNAs with 61 and 158 kb in size were demonstrated.
Advances in both theory and experimental conditions were increasingly seen in DLD, allowing shape-based focusing and separation of particles at sub-micrometer scale.76 In 2014, Ranjan et al. found that spherical and non-spherical bioparticles can be focused to different tight streams using DLD, allowing the shape-based separation of sub-micrometer bioparticles.77 More specifically, in the DLD device with an array of I-shaped pillars, non-spherical E. coli (∼0.50 μm wide and ∼2.0 μm long) mainly moved forward in a bumping mode from the inlet (Fig. 4a and b) to the outlet (Fig. 4b and c) while the spherical S. epidermidis (∼0.70 μm in diameter) followed a different path, resulting in the separation by shape. Similarly, the non-spherical K. pneumoniae (∼0.60 μm wide and ∼1.80 μm long) and Pseudomonas aeruginosa (P. aeruginosa, ∼0.60 μm wide and ∼1.60 μm long) showed the similar focusing behaviors to non-spherical E. coli. However, focusing particles smaller than 0.50 μm was shown to be challenging at that stage, as the effects such as diffusion and electrostatic forces become significant for smaller particles.78,79 In 2016, the focusing of PS particles as small as 0.051 μm was achieved using a DLD device with a large pore of 2.0 μm.74 More importantly, the focusing and size-based separation of exosomes ranging from 0.02 to 0.14 μm has been demonstrated using a nanometer spherical DLD array (Fig. 4d), opening the door for on-chip sorting and quantification of significant biological particles.80 Also, this work demonstrated the capability of fabricating DLD device with gaps as small as 0.025 μm (Fig. 4e). By adjusting the gap to 0.235 μm, Wunsch et al. demonstrated the focusing of 0.05 μm (yellow) and 0.11 μm (blue) PS particles in a pillar array (Fig. 4f). The detailed theories of DLD for nanometer particle focusing can be found in a study by Kim et al.,81 where a unified theoretical framework to predict particle trajectories in the whole pillar arrays was presented. This theory can be used not only to explain the unexpected particle trajectories but also to design DLD arrays for small particle focusing. Predictions from the theoretical framework were validated experimentally by focusing 0.05 μm PS particles with carboxylate groups. This work provides guidelines for modifying the geometry of pillar arrays to tune the critical diameter as well as the migration of small particles in an altered zig-zag mode.
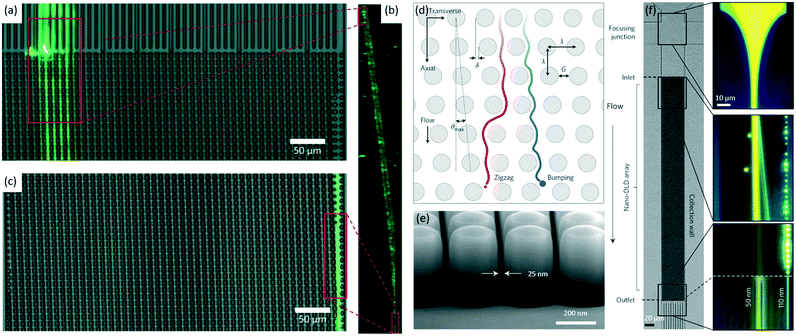 | ||
| Fig. 4 Sub-micrometer particle focusing using DLD pillars with two different shapes: spherical and I-shaped.77,80 (a–c) E. coli (green) in a DLD device with an array of I-shaped pillars mainly moves from the inlet (a) via a bumping mode (b) to the outlet (c). Reproduced from ref. 77 with permission from the Royal Society of Chemistry. (d) A schematic of a spherical pillar array. Particles with diameter DP, below the critical diameter, DC, follow a laminar flow in a zig-zag mode (red), whereas larger particles with DP ≥ DC follow maximum angle θmax in a bumping mode (blue). Reproduced from ref. 80 with permission from Springer Nature. (e) A scanning electron microscope image of an array with λ = 400 nm and G = 25 nm. Reproduced from ref. 80 with permission from Springer Nature. (f) The focusing of 0.05 (right-most jet, blue) and 0.11 μm (left-most jet, yellow) PS particles in an array with a gap of 0.235 um. The initially injected mixture of particles splits into two streamlines due to their differences in lateral displacements. Reproduced from ref. 80 with permission from Springer Nature. | ||
The resolution of DLD focusing has advanced from sub-micrometer scale to nanometer scale to date, enabling various biomedical and biological applications, e.g., separation of exosomes. DLD focusing shows advantages such as label-free operation, easy operation, and high focusing resolution. Although the theories of DLD focusing are improved greatly, its implementation is limited to some extent by clogging induced by particle–particle or particle–surface interactions.76 Anisotropic permeability, the microfluidic array's inherent tendency to induce an undesired lateral pressure gradient and unpredictable particle trajectories, becomes severe for arrays with unequal axial and lateral gaps or with highly asymmetric shapes.82 Moreover, the fabrication process for DLD devices is quite complex.
3. Active methods
Unlike passive techniques, active microfluidic techniques depend on the external fields to generate driving forces to direct sub-micrometer particles to their equilibrium focusing positions. This section discusses three main types of approaches including dielectrophoresis, acoustophoresis and thermophoresis, which use electric field, acoustic field and thermal field, respectively, to focus sub-micrometer particles.3.1 Dielectrophoresis
Dielectrophoretic (DEP) focusing arises from the translational motion of particles by the forces exerted by a non-uniform electric field on the electric dipoles of particles (Fig. 1e). If there are electric field non-uniformities normal to streamlines, particles can be deflected across the streamlines by DEP force (FDEP, eqn (8)), and finally focused to a tight streamline under the combined effect of DEP and hydrodynamic forces.83,84 The magnitude and direction of FDEP on a spherical particle depends on electric field strength (E), permittivity of particle and fluid, and Clausius–Mossotti factor (β), which are formulated as follows,85| FDEP = 2πεmR3 Re(β)∇E2 | (8) |
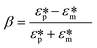 | (9) |
In 2010, Kayani et al. designed a microfluidic device (Fig. 5a) with curved electrodes to focus smaller (<1.0 μm) particles (Fig. 5b and c).87 Using deionized water with relative permittivity of 77.6 and electric conductivity of 2 × 10−4 S m−1, as the suspending medium, the focusing of 0.23 μm silica particles into a single stream has been demonstrated (Fig. 5d) at a flow rate of 10 μL min−1 (the magnitude and frequency of the alternating current, AC, field is 15 V and 5.0 MHz, respectively). Similarly, a narrow focal stream was observed for 0.45 μm silica particles at a flow rate of 2.0 μL min−1 (Fig. 5e). Further, Chrimes et al. demonstrated the focusing of ∼0.22 μm PS particles and ∼0.08 μm tungsten trioxide (WO3) particles using a similar electrode design.88 A study by Park et al. demonstrated the focusing of E. coli by negative DEP at the upstream channel, which facilitates the separation from blood cells by positive DEP at the downstream.89
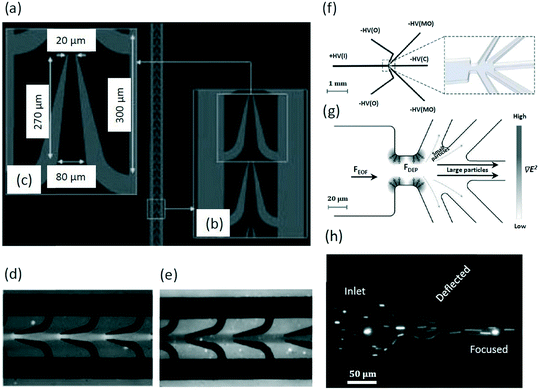 | ||
| Fig. 5 DEP devices for the focusing of silica particles87 and PSI crystals.26 (a) A DEP electrode array consists (b) curved electrodes with (c) specific dimensions. Reproduced from ref. 87 with permission from John Wiley and Sons. DEP focusing of (d) 0.23 and (e) 0.45 μm silica particles. Reproduced from ref. 87 with permission from John Wiley and Sons. (f) A schematic of the entire iDEP focusing and sorting device (without reservoirs for clarity). The zoom-in schematic shows the constriction region connecting the inlet channel to the outlets (right). Reproduced from ref. 26. Copyright 2013, American Chemical Society. (g) Larger particles are focused toward the center of the device while the smaller particles are deflected into the side outlet channels using iDEP. Reproduced from ref. 26. Copyright 2013, American Chemical Society. (h) A fluorescent image of PSI crystal focusing. Reproduced from ref. 26. Copyright 2013, American Chemical Society. | ||
In 2013, an simulation of iDEP by Abdallah et al. showed the separation of 0.09 μm particles from 0.90 μm ones by focusing the larger particles in the center while deflecting the smaller ones to the side channels.26 The microfluidic device consists of one inlet for sample injection, where positive potential is applied, and five outlets with negative potentials for the collection of the sorted fractions (Fig. 5f). The inlet channel and outlet channels are connected by a constriction region, which is used to produce an inhomogeneous electric field (Fig. 5f, right). When flowing from the inlet to outlets driven by electroosmosis, larger particles are focused to the channel centerline by negative DEP force (Fig. 5g). Based on this mechanism, larger photosystem I (PSI) crystals were focused into the center of the microchannel, while the smaller ones of around 0.10 μm were deflected to the outlets closer to the sidewalls (Fig. 5h).
