Open Access Article
Open Access ArticleBio-based solvents as entrainers for extractive distillation in aromatic/aliphatic and olefin/paraffin separation†
Thomas
Brouwer
 and
Boelo
Schuur
and
Boelo
Schuur
 *
*
Sustainable Process Technology Group, Process and Catalysis Engineering Cluster, Faculty of Science and Technology, University of Twente, Enschede, The Netherlands. E-mail: b.schuur@utwente.nl; Tel: +31 53 489 2891
First published on 7th July 2020
Abstract
The use of a wide range of bio-based solvents as entrainers in extractive distillation applications was investigated. The separation of hydrocarbon mixtures containing aromatic and aliphatic compounds is highly relevant, and the use of bio-based solvents for this separation was studied using the model system of methylcyclohexane and toluene. Additionally, the use of bio-based solvents for the difficult olefin/paraffin separation was studied using the model system of n-heptane and 1-heptene. From all of the bio-based solvents studied, Cyrene™ showed the highest relative volatility in the methylcyclohexane–toluene system. At compositions up to 40 wt% of methylcyclohexane in the hydrocarbon mixture, with a relative volatility of 3.17 ± 0.16 at 1000 mbar, the selectivity was comparable with the state-of-the-art industrial solvent Sulfolane™. At higher methylcyclohexane fractions, Cyrene™ outperforms Sulfolane™, resulting in a 43% reduction of the minimum reflux ratio, which is an excellent measure of energy efficiency. With regard to the relative volatility of n-heptane over 1-heptene, Cyrene™ also induces an increase in the relative volatility, but not as much as the industrial benchmark n-methylpyrrolidone (NMP). A relative volatility of 1.20 was measured at a solvent-to-feed ratio of 3 (mass basis), which can be further increased by the addition of extra Cyrene™. This leads to the prospect that Cyrene™ may be used for extractive distillation in olefin/paraffin separations, replacing NMP which is subject to severe environmental restrictions by the REACH agreement due to toxicity.
1. Introduction
Distillation is the workhorse in the chemical industry when it comes to separation. Although it is efficient and highly effective in many applications, this mature technology can also be energy intensive and thus costly when it comes to mixtures that are difficult to separate by distillation due to a pinch point or azeotrope, or simply due to low relative volatility. Up to 50% of the total costs of a chemical plant are due to separations,1 with much of it due to the use of heat as the separation agent.2 For mixtures that are difficult to separate by distillation, extractive distillation is commonly used, in which the addition of a high-boiling solvent helps to reduce the energy requirements by overcoming azeotropes and increasing the relative volatility.Well-known industrial solvents in the petroleum industry include Sulfolane™,3n-methylpyrrolidone (NMP)4,5 and N,N-dimethylformamide (DMF).6 The application of these traditional solvents is not always as benign as desired; for instance, NMP is going to be banned for certain industrial applications due to the REACH legislation.7 Therefore, increasingly more attention has been given to the search for alternative, more benign, solvents. For example ionic liquids (ILs),8 deep eutectic solvents (DESs)9 and switchable solvents10 are among the studied alternatives. Bio-based solvents may be considered another class of solvents, including both natural DESs (mixtures exhibiting an eutectic behaviour)11,12 and single-component molecular bio-based solvents.13,14 The single-component solvents are most similar to traditional solvents in terms of molecular properties but they differ in the feedstock.13,14
In this contribution, our study to find bio-based alternative solvents for extractive distillation is described, aiming at replacing fossil-based solvents to minimize the environmental impact associated with solvent production. In the comparison of the sustainability aspect of bio-based solvents and traditional solvents, the difference of the feedstock is apparent. In contrast to traditional solvents that are almost all derived from fossil oil,15 the feedstock for bio-based solvents is diverse, and includes lignocellulosic biomass,17 fermentation broths18 or (air-captured) carbon dioxide.19 On the condition that access to such bio-based chemicals involves clean processes, this approach can lessen the impact on the environment due to the use of carbon from the short carbon cycle.20
As lignocellulosic biomass consists mainly of C5- and C6-sugars and lignin, a large variety of platform chemicals may be derived from them. Access to highly interesting chemistry can be realized by pyrolysis; see for example the route to dihydrolevoglucosenone in Fig. 1. Upon further refinement, from biomass-derived sugars, for instance, propylene glycol, levulinic acid, γ-valerolactone, glycerol and furfural can be produced.17 With an additional synthetic step, the variety of accessible bio-based chemicals increases even further, e.g. nucleophilic addition of methanol to produce cyclopentyl methyl ether,21 fermentation of glycerol to propionic acid,22 trimerization of acetone to isophorone,23 and esterification of acetic acid and glycerol to triacetin24 and levulinic acid and ethanol to ethyl levulinate.25 Fast (catalytic) pyrolysis or hydrolysis of lignin can yield aromatic chemicals such as guaiacol,26 phenol27 and acetophenone.28 Ethylene carbonate can be produced by the cycloaddition of carbon dioxide to epoxides.19,29
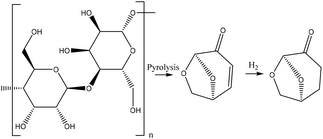 | ||
| Fig. 1 Synthetic steps of a bio-based solvent via pyrolysis of cellulose to levoglucosenone which is subsequently hydrogenated to dihydrolevoglucosenone (Cyrene™).16 | ||
Dihydrolevoglucosenone, or Cyrene™, shown in Fig. 1, has been mentioned as a promising bio-based alternative polar aprotic compound. It was synthesized in 1978 by Brimacombe et al.30 by the reduction of levoglucosenone and also shown by the group of Weckhuysen.31 Levoglucosenone itself can be obtained by the fast pyrolysis of cellulose.32–35 The recent rediscovery of Cyrene™ has resulted in various application assessments, including as a solvent for several reactions (fluorination,34 Menschutkins-,34 Sonogashira- and Cacchi-type annulation,16,36 basic reactions,16 acyl substitution,16 Suzuki–Miyaura cross-coupling,16,37 amide synthesis,16,38 urea synthesis,39 MOF synthesis,40 solid-phase synthesis41), as a starting material for platform chemicals,42 as a hydrotropic solvent due to the capabilities via its germinal diol43 and as a solvent for liquid exfoliation in graphene processing.44
We decided to include Cyrene™ in the aforementioned range of bio-based solvents to be evaluated as an entrainer in two highly relevant industrial extractive distillation processes. The separation of methylcyclohexane (MCH) and toluene is a model system for the separation of aromatics and aliphatics. Although this particular separation is challenging due to the close boiling nature of the binary mixture, it also represents a wider range of separations in a complex industrial hydrocarbon mixture. Relatively low-boiling aromatic compounds (BTX, i.e. benzene, toluene, and xylenes) are to be entrained from a wide range of aliphatic compounds which can be as volatile as the BTX compounds, but also much heavier. The addition of a solvent must therefore achieve a reversal of the boiling point order, hence separation of all aliphatic compounds over the top of the distillation column as the distillate.45,46 For this challenging task, many solvents do not show high-enough selectivity.46 The solvent screening results of this study will include a comparison with Sulfolane™ to identify which of the bio-based solvents perform similarly or better, and may be applied in a wider range of separations of aromatics and aliphatics. Furthermore, for promising solvents, the application as an entrainer in another challenging separation problem, the olefin/paraffin separation,47 will be investigated, for which n-heptane and 1-heptene were chosen as the model system.