Kim et al. reported that E. coli can be focused under high flow rate conditions (e.g., 25 μL min−1) using positive DEP.90 The focused E. coli can be captured by the electrodes to serve as sensors, showing the potential for the development of portable and highly sensitive chips that can enable rapid detection of bacteria in drinking water. In 2017, the focusing of λ-DNA of 48.5 kb was achieved, along with its separation from plasmid DNA of 10.2 kb.91 Interestingly, Rozynek et al. showed that conductive particles with diameters ranging from sub-micrometer to micrometer scale could be focused using DEP forces and then deposited onto the surface of any materials, e.g., glass, to form desired conductive patterns, holding promise for the manufacturing of circuits.92 By combining with accurate control over the conductivity of sub-micrometer particles, it may allow the use of microfluidics for precise particle patterning.
Although DEP focusing shows advantages such as high focusing resolution, real-time control, etc., it is still limited to the complicated process for sample preparation and the risk of sample damage. The main demerit of DEP is the demand for the sample with low conductivity, such as deionized water and sucrose solution.93,94 Unfortunately, most clinical samples and food matrices, e.g., whole human blood and cow's milk, have high conductivity,95,96 making them unsuitable for use in DEP devices. In summary, as one of the main pillars of particle manipulation techniques in microfluidics, DEP is widely used and still catching interests in the future.97–100
3.2 Acoustophoresis
Acoustophoretic focusing refers to the use of acoustic waves to induce particle lateral migration towards or away from pressure nodes or antinodes (Fig. 1f). It depends not only on the density and compressibility of the suspending medium but also on the size, density and compressibility of the particle. There are two types of acoustic standing waves: 1) bulk acoustic waves operating at the frequency usually in the range from 1.0 to 10 MHz, and 2) surface acoustic waves (SAWs) which can be further classified into traveling surface acoustic waves (TSAWs) and standing surface acoustic waves (SSAWs).101,102 Since the acoustic force is proportional to the SAW frequency, higher operating frequency (e.g., 636 MHz) is always used to counterbalance the drag force to focus particles smaller than 1.0 μm.103–106 A TSAW arises from an AC signal applied to interdigitated transducers (IDTs) on piezoelectric substrates, while an SSAW arises when two TSAWs interfere oppositely.102,107 In an SSAW, the acoustic force (Fa) can be calculated by the acoustic pressure (p) on a spherical particle with d in diameter as below,28,108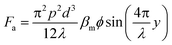 | (10) |
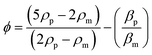 | (11) |
Using SSAW-induced acoustophoresis, it demonstrated that 0.87 μm PS particles can be focused along the sidewalls of the microchannel at flow rates ranging from 0.60 to 2.0 μL min−1.108 Continuous particle separation and a subsequent collection at the downstream have been achieved. Also, 0.50 μm PS particles are steadily aligned into a focal streamline by SAWs at a flow rate of 0.20 μL min−1.101 In 2014, Destgeer et al. designed a cross type particle sorter that could generate TSAWs by a focused IDT (Fig. 6a).102 This device enabled the separation of 0.71 and 3.0 μm PS particles by focusing them into different streamlines by TSAWs at 200 MHz (Fig. 6b). Also, a study by Collins et al. showed that 0.50 μm PS particles can be focused using SAWs.110 By taking advantage of the acoustic streaming induced drag force, Antfolk et al. achieved the focusing of E. coli and PS particles as small as 0.50 μm with a recovery rate of over 90% using bulk acoustic waves.27
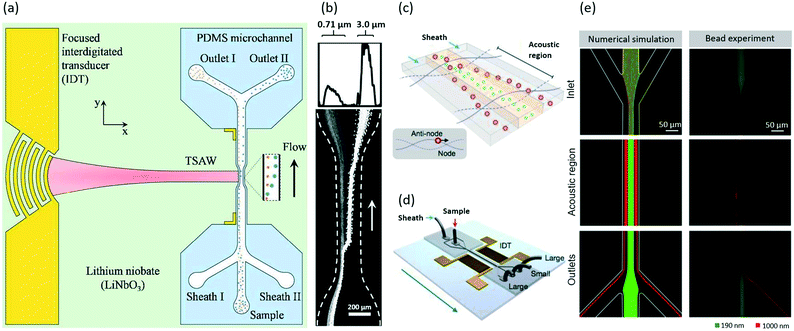 | ||
| Fig. 6 Acoustic focusing of PS particles102 and extracellular vesicles.28 (a) A schematic showing the cross type acoustic particle separator. The TSAWs generated by the IDT are coupled with the fluid inside the PDMS microchannel. The particles are collected at separate outlets. Reproduced from ref. 102 with permission from the Royal Society of Chemistry. (b) The focusing of 0.71 and 3.0 μm PS particles into different streamlines with TSAWs. The plot (top) shows the scattering light intensity from the particles across the width of the microchannel. Reproduced from ref. 102 with permission from the Royal Society of Chemistry. Schematics of (c) the operation mechanism with SSAWs and (d) the acoustic device. Reproduced from ref. 28. Copyright 2015, American Chemical Society. (e) Numerical and experimental results showing the focusing of 0.19 μm (green) and 1.0 μm (red) PS particles to focal streamlines at different lateral positions using SSAWs. Reproduced from ref. 28. Copyright 2015, American Chemical Society. | ||
In 2015, Lee et al. showed that exosomes are able to be focused using acoustic forces in an SSAW-based device,28 which consists of a pair of IDT electrodes to produce SSAWs across the flow, and two side outlets and one center outlet for collecting larger extracellular vesicles and exosomes, respectively (Fig. 6c and d). They also demonstrated the focusing of 0.19 and 1.0 μm PS particles in both numerical simulations and experiments (Fig. 6e). Then this device was utilized for selective isolation of exosomes smaller than 0.20 μm by displacing the larger extracellular vesicles to nodes of acoustic pressure region (Fig. 6c). During this process, the larger extracellular vesicles move faster compared to smaller ones due to larger acoustic force that is proportional to the volume of extracellular vesicles.
In 2018, Gautam et al. reported the use of SAWs for focusing E. coli and 0.25 μm PS particles by displacing them to different lateral positions within the microchannel.111 This work is important as it achieves high-speed (50 μL min−1) separation of micrometer and sub-micrometer particles by displacing particles to different size-dependent streamlines. Also, the focusing of E. coli was demonstrated using tilted-angle SSAWs.112 More recently, Wu et al. achieved the focusing of extracellular vesicles and lipoproteins using SSAWs, leading to continuous particle separation at the downstream.113 Please note that these two types of sub-micrometer particles are similar in size, but their acoustic properties, the tendency to move to the nodes or antinodes, are different.
Acoustophoretic focusing is potentially useful for health monitoring, disease diagnosis and personalized medicine. It shows merits such as non-contact, gentle and label-free manipulation, making it as a promising technique for sub-micrometer particle focusing. Although inherently limited by the attenuation of acoustic waves, the understanding of acoustic focusing in fluids has greatly advanced in parallel with development in devices and fundamentals of piezoelectricity, piezoelectric materials and transducers.114
3.3 Thermophoresis
Thermophoresis is a process in which suspended particles are driven towards the lower temperature region in non-isothermal mixtures. Thermophoretic focusing refers to the focusing of particles by adjusting thermophoretic force (FT) acting on the suspended particles when a temperature gradient exists (Fig. 1g). For example, the particles can be equilibrated to the isothermal layer due to FT (eqn (12)) within a channel. After the discovery of thermophoretic phenomenon by Maxwell, Epstein quantitated FT on a spherical particle in a gas by an equation, which was further improved by Brock and Talbot et al. for more general predictions.115–117FT can be expressed as below,118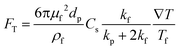 | (12) |
Thermophoretic behavior is similar to that of thermodiffusion as the size of the suspended phase is similar to the one of the molecules, indicating the key role of thermophoretic force on the particles with the size in the range of 0.10 to 1.0 μm.120 The simulation by Eslamian et al. demonstrated that 1.0 μm gold particles can be focused to a streamline close to channel center within a channel having a 20 K difference in temperature between the channel walls (a velocity of 50 mm s−1).121 By using a similar microchannel (Fig. 7a), the focusing of sub-micrometer particles ranging from 0.10 μm to 1.0 μm was achieved by thermophoretic effects.118 The numerical simulations in this work showed that 0.10, 0.50 and 1.0 μm particles can be focused to different lateral positions when there is a 10 K temperature difference between channel walls. Here, the velocity gradient across the width of the channel is almost constant (Fig. 7b), while the temperature gradient across the channel width shows a dramatic decrease, enabling the thermophoretic force to drive relatively larger particles upwards by counterbalancing the gravitational force (Fig. 7c). When the temperature difference between channel walls was changed to 2.0 K, it showed that 0.50 μm particles that randomly distributed at the inlet (Fig. 7d) can be focused at 3.0 mm downstream (Fig. 7e). It should be noted that both numerical simulations and experiments have been performed to demonstrate the successful focusing of sub-micrometer particles by thermophoresis. Thermophoretic focusing of sub-micrometer charged PS particles was also observed in experiment.122 Zhou et al. demonstrated that 0.74 μm charged PS particles can be focused and then sorted from the 2.0 μm ones in a size-dependent manner using thermophoresis.