A first estimate, or performance prediction, was performed using the modified UNIFAC (Do) model,48 known to be among the best predicting models for vapour–liquid equilibria.49
2. Experimental section
2.1. Materials
All the applied chemicals, detailed in the ESI,† were used without any additional purification.2.2. Experimental setup
Fischer Labodest VLE602 ebulliometers were used for the measurement of the isobaric vapour–liquid equilibria (VLE). Each mixture, containing the binary mixture and optionally a solvent, was heated to reach an equilibrium temperature at a pre-set pressure. For each measurement, a total amount of about 85 g of the liquid was added in the ebulliometer, which ensured adequate liquid and vapour flows throughout the ebulliometer system. Each measurement was left to equilibrate for approximately 60 min, and after reaching the equilibrium, 0.5–1.0 mL of liquid samples was taken from the liquid and condensed vapour flows. A solvent-to-feed ratio of 1 was used if not specified otherwise. A 50/50 wt% mixture of MCH and toluene was used for the screening of the bio-based solvents, while a 90/10 wt% mixture was used for the measurements with n-heptane/1-heptene. Distillation results are typically reported in mole fractions, so these were calculated as well.2.3. Analysis method
3. Results and discussion
The potential of various bio-based solvents to increase the relative volatility in the MCH–toluene binary mixture was evaluated experimentally by measuring the pseudo-binary vapor–liquid equilibrium (VLE), pseudo-binary meaning that the composition of the solvent is not taken into account in the calculations for the MCH–toluene binary mixture. The relative volatility (αij) is the ratio of the activity coefficients (γi) and the saturated vapour pressures (Poi) of both compounds, as shown in eqn (1).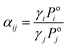 | (1) |
The saturated vapour pressures are pure component properties, whereas the activity coefficients are dependent on the mixture composition, and hence, are affected by the presence of a solvent. By predicting the activity coefficients using the mod. UNIFAC (Do) model,48 the corresponding effect of the solvent on the relative volatility can be predicted. All relative volatilities mentioned in this paper are pseudo-binary relative volatilities, i.e. the solvent is not taken into account. This is a common practice in studies on entrainer performance in extractive distillation, and mostly those solvents with much higher boiling points than the mixtures are selected.4,50
3.1. Relative volatility screening
The effect of the bio-based solvents in this study on the relative volatility in the MCH–toluene binary mixture was studied at a composition of 50/50 wt% of MCH and toluene. The predicted relative volatility using the mod. UNIFAC (Do) model is compared with the experimentally obtained values using a dynamic equilibrium measurement with an ebulliometer. The parity between the model and the experiment is shown in Fig. 2. Several bio-based solvents, such as furfural, γ-valerolactone, phenol and levulinic acid, perform very well, showing only slightly lower relative volatilities than was observed with Sulfolane™. Among these solvents, furfural is already a known extractive distillation solvent in the purification of C4-hydrocarbons,51 and phenol has been mentioned decades ago.52 Levulinic acid and γ-valerolactone have, to the best of our knowledge, not been identified as potential entrainers in the separation of aromatics and aliphatics by extractive distillation. Numerous other solvents induce significantly lower relative volatility, which is either the result of a lack of polarity or significant intramolecular hydrogen bond formation. Exceptionally well-performing is Cyrene™, which shows a relative volatility of MCH over toluene of 3.17 ± 0.16, which is higher than the relative volatility observed with Sulfolane™. This is an indication that Cyrene™ is likely to perform similarly to or even better than Sulfolane™.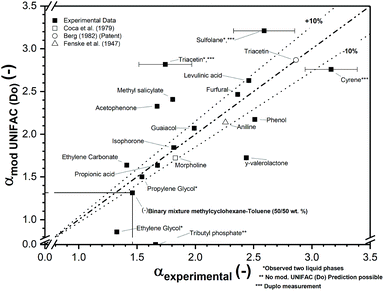 | ||
| Fig. 2 The screening results of sixteen bio-based solvents regarding the relative volatility of a 50/50 wt% MCH/toluene mixture with a solvent-to-feed ratio of 1 (mass basis) at 1000 mbar. On the x-axis, the experimental relative volatility is plotted against the relative volatility predicted by the mod. UNIFAC (Do) model on the y-axis. Additional literature values are included.53–55 The error bars indicate the standard deviation of duplo measurements. | ||
From Fig. 2, it can further be concluded that the mod. UNIFAC (Do) predictive model, even though being among the best predictive models for this task,49 shows significant deviations in the predictions. Although many predictions are accurate within a deviation of 10%, there are several solvents for which a larger inaccuracy was observed. From the previous work,49 at infinite dilution, the activity coefficient deviation of the mod. UNIFAC (Do) model was on an average 24.3%. This prediction is however more accurate for similar molecules, but can also be highly inaccurate, for instance between aliphatic compounds and aprotic and protic compounds (56.8% deviation). These trends are shown in Fig. 2, where the performance prediction of the aprotic polar solvent, acetophenone, is >10%. The deviation decreases if the polar character is decreased, such as in isophorone. Overall, these results are in agreement with the earlier conclusions at infinite dilution.49
3.2. Quasi-binary isobaric vapour liquid equilibrium diagram for methylcyclohexane and toluene with Cyrene™ as an entrainer
Based on the excellent results for the MCH–toluene separation, the use of Cyrene™ for extractive distillation of this mixture was further studied for the entire pseudo-binary composition range and compared with the effect of Sulfolane™ on the MCH–toluene pseudo-binary VLE. For each measurement, the solvent-to-feed (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F) ratio was maintained at 1 (mass basis) and the pressure was varied between 1000, 800 and 500 mbar. In Fig. 3, the composition profile at 1000 mbar can be seen. Similar results have been obtained for the other pressures and can be found in the ESI.† It can be seen that at smaller MCH mole fractions, until approximately 0.4, the relative volatility induced by Cyrene™ appears to be comparable with that of Sulfolane™, or slightly less. However, at higher fractions of MCH, with Sulfolane™, a distinct pinch point is observed, whereas with Cyrene™ in that part of the diagram, a much higher relative volatility is observed, resulting in the absence of the pinch point or at least a much less severe pinch point.
F) ratio was maintained at 1 (mass basis) and the pressure was varied between 1000, 800 and 500 mbar. In Fig. 3, the composition profile at 1000 mbar can be seen. Similar results have been obtained for the other pressures and can be found in the ESI.† It can be seen that at smaller MCH mole fractions, until approximately 0.4, the relative volatility induced by Cyrene™ appears to be comparable with that of Sulfolane™, or slightly less. However, at higher fractions of MCH, with Sulfolane™, a distinct pinch point is observed, whereas with Cyrene™ in that part of the diagram, a much higher relative volatility is observed, resulting in the absence of the pinch point or at least a much less severe pinch point.
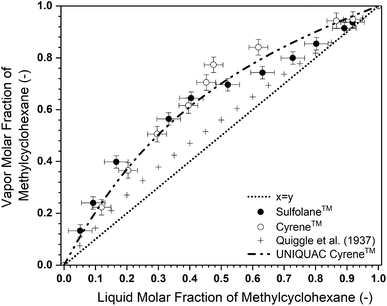 | ||
Fig. 3 The pseudo-binary isobaric vapour liquid equilibrium diagram with Sulfolane™ and Cyrene™ as the solvents with a solvent-to-feed (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F) ratio of 1 on mass basis at 1000 mbar. The literature values of Quiggle et al.56 were used as the binary reference. The UNIQUAC fit parameters are presented in the ESI.† F) ratio of 1 on mass basis at 1000 mbar. The literature values of Quiggle et al.56 were used as the binary reference. The UNIQUAC fit parameters are presented in the ESI.† | ||
This is likely due to phase splitting that can occur for Sulfolane™ at high MCH content, while for Cyrene™ this is not observed. Phase splitting reduces interactions of the solvent towards both toluene and MCH, hence diminishing the solvent effects on the relative volatility and resulting in a pinch point. Furthermore, an insignificant Cyrene™ fraction was found in the vapour phase, which varies between 0.07 and1.62 wt% mainly depending on the solvent-to-feed (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F) ratio and the operational pressure. The stability of Cyrene™ was confirmed by 1H NMR (see the ESI†) after its recovery using a rotary evaporator.
F) ratio and the operational pressure. The stability of Cyrene™ was confirmed by 1H NMR (see the ESI†) after its recovery using a rotary evaporator.
To explain the observations in the VLE experiments, the charge distributions in n-heptane, 1-heptene, toluene, MCH and Cyrene™ have been simulated using the COSMO-RS software (Conductor like Screening Model for Realistic Solvents). Based on density functional theory, the molecular geometries have been optimized, and then the screening charge around the surface of the molecules was calculated and plotted. For the five molecules in this study, the so-called σ-profiles are shown in Fig. 4, together with the surfaces. Negative screening charge density indicates an electropositive region, while positive screening charge density corresponds with an electronegative region. Cyrene™ is the most polar of the displayed molecules, which is reflected in both a peak at a positive screening charge density and a peak at a negative screening charge density. n-Heptane and MCH, in contrast, exhibit a single peak around 0, exemplary for their apolar character. This charge mismatch causes net repulsive interactions, resulting in high activity coefficients. The π-orbitals in the unsaturated hydrocarbons responsible for the electric quadrupole moments result in screening charge profiles that are off-centred, i.e. with clear maxima at the positive screening charge, and most pronounced for toluene, also at the negative screening charge. The presence of these positive and negative screening charges induces attractive dipole–dipole interactions, and for this reason both unsaturated hydrocarbons are less repelled by Cyrene™ than their corresponding saturated hydrocarbons. As a result, their activity coefficients are lower which results in an increased relative volatility, as indeed is shown in Fig. 3 for the MCH–toluene system.