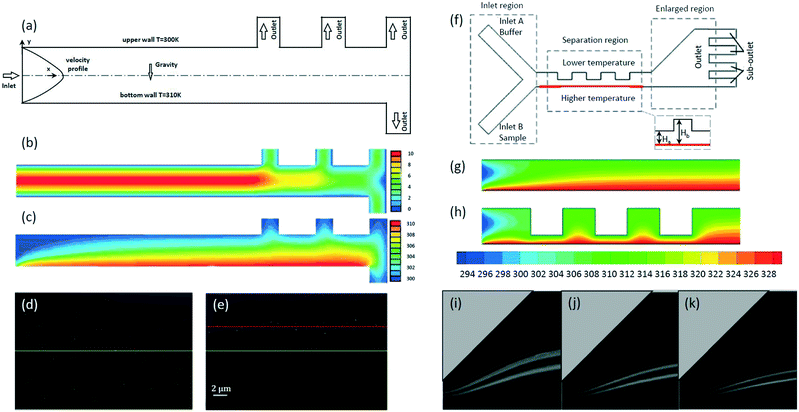 | ||
Fig. 7 The focusing of sub-micrometer particles using thermophoretic effects.118,140 (a) The microchannel with a temperature difference between sidewalls for thermophoretic focusing of sub-micrometer particles. Reproduced from ref. 118 with permission from Taylor & Francis. Simulation results of (b) velocity contours and (c) temperature contours in the microchannel. Reproduced from ref. 118 with permission from Taylor & Francis. Experimental results showing that 0.50 μm PS particles are randomly distributed at the inlet (d) but focused at 3.0 mm downstream (e). The yellow and red lines represent the center of the channel and the particle focusing streamlines, respectively. Reproduced from ref. 118 with permission from Taylor & Francis. (f) A schematic of the thermophoretic sorter with an expansion–contraction microchannel. Simulation results of temperature gradient contours with an expansion–contraction ratio of (g) Hb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ha = 1 and (h) Hb Ha = 1 and (h) Hb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ha = 4. Reproduced from ref. 140. Copyright 2019, Elsevier. Experimental results showing the focusing of 0.50 μm (up) and 1.0 μm (low) fluorescent particles at the inlet velocity ratio of (i) uA Ha = 4. Reproduced from ref. 140. Copyright 2019, Elsevier. Experimental results showing the focusing of 0.50 μm (up) and 1.0 μm (low) fluorescent particles at the inlet velocity ratio of (i) uA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) uB = 4, (j) uA uB = 4, (j) uA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) uB = 8 and (k) uA uB = 8 and (k) uA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) uB = 12. Reproduced from ref. 140. Copyright 2019, Elsevier. uB = 12. Reproduced from ref. 140. Copyright 2019, Elsevier. | ||
Based on the information provided above, we can see that the resolution in thermophoretic focusing has improved, indicating the potential of further applications in the detection and separation of nanometer particles in a label-free manner. However, thermophoresis may induce thermophysical changes in bioparticles, e.g., lipid vesicles.123
4. Other methods
Besides the six main microfluidic methods discussed above, there are a few more techniques applicable for sub-micrometer particle focusing, such as hydrophoresis, optical tweezers, magnetophoresis and electrophoresis. Hydrophoretic and optical methods have been demonstrated for focusing particles at sub-micrometer scale, however, we failed to find any reports on sub-micrometer particle focusing by either magnetophoresis or electrophoresis.Hydrophoresis refers to the movement of suspended particles using rotational flows induced by anisotropic obstacles (Fig. 1d).124 Geometric parameters such as channel width and oblique angle of grooves can affect focusing efficiency.125 By using anisotropic obstacles, 0.52 μm PS particles were shown to be focused by hydrophoretic effects at a flow rate of 5.1 mm s−1.124 The same device was also used for the separation of DNA molecules of 49 and 115 kb. Hydrophoresis is simple to operate, but it subjects to high probability of clogging since the obstacle height needs to be comparable to the particle size.2
Optical forces can be used to manipulate particles with size ranging from tens of nanometers to tens of micrometers.126,127 The underlying mechanism of the optical focusing technique is identical to that of optical tweezers for particle trapping (Fig. 1h). Zhao et al. provided a method based on optical gradient forces to guide and focus particles in fluid flows by numerical simulations and particles as small as 0.10 μm were focused close to the center of the microchannel.128 This method features non-contact, easy fabrication and operation, but it is limited by low flow rates (e.g., 1.0 mm s−1).
Magnetophoresis demonstrates its capability for the focusing of particles at micrometer scale, e.g., superparamagnetic and diamagnetic particles and cells,129–132 where the forces exerted on the particles are generated due to the difference in permeability between particles and the suspending medium. Electrophoresis, in which a uniform electric field is applied, enables the focusing of charged particles with different sizes into different streamlines in a size-dependent manner, and facilitates downstream separation in microfluidics.133 A study by Kawamata et al. demonstrated that 1.0 and 2.1 μm particles are focused into different streamlines by electrophoretic forces.134 Compared with DEP, electrophoresis is limited to charged particles.
Hybrid methods, which normally combine active and passive techniques, are emerging techniques that feature better performance in terms of stability, versatility and convenience.135,136 The integration of 1) DLD with DEP, and 2) inertial microfluidics with thermophoresis have been demonstrated for the focusing of sub-micrometer particles. By introducing an electric field to DLD, the focusing of PS particles and extracellular vesicles was achieved in a tunable manner.137,138 The integration of DEP with DLD can decrease the critical size of a DLD chip in a controlled manner, enabling the focusing not only depends on size but also on dielectric properties of target particles.139 Also, this hybrid method shows other benefits, such as the decrease in the probability of clogging, as the Dc can be decreased with the aid of DEP without the need to reduce the size of the gaps between the pillars.
The integration of inertial microfluidics and thermophoresis enables the focusing of sub-micrometer particles at a relatively low flow rate.140 Wang et al. achieved the thermophoretic sorting of 0.50 and 1.0 μm fluorescent particles by focusing them into different streamlines in an expansion–contraction microchannel with the aid of inertial microfluidics (Fig. 7f). Numerical simulations demonstrated that the contraction region with an expansion–contraction ratio of 4 (Fig. 7h) enhances the temperature gradient compared to the one with an expansion–contraction ratio of 1 (Fig. 7g). The enhanced temperature gradient increases the magnitude of the thermophoretic force exerted on particles, leading to the better focusing performance in the expansion region. Experimental results showed that 0.50 and 1.0 μm fluorescent particles were focused to upper and lower streamlines, respectively (Fig. 7i–k) at different inlet velocity ratios (ΔT = 5.0 K, uB = 2.0 mm s−1, Hb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ha = 4). Hybrid methods seem to be more promising for particle focusing as the research is becoming increasingly interdisciplinary.
Ha = 4). Hybrid methods seem to be more promising for particle focusing as the research is becoming increasingly interdisciplinary.
5. Discussion
Microfluidics is capable of focusing sub-micrometer particles, such as PS beads, bacteria, exosomes and DNA, which are significant in biomedical, environmental and industrial fields. A variety of microfluidic techniques have been demonstrated for particle focusing with resolution at sub-micrometer scale, although it is still challenging for a couple of techniques such as hydrophoresis and inertial microfluidics to focus the particles as small as 0.10 μm. New physics begin to emerge and need to be considered as particle size decreases to sub-micrometer scale. In this section, we firstly talk about four types of particles within sub-micrometer range, and then the challenges including diffusion, throughput and resolution are discussed. We also talk about the ability of each method to integrate with downstream separation components. Lastly, design guidelines for the focusing of sub-micrometer particles using each technique are presented, which are expected to be useful for lab-on-a-chip researchers in both academia and industry.5.1 Sub-micrometer particles
Inertial microfluidics and hydrophoresis do not show high focusing resolution, as it calls for a significant increase in minimum channel length for inertial focusing and comparable obstacle height to particle size for hydrophoresis. DLD shows better performance in precise particle focusing and separation, especially for nanometer PS particles, but the throughput is quite low (e.g., 0.05 μL min−1),74 which may limit its applications. Acoustophoresis is promising for the focusing of PS particles at sub-micrometer scale due to its high compatibility and advances in piezoelectric thin films.141
DLD device with I-shaped pillars realizes the shape-based focusing and separation of the bacteria, by focusing the spherical S. epidermidis into the streamlines different to that of the rod-shaped bacteria including E. coli, K. pneumoniae and P. aeruginosa.77 Although inertial microfluidics has been demonstrated for focusing of particles at micrometer scale by shape,144,145 there is no report on sub-micrometer particles yet. Please note that other methods such as magnetophophoresis146 and nanofluidics147 have been demonstrated for the separation of bacteria.