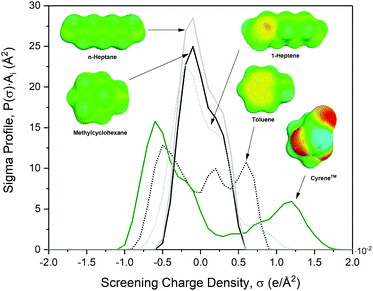 | ||
| Fig. 4 The charge distribution (σ-profile) of toluene, methylcyclohexane, n-heptane, 1-heptene and Cyrene™. Calculated with COSMOthermX C30_1705 using the TZVP-parameterization. | ||
The energy requirements of a distillation column (reboiler and condenser) are highly dependent on the minimal reflux ratio (Rmin), which influences the amount of liquid that needs to be evaporated in the reboiler. The Rmin was estimated by the graphical McCabe–Thiele approach57 and found to be 2.21 for Sulfolane™ and only 1.25 for Cyrene™. This shows the strong effect of removing the pinch point when replacing Sulfolane™ with Cyrene™, resulting in a significant decrease of 43% in Rmin, which could correspond to a reduction in the reboiler duty of approximately 30%, depending on the exact conditions of operation.
3.3. Olefin/paraffin separation: n-heptane and 1-heptene
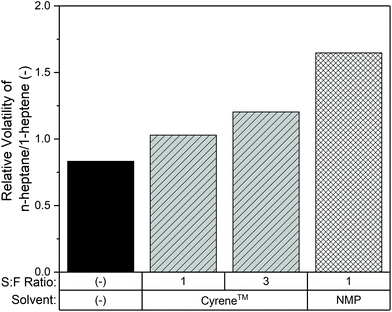
]Based on the potential of Cyrene™ as a bio-based alternative for Sulfolane™, there may be other applications where Cyrene™ can replace polar non-protic entrainers. An important class is the separation of olefin/paraffin. Therefore the evaluation of Cyrene™ was extended towards olefin/paraffin separation. The model system of n-heptane and 1-heptene was selected because of the experimentally convenient boiling temperatures, and was examined at a single binary composition of 90 wt% n-heptane and 10 wt% 1-heptene. This is the composition where the solvent effect for the MCH–toluene separation with Cyrene™ was the largest. As can be seen in Fig. 5, Cyrene™ increases the relative volatility from 0.83 to 1.03 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F = 1 on mass basis) and 1.20 (S
F = 1 on mass basis) and 1.20 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F = 3) due to the impact of the difference in screening charge distributions between n-heptane and 1-heptene, as shown in Fig. 4. The experiments thus showed that it was possible to achieve the desired natural boiling order reversal effect. In comparison with one of the industrial standards, NMP, which induces a higher relative volatility of 1.65 (S
F = 3) due to the impact of the difference in screening charge distributions between n-heptane and 1-heptene, as shown in Fig. 4. The experiments thus showed that it was possible to achieve the desired natural boiling order reversal effect. In comparison with one of the industrial standards, NMP, which induces a higher relative volatility of 1.65 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F ratio = 1), the performance of Cyrene™ is clearly lower. This is due to the less pronounced positive screening charge area of 1-heptene compared to toluene; see Fig. 4. Nevertheless, the effect of Cyrene™ can be further enhanced by using a larger S
F ratio = 1), the performance of Cyrene™ is clearly lower. This is due to the less pronounced positive screening charge area of 1-heptene compared to toluene; see Fig. 4. Nevertheless, the effect of Cyrene™ can be further enhanced by using a larger S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F ratio.
F ratio.
Furthermore, we speculate that the effect of the solvent will be more pronounced in the industrially relevant separation of butadiene,47 as butadiene has twice the amount of unsaturated bonds in comparison with 1-heptene. This allows for significantly more dipole interactions of the solvent via the π-bonds, which lowers the activity coefficient, and thus increases the relative volatility towards the saturated compound. This has been shown by De Oliveira et al. for 1,3-butadiene and isobutene in the presence of NMP.58,59
Based on the observed results for the studied systems, we conclude that the bio-based solvent Cyrene™ has the potential of phasing out toxic solvents such as NMP60 in extractive distillation applications.