5.2 Challenges
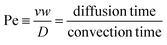 | (13) |
 | (14) |
A common strategy to focus small particles is to scale down the channel dimensions. This usually works for passive methods due to their dependence on the intrinsic properties of fluids and channel geometry. For example, the downscaling of channel cross section contributes to the focusing of 0.92 μm PS particles at a flow rate of 500 μL min−1.50 The throughput of viscoelastic microfluidics is generally lower than that of inertial microfluidics, as the viscoelastic medium causes an increase in fluidic resistance. The ratio of surface to volume is relatively high for the fluid flowing in a DLD device, thus increasing fluidic resistance and contributing to the extremely low throughput. For example, a study by Wunsch et al. showed that the flow rate used for the focusing of exosomes (0.02 to 0.14 μm) ranges from 0.1 to 0.2 nL min−1.80 Besides, ΔP will increase with the increase of surface to volume ratio, resulting in a higher risk of device damage. Anisotropic obstacles are employed in hydrophoresis to induce rotational flows to arrange particles, leading to a relatively high ratio of surface to volume. Thus, the resultant fluidic resistance is high, which may be a reason for low throughput in hydrophoretic focusing of sub-micrometer PS particles.124
Active methods also encounter fluidic resistance with respect to the focusing of sub-micrometer particles. Further, particle focusing is influenced by the combined effects of hydrodynamic forces and driving forces enabled by external fields, thus the throughput cannot increase unlimitedly. Kim et al. achieved the focusing of E. coli using DEP at a moderate flow rate (25 μL min−1),90 which is similar to the flow rate used in thermophoresis (24 μL min−1).118 The flow rate applied in acoustophoresis (0.20 to 2.0 μL min−1) is not high in the focusing of sub-micrometer PS particles,27,101,102,108,110 except the one used by Gautam et al. (50 μL min−1).111 Although optical focusing of 0.10 μm particles has been achieved, the throughput is extremely low (0.24 nL min−1).128
5.3 Capability of integration with downstream separation components
Various microfluidic focusing techniques, such as inertial microfluidics,164 DLD,165 hydrodynamic sheath focusing,166,167 DEP168 and acoustophoresis169,170 have demonstrated the capability of integrating with downstream sorting components for the separation of micrometer scale particles (e.g., circulating tumor cells, microalgae cells, and PS particles). Unfortunately, there are only a few reports on the integration of upstream focusing with downstream sorting for applications to sub-micrometer particles. The challenges, such as fluidic resistance, diffusion and detection, may be accountable for this.Hydrodynamic sheath focusing has been integrated with optical tweezers for the separation of sub-micrometer microbial cells.171 In 2017, Wu et al. realized the separation of exosomes directly from whole blood using a microfluidic device with two sets of acoustic units (Fig. 8).163 The upstream unit is responsible for the focusing of apoptotic bodies, exosomes and microvesicles while deflecting larger particles including white blood cells, red blood cells and platelets. Then, the downstream unit achieves the separation of exosomes, apoptotic bodies and microvesicles (Fig. 8a and b). Larger particles are subjected to a larger acoustic force enabled by the SAW field, and are driven toward the pressure node (Fig. 8c). This work demonstrates the ability of the acoustic focusing technique to integrate with the downstream separation components. We expect that this device can further advance exosome-related biomedical research with potential applications in health monitoring, disease diagnostics and therapeutics.
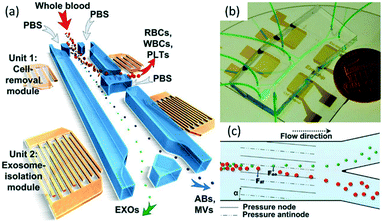 | ||
| Fig. 8 Schematic illustration and mechanisms for isolating exosomes from blood using an acoustic microfluidic device.163 (a) RBCs, WBCs, and PLTs are filtered by the cell-removal module, and then subgroups of EVs (ABs: apoptotic bodies; EXOs: exosomes; MVs: microvesicles) are separated. Reproduced from ref. 163. Copyright 2017, National Academy of Sciences, USA. (b) An optical image of the device. Two acoustic modules are integrated on a single chip. Reproduced from ref. 163. Copyright 2017, National Academy of Sciences, USA. (c) Size-based separation due to the lateral deflection induced by an acoustic field. The periodic distribution of pressure nodes and antinodes produces an acoustic radiation force to push larger particles toward node planes. Reproduced from ref. 163. Copyright 2017, National Academy of Sciences, USA. | ||
5.4 Design guidelines
The mechanisms underlying each technique are different, thus leading to the difficulties in the development of overall design guidelines for the focusing of sub-micrometer particles. Here, we summarize general design guidelines for each technique based on its operating principles.In inertial microfluidics, required minimum channel length and flow rate should be designed to achieve effective focusing of particles to equilibrium positions in straight rectangular or continuously curving channels.49 To avoid trade-off between these two parameters, using a channel with varying cross sections is a promising method.55 For viscoelastic microfluidics, precise control of the rheological properties of the carrier solution is the key.58 Critical diameter plays an essential role in a DLD device, so its design should focus on the physics between pillars. Moreover, the integration of DLD with external fields provides an alternative approach to promote focusing performance.138 Appropriate anisotropic obstacles are used for hydrophoretic focusing, and the height of the obstacles need to be comparable to particle size.125
Non-uniform distributed electrical field is the key for DEP focusing, and careful design of electrode arrays within channels or insulating structures is necessary.172 A general approach for effective focusing of small-sized particles by acoustophoresis is to use high-frequency acoustic radiation. Also, the layout of piezoelectric films needs to be considered. Particle focusing using thermophoresis is at a nascent stage, and several factors, such as temperature gradient, flow rate and channel geometry can affect thermophoretic focusing. The mechanism of focusing sub-micrometer particles using optical force is identical to optical tweezers.128,173 Please refer to comprehensive and in-depth studies on inertial microfluidics,49,55,56,174 viscoelastic microfluidics,58,175,176 DLD,76,138,177 hydrophoresis,124,125 DEP,172,178 acoustophoresis,107,141,179 thermophoresis118,180 and optical focusing128,173 to obtain detailed design guidelines.
6. Conclusion
Due to the significance of sub-micrometer particles (e.g., microvesicles, protein aggregates and E. coli) in various fields such as biology, biomedicine, chemistry and environment, the focusing of sub-micrometer particles, a prerequisite step for downstream detection, separation and manipulation, draws great interest in microfluidic devices. With improvements in theoretical fundamentals and fabrication technology, the focusing resolution has advanced from micrometer to sub-micrometer and even to nanometer scale. It also allows the integration of particle focusing techniques to lab-on-a-chip systems for wider applications.In this work, eight main microfluidic techniques, either passive or active, for focusing sub-micrometer particles have been discussed along with hybrid methods. Their characteristics are summarized and compared in Table 1. The selection of the optimum approach usually depends on the needs of applications. Each microfluidic technique has its merits and demerits, for example, inertial microfluidics can achieve high throughput but it is limited by low focusing resolution. Further, hybrid microfluidics integrates the strength of each approach, making it attractive for particle focusing. Although great advances in sub-micrometer particle focusing have been achieved in microfluidic devices recently, it calls for further optimization of these techniques to improve focusing resolution, simplify sample preparation, reduce the risk of sample damage, enhance throughput, etc. for practical applications. We expect that better performance of sub-micrometer particle focusing in terms of accuracy, throughput and reproducibility can be achieved with future advances in microfluidic techniques.