4. Conclusion
From a wide-range screening of bio-based solvents to separate a 50/50 wt% mixture of MCH and toluene, Cyrene™ was seen to most effectively entrain toluene to induce an excellent relative volatility of 3.17 ± 0.16, being even higher than the industrial state-of-the-art Sulfolane™. Especially at higher MCH fractions, Cyrene™ significantly induces the relative volatility in the system, whereas the use of Sulfolane™ in this composition range results in a pinch point. The absence of the pinch point when using Cyrene™ lowers the minimum reflux ratio from 2.21 for Sulfolane™ to 1.25 for Cyrene™, corresponding to an expected energy usage reduction of approximately 30%.The potential of Cyrene™ was additionally evaluated for the olefin/paraffin separation of n-heptane and 1-heptene. Based on the observed relative volatility towards n-heptane of 1.03 and 1.20 for the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F ratio of 1 and 3 respectively, we expect that the use of Cyrene™ for the industrially highly relevant butadiene splitting is also suitable. This offers the opportunity to replace NMP, which is subject to strong environmental restrictions.
F ratio of 1 and 3 respectively, we expect that the use of Cyrene™ for the industrially highly relevant butadiene splitting is also suitable. This offers the opportunity to replace NMP, which is subject to strong environmental restrictions.
Abbreviations
| Cyrene™ | Dihydrolevoglucosenone |
| DMF | N,N-Dimethylformamide |
| FID | Flame ionization detector |
| MCH | Methylcyclohexane |
| Mod. UNIFAC (Do) | Dortmund modification of UNIFAC |
| NMP | N-Methylpyrolidone |
| NMR | Nuclear magnetic resonance |
| REACH | Registration, Evaluation, Authorisation and Restriction of Chemicals |
S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F ratio F ratio | Solvent-to-feed ratio (on mass basis) |
| Sulfolane™ | Tetrahydrothiophene-1,1-dioxide |
| TOL | Toluene |
| TZVP | Triple valence plus polarization |
| UNIFAC | UNIQUAC functional-group activity coefficients |
| UNIQUAC | Universal quasichemical |
| VLE | Vapour liquid equilibrium |
| α ij | Relative volatility (−) |
| P o | Pure component vapour pressure (bar) |
| x i | Molar fraction of compound i |
Conflicts of interest
A patent application has been filed for the finding as described in the publication, claiming “the use of Cyrene™ as solvent in fluid separations”. The authors have transferred the rights to a third party, and did not have a conflict of interest from the moment the manuscript was submitted for publication.Acknowledgements
This has been an ISPT (Institute for Sustainable Process Technology) project (TEEI314006/BL-20-07), co-funded by the Topsector Energy by the Dutch Ministry of Economic Affairs and Climate Policy.References
- A. A. Kiss, J.-P. Lange, B. Schuur, D. W. F. Brilman, A. G. van der Ham and S. R. Kersten, Biomass Bioenergy, 2016, 95, 296–309 CrossRef CAS.
- M. Blahušiak, A. A. Kiss, S. R. Kersten and B. Schuur, Energy, 2016, 116, 20–31 CrossRef.
- Y. J. Choi, K. W. Cho, B. W. Cho and Y.-K. Yeo, Ind. Eng. Chem. Res., 2002, 41, 5504–5509 CrossRef CAS.
- M. Fahim and A. Elkilani, Sep. Sci. Technol., 1990, 25, 1803–1815 CrossRef CAS.
- R. J. L. Noe, B. R. Beadle and L. E. Sullivan, United States Pat., US8552247B2, 2013 Search PubMed.
- Z. Lei, R. Zhou and Z. Duan, Chem. Eng. J., 2002, 85, 379–386 CrossRef CAS.
- J. Sherwood, T. J. Farmer and J. H. Clark, Chem, 2018, 4, 2010–2012 CAS.
- Z. Lei, C. Dai, J. Zhu and B. Chen, AIChE J., 2014, 60, 3312–3329 CrossRef CAS.
- N. R. Rodriguez, A. S. Gonzalez, P. M. Tijssen and M. C. Kroon, Fluid Phase Equilib., 2015, 385, 72–78 CrossRef CAS.