| Classification | Method | Criteria | Sample | Size (μm) | Throughput | Merits | Demerits |
|---|---|---|---|---|---|---|---|
| Inertial focusing50,54–56 | Inertial fluid | Size | PS particles, E. coli, S. typhimurium and K. pneumoniae | 0.50 to 0.92 | 30 to 500 μL min−1 | High throughput | Low resolution |
| Viscoelastic microfluidics65,68,69,71–73 | Viscoelastic fluid | Viscoelasticity and size | PS particles, E. coli, λ-DNA and T4-DNA, exosomes, microvesicles | 0.10 to ∼1.0 | 0.27 nL to 25 μL min−1 | Easy to operate | Particle–particle interactions |
| DLD74,75,77,80 | DLD pillars | Size and shape | PS particles, DNA, E. coli, S. epidermidis, K. pneumoniae | 0.02 to 1.50 | 0.10 nL to 0.05 μL min−1 | High resolution and shape-dependent | Complex fabrication and anisotropic permeability |
| P. aeruginosa, exosomes | |||||||
| Hydrophoresis124 | Anisotropic obstacles | Size | PS particles and DNA | 0.52 to 1.10 | 0.01 μL min−1 | Easy control | Low throughput and clogging |
| DEP26,87–89,91 | Non-uniform electric field | Polarizability and size | Silica, PS and WO3 particles, E. coli, PSI crystals, λ-DNA | 0.08 to 0.90 | 0.022 to 25 μL min−1 | High resolution and real-time control | Complicated preparation process and risk for sample damage |
| Acoustophoresis97,108,63 | Acoustic field | Compressibility, acoustic properties, density and size | PS particles, exosomes, E. coli, chylomicrons, and lipoproteins | 0.11 to 0.87 | 0.20 to 4.0 μL min−1 | Versatile and non-contact | Attenuation of acoustic waves |
| Thermophoresis118 | Non-isothermal field | Size | PS particles | 0.10 to 1.0 | 24 μL min−1 (10 mm s−1) | Non-contact | Risk of changes in structures |
| Optical tweezers128 | Optical field | Size | N.A. | 0.10 to 0.20 | 0.24 nL min−1 | No need for sheath flow and simple fabrication | Low throughput |
| DEP + DLD137,138 | Non-uniform electric field + DLD pillars | Polarizability and size | Extracellular vesicles, PS particles | 0.05 to 0.50 | 0.30 nL min−1 | Tuneable critical diameter | Low throughput |
| Inertial + thermophoresis140 | Inertial fluid + non-isothermal field | Size | Fluorescent particles | 0.50 to 1.0 | N.A. | Non-contact | Low throughput |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by Amada Foundation and NSG Foundation of Japan and MQ-NAIST Cotutelle Program. We also acknowledge the support of JSPS Core-to-Core program and Macquarie University New Staff Grant.References
- J. Shi, S. Yazdi, S. C. S. Lin, X. Ding, I. K. Chiang, K. Sharp and T. J. Huang, Lab Chip, 2011, 11, 2319–2324 RSC
.
- X. Xuan, J. Zhu and C. Church, Microfluid. Nanofluid., 2010, 9, 1–16 CrossRef
.
- N. Nitta, T. Sugimura, A. Isozaki, H. Mikami, K. Hiraki, S. Sakuma, T. Iino, F. Arai, T. Endo, Y. Fujiwaki, H. Fukuzawa, M. Hase, T. Hayakawa, K. Hiramatsu, Y. Hoshino, M. Inaba, T. Ito, H. Karakawa, Y. Kasai, K. Koizumi, S. Lee, C. Lei, M. Li, T. Maeno, S. Matsusaka, D. Murakami, A. Nakagawa, Y. Oguchi, M. Oikawa, T. Ota, K. Shiba, H. Shintaku, Y. Shirasaki, K. Suga, Y. Suzuki, N. Suzuki, Y. Tanaka, H. Tezuka, C. Toyokawa, Y. Yalikun, M. Yamada, M. Yamagishi, T. Yamano, A. Yasumoto, Y. Yatomi, M. Yazawa, D. Di Carlo, Y. Hosokawa, S. Uemura, Y. Ozeki and K. Goda, Cell, 2018, 175, 266–276.e13 CrossRef CAS PubMed
.
- C. Lei, H. Kobayashi, Y. Wu, M. Li, A. Isozaki, A. Yasumoto, H. Mikami, T. Ito, N. Nitta, T. Sugimura, M. Yamada, Y. Yatomi, D. Di Carlo, Y. Ozeki and K. Goda, Nat. Protoc., 2018, 13, 1603–1631 CrossRef CAS
.
- K. Goda, A. Ayazi, D. R. Gossett, J. Sadasivam, C. K. Lonappan, E. Sollier, A. M. Fard, S. C. Hur, J. Adam, C. Murray, C. Wang, N. Brackbill, D. Di Carlo and B. Jalali, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 11630–11635 CrossRef CAS
.
- D. R. Gossett, H. T. K. Tse, S. A. Lee, Y. Ying, A. G. Lindgren, O. O. Yang, J. Rao, A. T. Clark and D. Di Carlo, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 7630–7635 CrossRef CAS
.
- H. T. K. Tse, D. R. Gossett, Y. S. Moon, M. Masaeli, M. Sohsman, Y. Ying, K. Mislick, R. P. Adams, J. Rao and D. Di Carlo, Sci. Transl. Med., 2013, 5, 212ra163 CrossRef
.
- S. H. Yang, D. J. Lee, J. R. Youn and Y. S. Song, Anal. Chem., 2017, 89, 3639–3647 CrossRef CAS
.
- E. K. Sackmann, A. L. Fulton and D. J. Beebe, Nature, 2014, 507, 181–189 CrossRef CAS
.
- D. Mark, S. Haeberle, G. Roth, F. Von Stetten and R. Zengerle, Chem. Soc. Rev., 2010, 39, 1153–1182 RSC
.
- N. Ota, Y. Owa, T. Kawai and Y. Tanaka, J. Chromatogr. A, 2016, 1455, 172–177 CrossRef CAS
.
- A. M. Gañán-Calvo, J. M. Montanero, L. Martín-Banderas and M. Flores-Mosquera, Adv. Drug
Delivery Rev., 2013, 65, 1447–1469 CrossRef PubMed
.
- K. S. Elvira, X. C. I. Solvas, R. C. R. Wootton and A. J. deMello, Nat. Chem., 2013, 5, 905–915 CrossRef CAS
.
- W. Jung, J. Han, J. W. Choi and C. H. Ahn, Microelectron. Eng., 2015, 132, 46–57 CrossRef CAS
.
- Y. Shen, Y. Yalikun and Y. Tanaka, Sens. Actuators, B, 2019, 282, 268–281 CrossRef CAS
.
- T. A. Brown, R. D. Fetter, A. N. Tkachuk and D. A. Clayton, Methods, 2010, 51, 458–463 CrossRef CAS
.
- M. Navratil, A. Terman and E. A. Arriaga, Exp. Cell Res., 2008, 314, 164–172 CrossRef CAS PubMed
.
- H. S. Horowitz, S. J. Mclain, A. W. Sleight, J. D. Druliner, P. L. Gai, M. J. Vankavelaar, J. L. Wagner, B. D. Biggs and S. J. Poon, Science, 1989, 243, 66–69 CrossRef CAS
.
- S. Hong, J. Bhardwaj, C. H. Han and J. Jang, Environ. Sci. Technol., 2016, 50, 12365–12372 CrossRef CAS
.
- C. J. Tan and Y. W. Tong, Anal. Chem., 2007, 79, 299–306 CrossRef CAS
.
- T. Bollhorst, T. Grieb, A. Rosenauer, G. Fuller, M. Maas and K. Rezwan, Chem. Mater., 2013, 25, 3464–3471 CrossRef CAS
.
- N. Lewinski, V. Colvin and R. Drezek, Small, 2008, 4, 26–49 CrossRef CAS PubMed
.
- S. Foss Hansen, B. H. Larsen, S. I. Olsen and A. Baun, Nanotoxicology, 2007, 1, 243–250 CrossRef
.
- M. Auffan, J. Rose, J. Y. Bottero, G. V. Lowry, J. P. Jolivet and M. R. Wiesner, Nat. Nanotechnol., 2009, 4, 634–641 CrossRef CAS
.
- R. Vasudev, S. Mathew and N. Afonina, J. Pharm. Sci., 2015, 104, 1622–1631 CrossRef CAS
.
- B. G. Abdallah, T. C. Chao, C. Kupitz, P. Fromme and A. Ros, ACS Nano, 2013, 7, 9129–9137 CrossRef CAS PubMed
.
- M. Antfolk, P. B. Muller, P. Augustsson, H. Bruus and T. Laurell, Lab Chip, 2014, 14, 2791–2799 RSC
.
- K. Lee, H. Shao, R. Weissleder and H. Lee, ACS Nano, 2015, 9, 2321–2327 CrossRef CAS PubMed
.
- S. EL Andaloussi, I. Mäger, X. O. Breakefield and M. J. A. Wood, Nat. Rev. Drug Discovery, 2013, 12, 347–357 CrossRef CAS
.
- B. György, T. G. Szabó, M. Pásztói, Z. Pál, P. Misják, B. Aradi, V. László, É. Pállinger, E. Pap, Á. Kittel, G. Nagy, A. Falus and E. I. Buzás, Cell. Mol. Life Sci., 2011, 68, 2667–2688 CrossRef
.
- M. A. Antonyak, B. Li, L. K. Boroughs, J. L. Johnson, J. E. Druso, K. L. Bryant, D. A. Holowka and R. A. Cerione, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 4852–4857 CrossRef CAS
.
- E. Boilard, P. A. Nigrovic, K. Larabee, G. F. M. Watts, J. S. Coblyn, M. E. Weinblatt, E. M. Massarotti, E. Remold-O'Donnell, R. W. Farndale, J. Ware and D. M. Lee, Science, 2010, 327, 580–583 CrossRef CAS
.
- B. György, K. Módos, E. Pállinger, K. Pálóczi, M. Pásztói, P. Misják, M. A. Deli, A. Sipos, A. Szalai, I. Voszka, A. Polgár, K. Tóth, M. Csete, G. Nagy, S. Gay, A. Falus, A. Kittel and E. I. Buzás, Blood, 2011, 117, e39–48 CrossRef
.
- C. Soto, L. Estrada and J. Castilla, Trends Biochem. Sci., 2006, 31, 150–155 CrossRef CAS
.
- M. Jucker and L. C. Walker, Nature, 2013, 501, 45–51 CrossRef CAS
.
- C. Lin, R. Jungmann, A. M. Leifer, C. Li, D. Levner, G. M. Church, W. M. Shih and P. Yin, Nat. Chem., 2012, 4, 832–839 CrossRef CAS
.
- M. Otto, Nat. Rev. Microbiol., 2009, 7, 555–567 CrossRef CAS
.
- F. D. Lowy, N. Engl. J. Med., 1998, 339, 520–532 CrossRef CAS PubMed
.
- A. Morel, Y. H. Ahn, F. Partensky, D. Vaulot and H. Claustre, J. Mar. Res., 1993, 51, 617–649 CrossRef CAS
.
- F. Partensky, W. R. Hess and D. Vaulot, Microbiol. Mol. Biol. Rev., 1999, 63, 106–127 CAS
.
- E. T. Buitenhuis, W. K. W. Li, D. Vaulot, M. W. Lomas, M. R. Landry, F. Partensky, D. M. Karl, O. Ulloa, L. Campbell, S. Jacquet, F. Lantoine, F. Chavez, D. Macias, M. Gosselin and G. B. McManus, Earth Syst. Sci. Data, 2012, 4, 37–46 CrossRef
.
- S. J. Biller, P. M. Berube, D. Lindell and S. W. Chisholm, Nat. Rev. Microbiol., 2015, 13, 13–27 CrossRef CAS PubMed
.
- S. W. Chisholm, Curr. Biol., 2017, 27, R447–R448 CrossRef CAS PubMed
.
- G. M. Hennon, J. J. Morris, S. T. Haley, E. R. Zinser, A. R. Durrant, E. Entwistle, T. Dokland and S. T. Dyhrman, ISME J., 2018, 12, 520–531 CrossRef PubMed
.
- N. Joumaa, M. Lansalot, A. Théretz, A. Elaissari, A. Sukhanova, M. Artemyev, I. Nabiev and J. H. M. Cohen, Langmuir, 2006, 22, 1810–1816 CrossRef CAS
.
- C. Loos, T. Syrovets, A. Musyanovych, V. Mailänder, K. Landfester, G. U. Nienhaus and T. Simmet, Beilstein J. Nanotechnol., 2014, 5, 2403–2412 CrossRef PubMed
.
- A. Rogach, A. Susha, F. Caruso, G. Sukhorukov, A. Kornowski, S. Kershaw, H. Möhwald, A. Eychmüller and H. Weller, Adv. Mater., 2000, 12, 333–337 CrossRef CAS
.
- S. W. Ahn, S. S. Lee, S. J. Lee and J. M. Kim, Chem. Eng. Sci., 2015, 126, 237–243 CrossRef CAS
.
- D. Di Carlo, Lab Chip, 2009, 9, 3038–3046 RSC
.
- L. Wang and D. S. Dandy, Adv. Sci., 2017, 4, 1700153 CrossRef PubMed
.
- M. Li, M. van Zee, K. Goda and D. Di Carlo, Lab Chip, 2018, 18, 2575–2582 RSC
.
- M. Li, H. E. Muñoz, A. Schmidt, B. Guo, C. Lei, K. Goda and D. Di Carlo, Lab Chip, 2016, 16, 4458–4465 RSC
.
- J. M. Martel and M. Toner, Annu. Rev. Biomed. Eng., 2014, 16, 371–396 CrossRef CAS PubMed
.
- J. Cruz, S. Hooshmand Zadeh, T. Graells, M. Andersson, J. Malmström, Z. G. Wu and K. Hjort, J. Micromech. Microeng., 2017, 27, 084001 CrossRef
.
- B. R. Mutlu, J. F. Edd and M. Toner, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 7682–7687 CrossRef CAS PubMed
.
- J. Cruz, T. Graells, M. Walldén and K. Hjort, Lab Chip, 2019, 19, 1257–1266 RSC
.
- Y. Gou, Y. Jia, P. Wang and C. Sun, Sensors, 2018, 18, 1762 CrossRef PubMed
.
- A. M. Leshansky, A. Bransky, N. Korin and U. Dinnar, Phys. Rev. Lett., 2007, 98, 234501 CrossRef CAS PubMed
.
- E. S. Asmolov, J. Fluid Mech., 1999, 381, 63–87 CrossRef CAS
.
- S. Yang, J. Y. Kim, S. J. Lee, S. S. Lee and J. M. Kim, Lab Chip, 2011, 11, 266–273 RSC
.
-
R. J. Poole, The Deborah and Weissenberg numbers, 2012, vol. 53 Search PubMed
.
- D. Yuan, Q. Zhao, S. Yan, S. Y. Tang, G. Alici, J. Zhang and W. Li, Lab Chip, 2018, 18, 551–567 RSC
.
- J. Zhang, S. Yan, D. Yuan, G. Alici, N. T. Nguyen, M. Ebrahimi Warkiani and W. Li, Lab Chip, 2016, 16, 10–34 RSC
.
- T. M. Squires and S. R. Quake, Rev. Mod. Phys., 2005, 77, 977–1026 CrossRef CAS
.
- J. Y. Kim, S. W. Ahn, S. S. Lee and J. M. Kim, Lab Chip, 2012, 12, 2807–2814 RSC
.
- K. Sozański, A. Wiśniewska, T. Kalwarczyk and R. Hołyst, Phys. Rev. Lett., 2013, 111, 228301 CrossRef PubMed
.
- A. Nikoubashman, N. A. Mahynski, A. H. Pirayandeh and A. Z. Panagiotopoulos, J. Chem. Phys., 2014, 140, 094903 CrossRef PubMed
.
- I. De Santo, G. D'Avino, G. Romeo, F. Greco, P. A. Netti and P. L. Maffettone, Phys. Rev. Appl., 2014, 2, 064001 CrossRef
.
- C. Liu, C. Xue, X. Chen, L. Shan, Y. Tian and G. Hu, Anal. Chem., 2015, 87, 6041–6048 CrossRef CAS PubMed
.
- C. Liu, B. Ding, C. Xue, Y. Tian, G. Hu and J. Sun, Anal. Chem., 2016, 88, 12547–12553 CrossRef CAS PubMed
.
- C. Liu, J. Guo, F. Tian, N. Yang, F. Yan, Y. Ding, J. Wei, G. Hu, G. Nie and J. Sun, ACS Nano, 2017, 11, 6968–6976 CrossRef CAS PubMed
.
- C. Liu, J. Zhao, F. Tian, J. Chang, W. Zhang and J. Sun, J. Am. Chem. Soc., 2019, 141, 3817–3821 CrossRef CAS
.
- Y. Zhou, Z. Ma, M. Tayebi and Y. Ai, Anal. Chem., 2019, 91, 4577–4584 CrossRef CAS
.
- K. K. Zeming, N. V. Thakor, Y. Zhang and C. H. Chen, Lab Chip, 2016, 16, 75–85 RSC
.
- L. R. Huang, E. C. Cox, R. H. Austin and J. C. Sturm, Science, 2004, 304, 987–990 CrossRef CAS
.
- J. McGrath, M. Jimenez and H. Bridle, Lab Chip, 2014, 14, 4139–4158 RSC
.
- S. Ranjan, K. K. Zeming, R. Jureen, D. Fisher and Y. Zhang, Lab Chip, 2014, 14, 4250–4262 RSC
.
- S. M. Santana, M. A. Antonyak, R. A. Cerione and B. J. Kirby, Biomed. Microdevices, 2014, 16, 869–877 CrossRef CAS
.
- J. C. Sturm, E. C. Cox, B. Comella and R. H. Austin, Interface Focus, 2014, 4, 20140054 CrossRef
.