- B. Schuur, M. Nijland, M. Blahušiak and A. Juan, ACS Sustainable Chem. Eng., 2018, 6, 10429–10435 CrossRef CAS PubMed.
- B. Sharma, N. Singh and J. P. Kushwaha, J. Chem. Eng. Data, 2020, 65(4), 1497–1505 CrossRef CAS.
- Y. Liu, J. B. Friesen, J. B. McAlpine, D. C. Lankin, S.-N. Chen and G. F. Pauli, J. Nat. Prod., 2018, 81, 679–690 CrossRef CAS PubMed.
- B. Schuur, T. Brouwer, D. Smink and L. M. J. Sprakel, Curr. Opin. Green Sustainable Chem., 2019, 18, 57–65 CrossRef.
- F. Jérôme and R. Luque, Bio-Based Solvents, John Wiley & Sons, Hoboken, New Jersey, 2017 Search PubMed.
- E. Clark, Sulfolane and sulfones, in Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc., Hoboken, New Jersey, 2000, vol. 21, p. 11 Search PubMed.
- J. E. Camp, ChemSusChem, 2018, 11, 3048–3055 CrossRef CAS PubMed.
- F. H. Isikgor and C. R. Becer, Polym. Chem., 2015, 6, 4497–4559 RSC.
- J. Y. Dai, Y. Q. Sun and Z. L. Xiu, Eng. Life Sci., 2014, 14, 108–117 CrossRef CAS.
- M. North, P. Villuendas and C. Young, Chem. – Eur. J., 2009, 15, 11454–11457 CrossRef CAS PubMed.
- M. Patel, X. Zhang and A. Kumar, Renewable Sustainable Energy Rev., 2016, 53, 1486–1499 CrossRef CAS.
- K. Watanabe, N. Yamagiwa and Y. Torisawa, Org. Process Res. Dev., 2007, 11, 251–258 CrossRef CAS.
- A. Ekman and P. Börjesson, J. Cleaner Prod., 2011, 19, 1257–1265 CrossRef CAS.
- C. Kelkar and A. Schutz, Appl. Clay Sci., 1998, 13, 417–432 CrossRef CAS.
- S. Kale, U. Armbruster, S. Umbarkar, M. Dongare and A. Martin, Presented in part at the 10th Green Chemistry Conference, Barcelona, 2013.
- D. Fernandes, A. Rocha, E. Mai, C. J. Mota and V. T. Da Silva, Appl. Catal., A, 2012, 425, 199–204 CrossRef.
- X. Li, L. Su, Y. Wang, Y. Yu, C. Wang, X. Li and Z. Wang, Front. Environ. Sci. Eng., 2012, 6, 295–303 CrossRef CAS.
- S. Nenkova, T. Vasileva and K. Stanulov, Chem. Nat. Compd., 2008, 44, 182–185 CrossRef CAS.
- C. Amen-Chen, H. Pakdel and C. Roy, Bioresour. Technol., 2001, 79, 277–299 CrossRef CAS PubMed.
- Y. P. Patil, P. J. Tambade, S. R. Jagtap and B. M. Bhanage, Front. Chem. Sci. Eng., 2010, 4, 213–235 CAS.
- J. S. Brimacombe, F. Hunedy and L. C. Tucker, Carbohydr. Res., 1978, 60, 11–12 CrossRef.
- C. Sener, D. D. Petrolini, D. J. McClelland, J. He, M. R. Ball, Y. Liu, L. Martins, J. A. Dumesic, G. W. Huber and B. M. Weckhuysen, Green Chem., 2019, 21, 5000–5007 RSC.
- F. Cao, T. J. Schwartz, D. J. McClelland, S. H. Krishna, J. A. Dumesic and G. W. Huber, Energy Environ. Sci., 2015, 8, 1808–1815 RSC.
- S. Kudo, N. Goto, J. Sperry, K. Norinaga and J.-I. Hayashi, ACS Sustainable Chem. Eng., 2016, 5, 1132–1140 CrossRef.
- J. Sherwood, A. Constantinou, L. Moity, C. R. McElroy, T. J. Farmer, T. Duncan, W. Raverty, A. J. Hunt and J. H. Clark, Chem. Commun., 2014, 50, 9650–9652 RSC.