- B. H. Wunsch, J. T. Smith, S. M. Gifford, C. Wang, M. Brink, R. L. Bruce, R. H. Austin, G. Stolovitzky and Y. Astier, Nat. Nanotechnol., 2016, 11, 936–940 CrossRef CAS
.
- S. C. Kim, B. H. Wunsch, H. Hu, J. T. Smith, R. H. Austin and G. Stolovitzky, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E5034–E5041 CAS
.
- R. Vernekar, T. Krüger, K. Loutherback, K. Morton and D. W. Inglis, Lab Chip, 2017, 17, 3318–3330 RSC
.
- S. Fiedler, S. G. Shirley, T. Schnelle and G. Fuhr, Anal. Chem., 1998, 70, 1909–1915 CrossRef CAS
.
- P. R. C. Gascoyne and J. Vykoukal, Electrophoresis, 2002, 23, 1973–1983 CrossRef CAS
.
- R. Martinez-Duarte, Electrophoresis, 2012, 33, 3110–3132 CrossRef CAS
.
- S. Ozuna-Chacón, B. H. Lapizco-Encinas, M. Rito-Palomares, S. O. Martínez-Chapa and C. Reyes-Betanzo, Electrophoresis, 2008, 29, 3115–3122 CrossRef PubMed
.
- A. A. Kayani, C. Zhang, K. Khoshmanesh, J. L. Campbell, A. Mitchell and K. Kalantar-zadeh, Electrophoresis, 2010, 31, 1071–1079 CrossRef CAS
.
- A. F. Chrimes, A. A. Kayani, K. Khoshmanesh, P. R. Stoddart, P. Mulvaney, A. Mitchell and K. Kalantar-zadeh, Lab Chip, 2011, 11, 921–928 RSC
.
- S. Park, Y. Zhang, T. H. Wang and S. Yang, Lab Chip, 2011, 11, 2893–2900 RSC
.
- M. Kim, T. Jung, Y. Kim, C. Lee, K. Woo, J. H. Seol and S. Yang, Biosens. Bioelectron., 2015, 74, 1011–1015 CrossRef CAS
.
- P. V. Jones, G. L. Salmon and A. Ros, Anal. Chem., 2017, 89, 1531–1539 CrossRef CAS
.
- Z. Rozynek, M. Han, F. Dutka, P. Garstecki, A. Józefczak and E. Luijten, Nat. Commun., 2017, 8, 15255 CrossRef CAS PubMed
.
- B. H. Lapizco-Encinas, B. A. Simmons, E. B. Cummings and Y. Fintschenko, Anal. Chem., 2004, 76, 1571–1579 CrossRef CAS PubMed
.
- L. Wu, L. Y. Lanry Yung and K. M. Lim, Biomicrofluidics, 2012, 6, 14113 CrossRef PubMed
.
- M. Koklu, S. Park, S. D. Pillai and A. Beskok, Biomicrofluidics, 2010, 4, 034107 CrossRef PubMed
.
- S. H. Liao, C. Y. Chang and H. C. Chang, Biomicrofluidics, 2013, 7, 24110 CrossRef
.
- L. Liu, K. Chen, N. Xiang and Z. Ni, Electrophoresis, 2019, 40, 873–889 CrossRef CAS PubMed
.
- Q. Chen and Y. J. Yuan, RSC Adv., 2019, 9, 4963–4981 RSC
.
- R. Pethig, J. Electrochem. Soc., 2017, 164, B3049–B3055 CrossRef CAS
.
- B. H. Lapizco-Encinas, Electrophoresis, 2017, 38, 1405–1406 CrossRef CAS PubMed
.
- V. Yantchev, J. Enlund, I. Katardjiev and L. Johansson, J. Micromech. Microeng., 2010, 20, 035031 CrossRef
.
- G. Destgeer, B. H. Ha, J. H. Jung and H. J. Sung, Lab Chip, 2014, 14, 4665–4672 RSC
.
- D. J. Collins, Z. Ma and Y. Ai, Anal. Chem., 2016, 88, 5513–5522 CrossRef CAS
.
- S. M. Woodside, B. D. Bowen and J. M. Piret, AIChE J., 1997, 43, 1727–1736 CrossRef CAS
.
- H. Bruus, Lab Chip, 2012, 12, 1014–1021 RSC
.
- X. Ding, S. C. S. Lin, B. Kiraly, H. Yue, S. Li, I. K. Chiang, J. Shi, S. J. Benkovic and T. J. Huang, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 11105–11109 CrossRef CAS
.
- L. Y. Yeo and J. R. Friend, Annu. Rev. Fluid Mech., 2014, 46, 379–406 CrossRef
.
- J. Shi, H. Huang, Z. Stratton, Y. Huang and T. J. Huang, Lab Chip, 2009, 9, 3354–3359 RSC
.
- J. Shi, X. Mao, D. Ahmed, A. Colletti and T. J. Huang, Lab Chip, 2008, 8, 221–223 RSC
.
- D. J. Collins, T. Alan and A. Neild, Lab Chip, 2014, 14, 1595–1603 RSC
.
- G. P. Gautam, R. Gurung, F. A. Fencl and M. E. Piyasena, Anal. Bioanal. Chem., 2018, 410, 6561–6571 CrossRef CAS PubMed
.
- S. Li, F. Ma, H. Bachman, C. E. Cameron, X. Zeng and T. J. Huang, J. Micromech. Microeng., 2017, 27, 015031 CrossRef PubMed
.
- M. Wu, C. Chen, Z. Wang, H. Bachman, Y. Ouyang, P. H. Huang, Y. Sadovsky and T. J. Huang, Lab Chip, 2019, 19, 1174–1182 RSC
.
- W. Connacher, N. Zhang, A. Huang, J. Mei, S. Zhang, T. Gopesh and J. Friend, Lab Chip, 2018, 18, 1952–1996 RSC
.
- P. S. Epstein, Z. Phys., 1929, 54, 537–563 CrossRef
.
- J. R. Brock, J. Colloid Sci., 1962, 17, 768–780 CrossRef CAS
.
- L. Talbot, R. K. Cheng, R. W. Schefer and D. R. Willis, J. Fluid Mech., 1980, 101, 737–758 CrossRef
.
- R. Wang, J. Du, W. Guo and Z. Zhu, Nanoscale Microscale Thermophys. Eng., 2016, 20, 51–65 CrossRef CAS
.
- G. S. McNab and A. Meisen, J. Colloid Interface Sci., 1973, 44, 339–346 CrossRef CAS
.
- M. Eslamian, Front. Heat Mass Transfer, 2011, 2, 1–20 Search PubMed
.
- M. Eslamian and M. Z. Saghir, Appl. Therm. Eng., 2013, 59, 527–534 CrossRef CAS
.
- Y. Zhou, C. Yang, Y. C. Lam and X. Huang, Int. J. Heat Mass Transfer, 2016, 101, 1283–1291 CrossRef CAS
.
- E. H. Hill, J. Li, L. Lin, Y. Liu and Y. Zheng, Langmuir, 2018, 34, 13252–13262 CrossRef CAS PubMed
.
- S. Choi, S. Song, C. Choi and J. K. Park, Anal. Chem., 2009, 81, 50–55 CrossRef CAS PubMed
.
- S. Song and S. Choi, J. Chromatogr. A, 2013, 1302, 191–196 CrossRef CAS PubMed
.
- D. G. Grier, Nature, 2003, 424, 810–816 CrossRef CAS PubMed
.
- K. Dholakia and T. Čižmár, Nat. Photonics, 2011, 5, 335–342 CrossRef CAS
.
- Y. Zhao, B. S. Fujimoto, G. D. Jeffries, P. G. Schiro and D. T. Chiu, Opt. Express, 2007, 15, 6167–6176 CrossRef PubMed
.
- T. Zhu, R. Cheng and L. Mao, Microfluid. Nanofluid., 2011, 11, 695–701 CrossRef
.
- R. Afshar, Y. Moser, T. Lehnert and M. A. M. Gijs, Anal. Chem., 2011, 83, 1022–1029 CrossRef CAS PubMed
.
- J. Zeng, C. Chen, P. Vedantam, V. Brown, T. R. J. Tzeng and X. Xuan, J. Micromech. Microeng., 2012, 22, 105018 CrossRef
.
- R. Zhou and C. Wang, Biomicrofluidics, 2016, 10, 034101 CrossRef PubMed
.
- S. M. Kenyon, M. M. Meighan and M. A. Hayes, Electrophoresis, 2011, 32, 482–493 CrossRef CAS PubMed
.
- T. Kawamata, M. Yamada, M. Yasuda and M. Seki, Electrophoresis, 2008, 29, 1423–1430 CrossRef CAS PubMed
.
- S. Yan, J. Zhang, Y. Yuan, G. Lovrecz, G. Alici, H. Du, Y. Zhu and W. Li, Electrophoresis, 2015, 36, 284–291 CrossRef CAS PubMed
.