- S. H. Krishna, D. J. McClelland, Q. A. Rashke, J. A. Dumesic and G. W. Huber, Green Chem., 2017, 19, 1278–1285 RSC.
- K. L. Wilson, A. R. Kennedy, J. Murray, B. Greatrex, C. Jamieson and A. J. Watson, Beilstein J. Org. Chem., 2016, 12, 2005–2011 CrossRef CAS PubMed.
- K. L. Wilson, J. Murray, C. Jamieson and A. J. Watson, Synlett, 2018, 29, 650–654 CrossRef CAS.
- K. L. Wilson, J. Murray, C. Jamieson and A. J. Watson, Org. Biomol. Chem., 2018, 16, 2851–2854 RSC.
- L. Mistry, K. Mapesa, T. W. Bousfield and J. E. Camp, Green Chem., 2017, 19, 2123–2128 RSC.
- J. Zhang, G. B. White, M. D. Ryan, A. J. Hunt and M. J. Katz, ACS Sustainable Chem. Eng., 2016, 4, 7186–7192 CrossRef CAS.
- S. Lawrenson, M. North, F. Peigneguy and A. Routledge, Green Chem., 2017, 19, 952–962 RSC.
- G. Bonneau, A. A. Peru, A. L. Flourat and F. Allais, Green Chem., 2018, 20, 2455–2458 RSC.
- M. De Bruyn, V. L. Budarin, A. Misefari, S. Shimizu, H. Fish, M. Cockett, A. J. Hunt, H. Hofstetter, B. M. Weckhuysen and J. H. Clark, ACS Sustainable Chem. Eng., 2019, 7878–7883 CrossRef CAS.
- H. J. Salavagione, J. Sherwood, V. Budarin, G. Ellis, J. Clark and P. Shuttleworth, Green Chem., 2017, 19, 2550–2560 RSC.
- F. Abushwireb, H. Elakrami and M. Emtir, Comput.-Aided Chem. Eng., 2007, 24, 1071–1076 CAS.
- M. Blahušiak, A. A. Kiss, K. Babic, S. R. Kersten, G. Bargeman and B. Schuur, Sep. Purif. Technol., 2018, 194, 301–318 CrossRef.
- R. B. Eldridge, Ind. Eng. Chem. Res., 1993, 32, 2208–2212 CrossRef.
- U. Weidlich and J. Gmehling, Ind. Eng. Chem. Res., 1987, 26, 1372–1381 CrossRef CAS.
- T. Brouwer and B. Schuur, Ind. Eng. Chem. Res., 2019, 58, 8903–8914 CrossRef CAS.
- Q. Wang, J. Y. Chen, M. Pan, C. He, C. C. He, B. J. Zhang and Q. L. Chen, Chem. Eng. Process., 2018, 128, 80–95 CrossRef CAS.
- C. Buell and R. Boatright, Ind. Eng. Chem., 1947, 39, 695–705 CrossRef CAS.
- G. R. Lake, United States Pat., US2406695, 1946 Search PubMed.
- J. Coca and J. J. Pis, J. Chem. Eng. Data, 1979, 24, 103–105 CrossRef CAS.
- L. Berg, United States Pat., US4363704A, 1982 Search PubMed.
- M. Fenske, C. Carlson and D. Quiggle, Ind. Eng. Chem., 1947, 39, 1322–1328 CrossRef CAS.
- D. Quiggle and M. R. Fenske, J. Am. Chem. Soc., 1937, 59, 1829–1832 CrossRef CAS.
- W. L. McCabe, J. C. Smith and P. Harriott, Unit operations of chemical engineering, McGraw-Hill, New York, 1993 Search PubMed.
- J. V. De Oliveira and A. C. Uller, Fluid Phase Equilib., 1989, 46, 267–280 CrossRef CAS.
- J. V. De Oliveira and A. C. Uller, Fluid Phase Equilib., 1996, 118, 133–141 CrossRef CAS.
- L. Malley, G. Kennedy, G. Elliott, T. Slone, W. Mellert, K. Deckardt, K. Kuttler, B. Hildebrand, M. Banton and R. Parod, Drug Chem. Toxicol., 2001, 24, 315–338 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0gc01769h |
| This journal is © The Royal Society of Chemistry 2020 |