- S. Yan, J. Zhang, D. Yuan and W. Li, Electrophoresis, 2017, 38, 238–249 CrossRef CAS PubMed
.
- Y. Hattori, T. Shimada, T. Yasui, N. Kaji and Y. Baba, Anal. Chem., 2019, 91, 6514–6521 CAS
.
- V. Calero, P. Garcia-Sanchez, C. Honrado, A. Ramos and H. Morgan, Lab Chip, 2019, 19, 1386–1396 RSC
.
- J. P. Beech, P. Jönsson and J. O. Tegenfeldt, Lab Chip, 2009, 9, 2698–2706 RSC
.
- R. Wang, S. Sun, W. Wang and Z. Zhu, Int. J. Heat Mass Transfer, 2019, 133, 912–919 CrossRef
.
- Y. Q. Fu, J. K. Luo, N. T. Nguyen, A. J. Walton, A. J. Flewitt, X. Zu, Y. Li, G. McHale, A. Matthews, E. Iborra, H. Du and W. I. Milne, Prog. Mater. Sci., 2017, 89, 31–91 CrossRef CAS
.
- M. A. Croxen, R. J. Law, R. Scholz, K. M. Keeney, M. Wlodarska and B. B. Finlay, Clin. Microbiol. Rev., 2013, 26, 822–880 CrossRef CAS PubMed
.
- N. Ota, Y. Yalikun, T. Suzuki, S. W. Lee, Y. Hosokawa, K. Goda and Y. Tanaka, R. Soc. Open Sci., 2019, 6, 181776 CrossRef CAS PubMed
.
- M. Li, H. E. Muñoz, K. Goda and D. Di Carlo, Sci. Rep., 2017, 7, 10802 CrossRef PubMed
.
- M. Masaeli, E. Sollier, H. Amini, W. Mao, K. Camacho, N. Doshi, S. Mitragotri, A. Alexeev and D. Di Carlo, Phys. Rev. X, 2012, 2, 031017 Search PubMed
.
- J. J. Lee, K. J. Jeong, M. Hashimoto, A. H. Kwon, A. Rwei, S. A. Shankarappa, J. H. Tsui and D. S. Kohane, Nano Lett., 2014, 14, 1–5 CrossRef CAS
.
- Ö. Baltekin, A. Boucharin, E. Tano, D. I. Andersson and J. Elf, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 9170–9175 CrossRef
.
- J. Skog, T. Würdinger, S. van Rijn, D. H. Meijer, L. Gainche, M. Sena-Esteves, W. T. Curry, B. S. Carter, A. M. Krichevsky and X. O. Breakefield, Nat. Cell Biol., 2008, 10, 1470–1476 CrossRef CAS PubMed
.
- P. D. Robbins and A. E. Morelli, Nat. Rev. Immunol., 2014, 14, 195–208 CrossRef CAS PubMed
.
- B. T. Pan, K. Teng, C. Wu, M. Adam and R. M. Johnstone, J. Cell Biol., 1985, 101, 942–948 CrossRef CAS PubMed
.
- L. Barile and G. Vassalli, Pharmacol. Ther., 2017, 174, 63–78 CrossRef CAS PubMed
.
- M. Tkach and C. Théry, Cell, 2016, 164, 1226–1232 CrossRef CAS PubMed
.
- K. W. Witwer, E. I. Buzás, L. T. Bemis, A. Bora, C. Lässer, J. Lötvall, E. N. Nolte-'t Hoen, M. G. Piper, S. Sivaraman, J. Skog, C. Théry, M. H. Wauben and F. Hochberg, J. Extracell. Vesicles, 2013, 2, 20360 CrossRef PubMed
.
- K. K. Jella, T. H. Nasti, Z. Li, S. R. Malla, Z. S. Buchwald and M. K. Khan, Vaccines, 2018, 6, 69 CrossRef CAS PubMed
.
- R. C. Lai, R. W. Y. Yeo, K. H. Tan and S. K. Lim, Biotechnol. Adv., 2013, 31, 543–551 CrossRef CAS PubMed
.
- J. C. Contreras-Naranjo, H. J. Wu and V. M. Ugaz, Lab Chip, 2017, 17, 3558–3577 RSC
.
- P. Li, M. Kaslan, S. H. Lee, J. Yao and Z. Gao, Theranostics, 2017, 7, 789–804 CrossRef CAS PubMed
.
- T. Salafi, K. K. Zeming and Y. Zhang, Lab Chip, 2017, 17, 11–33 RSC
.
- L. Rems, D. Kawale, L. J. Lee and P. E. Boukany, Biomicrofluidics, 2016, 10, 043403 CrossRef PubMed
.
- J. L. Viovy, Rev. Mod. Phys., 2000, 72, 813–872 CrossRef CAS
.
- M. Viefhues, J. Regtmeier and D. Anselmetti, Analyst, 2013, 138, 186–196 RSC
.
- D. J. Beebe, G. A. Mensing and G. M. Walker, Annu. Rev. Biomed. Eng., 2002, 4, 261–286 CrossRef CAS PubMed
.
- M. Wu, Y. Ouyang, Z. Wang, R. Zhang, P. H. Huang, C. Chen, H. Li, P. Li, D. Quinn, M. Dao, S. Suresh, Y. Sadovsky and T. J. Huang, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 10584–10589 CrossRef CAS PubMed
.
- Y. Chen, A. J. Chung, T. H. Wu, M. A. Teitell, D. Di Carlo and P. Y. Chiou, Small, 2014, 10, 1746–1751 CrossRef CAS PubMed
.
- E. Ozkumur, A. M. Shah, J. C. Ciciliano, B. L. Emmink, D. T. Miyamoto, E. Brachtel, M. Yu, P. Chen, B. Morgan, J. Trautwein, A. Kimura, S. Sengupta, S. L. Stott, N. M. Karabacak, T. A. Barber, J. R. Walsh, K. Smith, P. S. Spuhler, J. P. Sullivan, R. J. Lee, D. T. Ting, X. Luo, A. T. Shaw, A. Bardia, L. V. Sequist, D. N. Louis, S. Maheswaran, R. Kapur, D. A. Haber and M. Toner, Sci. Transl. Med., 2013, 5, 179ra47 Search PubMed
.
- T. H. Wu, Y. Chen, S. Y. Park, J. Hong, T. Teslaa, J. F. Zhong, D. Di Carlo, M. A. Teitell and P. Y. Chiou, Lab Chip, 2012, 12, 1378 RSC
.
- S. Sakuma, Y. Kasai, T. Hayakawa and F. Arai, Lab Chip, 2017, 17, 2760–2767 RSC
.
-
X. Zhu, Y. C. Kung, T. H. Wu, M. A. Teitell and P. Y. E. Chiou, in Optical Trapping and Optical Micromanipulation XIV, ed. K. Dholakia and G. C. Spalding, SPIE, 2017, vol. 10347, p. 91 Search PubMed
.
- L. Ren, S. Yang, P. Zhang, Z. Qu, Z. Mao, P. H. Huang, Y. Chen, M. Wu, L. Wang, P. Li and T. J. Huang, Small, 2018, 14, 1801996 CrossRef PubMed
.
- O. Jakobsson, C. Grenvall, M. Nordin, M. Evander and T. Laurell, Lab Chip, 2014, 14, 1943–1950 RSC
.
- K. S. Lee, M. Palatinszky, F. C. Pereira, J. Nguyen, V. I. Fernandez, A. J. Mueller, F. Menolascina, H. Daims, D. Berry, M. Wagner and R. Stocker, Nat. Microbiol., 2019, 4, 1035–1048 CrossRef CAS PubMed
.
- B. Çetin and D. Li, Electrophoresis, 2011, 32, 2410–2427 CrossRef PubMed
.
- J. R. Moffitt, Y. R. Chemla, S. B. Smith and C. Bustamante, Annu. Rev. Biochem., 2008, 77, 205–228 CrossRef CAS PubMed
.
- H. Amini, W. Lee and D. Di Carlo, Lab Chip, 2014, 14, 2739 RSC
.
- D. Stoecklein and D. Di Carlo, Anal. Chem., 2019, 91, 296–314 CrossRef CAS PubMed
.
- G. D'Avino, F. Greco and P. L. Maffettone, Annu. Rev. Fluid Mech., 2017, 49, 341–360 CrossRef
.
- D. W. Inglis, J. A. Davis, R. H. Austin and J. C. Sturm, Lab Chip, 2006, 6, 655–658 RSC
.
- S. Dash and S. Mohanty, Electrophoresis, 2014, 35, 2656–2672 CrossRef CAS PubMed
.
- J. Friend and L. Y. Yeo, Rev. Mod. Phys., 2011, 83, 647–704 CrossRef
.
- Z. Liu, Z. Chen and M. Shi, Appl. Therm. Eng., 2009, 29, 1020–1025 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2020 |