Open Access Article
Open Access ArticleContinuous hydroformylation of 1-decene in an aqueous biphasic system enabled by methylated cyclodextrins†
K. U.
Künnemann
 a,
L.
Schurm
a,
L.
Schurm
 a,
D.
Lange
a,
T.
Seidensticker
a,
D.
Lange
a,
T.
Seidensticker
 a,
S.
Tilloy
a,
S.
Tilloy
 b,
E.
Monflier
b,
E.
Monflier
 b,
D.
Vogt
b,
D.
Vogt
 a and
J. M.
Dreimann
*a
a and
J. M.
Dreimann
*a
aLaboratory of Industrial Chemistry, Department of Biochemical and Chemical Engineering, TU Dortmund University, Emil-Figge-Straße 66, 44227 Dortmund, Germany. E-mail: jens.dreimann@tu-dortmund.de
bUniv. Artois, CNRS, Centrale Lille, Univ. Lille, UMR 8181 – UCCS – Unité de Catalyse et Chimie du Solide, F-62300 Lens, France
First published on 14th April 2020
Abstract
For the first time, randomly methylated β-cyclodextrin was applied as the mass transfer agent in a continuous process. Considering the example of the Rh-catalyzed hydroformylation of 1-decene, process development was shown, where cyclodextrin was used together with a catalyst system that was continuously recovered and recycled using an aqueous biphasic system. In initial experiments, water-soluble and commercially available Rh/TPPTS and Rh/sulfoxantphos catalyst systems were scaled up from 50 ml into 1000 ml high-pressure autoclave systems to demonstrate their scalability. Both these systems were compared, and they afforded excellent chemoselectivity (>99%) toward the desired linear aldehyde product. In particular, higher regioselectivity (up to 31) was achieved for the Rh/sulfoxantphos system. Investigations regarding the long-term stability of the mass transfer agent and both catalyst systems were carried out in a continuously operated miniplant process. It was shown that the process could be successfully operated under the steady state for over 200 h with chemoselectivity of >97% toward the desired aldehyde product. Simultaneously, extremely low Rh leaching (total: 0.59%) was observed over the entire period of 200 h.
1. Introduction
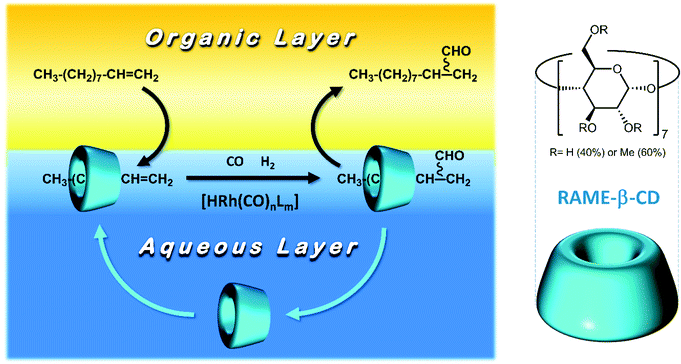
Hydroformylation is widely used in industry to manufacture high-value-added aldehydes and alcohols, and it is considered to be one of the largest homogenously catalyzed reactions in industry. A major issue persisting with the use of homogenous catalysts is their recovery and separation from the product mixture, particularly for higher olefins; this is important due to the cost of the employed transition metals. These days, distillation has become the most commonly used separation method in the hydroformylation of alkenes and therefore the most well-known method.1 However, distillation conditions that are required to separate high-boiling products may lead to catalyst decomposition along with concomitant loss of metal. Hence, cheaper cobalt catalysts are used, ensuring that the processes remain economically viable.2 However, cobalt catalysts are less active than rhodium catalysts, requiring higher reaction temperatures and pressures.3 Therefore, the challenge involved in formulating techniques to convert higher olefins in hydroformylation persist, particularly in a manner that can address the separation problem and minimize environmental impact, while not compromising on catalyst selectivity and activity. Some concepts focused on this involve the intensification of the monophasic flow process for hydroformylation.4 Other approaches have been developed in both industry and academia to tackle the main issue of catalyst separation from the product by using rhodium catalysts. Examples of these are the use of supercritical CO2 (scCO2),5 CO2-expanded liquid (CXL),6,7 supported ionic liquid phase (SILP),7 organic solvent nanofiltration (OSN),8 thermomorphic multiphase systems (TMS),9 microemulsions,10 or biphasic systems.11 Some of them use abundant resources (such as carbon dioxide and the environmentally friendly solvent, water), but others do not. The use of water as a solvent for process applications is particularly beneficial from the economic and environmental impact viewpoints since water is fairly accessible, nontoxic, nonflammable, odorless, and has a high heat capacity and heat of vaporization.12 Nevertheless, these benefits have to compete with its intrinsic limitation of low organic substrate solubility in the aqueous catalyst phase, which have also been confirmed by early kinetic studies.13,14 Therefore, several approaches have been developed to tackle the issue of low space–time yield in biphasic aqueous–organic reaction systems involving poorly water-soluble substrates, namely, by using surfactants, cosolvents, thermoregulated ligands, and cyclodextrins (CDs) to achieve improved hydroformylation, as summarized in the review article by Matsinha et al.14From this perspective, this work further focuses on the use of CDs as the mass transfer agent to overcome mass transfer limitations in the biphasic hydroformylation of 1-decene. CDs are cyclic oligosaccharides comprising six (α-CD), seven (β-CD), or eight (γ-CD) α-D-glucopyranose units.15 They form conical cylinders with the outer hydrophilic surface and inner hydrophobic surface. The inner surface forms a cavity and allows for the formation of an inclusion complex when it binds with a hydrophobic substrate (Fig. 1).16 To vary their size and shape, native CDs can be modified by substituting their hydroxyl groups with various other functional groups. These are referred to as mono- or polysubstituted CDs. Due to their ability to form inclusion complexes with a wide range of compounds, native and modified CDs have applications in various fields.17 In the field of aqueous biphasic organometallic catalysis, CDs have mainly been employed as mass transfer agents16,18 in order to increase the solubility of hydrophobic substrates in water. For example, in 1995, Monflier et al.16,19–22 described rhodium-catalyzed hydroformylation in an aqueous/organic two-phase system of 1-decene in the presence of randomly methylated β-CD (RAME-β-CD; Fig. 1).21,23 The phase separation between the organic and aqueous phases was described to be fast; further, the rhodium and phosphorus contents in the organic phase were found to be less than 0.5 and 1.2 ppm, respectively. Moreover, in 1999, Tilloy et al. proposed the reusability of the Rh/TPPTS/RAME-β-CD catalytic system by performing the rhodium-catalyzed hydroformylation of 1-decene with 5 catalytic recycle runs without any loss of activity.24 Later, in 2004, Leclercq et al. investigated the same reaction using the sulfoxantphos ligand, which led to improved performances in terms of chemoselectivity and regioselectivity.20 Although CDs are highly promising as mass transfer agents in aqueous biphasic systems for the selective hydroformylation of higher olefins, they have never been applied in a continuously operated process. As our research group has gained expertise in various continuously operated recycling strategies for homogeneous catalysts with regard to hydroformylation25,26 in earlier years, we combined our knowledge with the group of Monflier to understand if CDs can be employed for such applications. Therefore, in this work, we transfer two catalytic systems (Rh/TPPTS/RAME-β-CD and Rh/sulfoxantphos/RAME-β-CD) into one of our continuously operated miniplants for the purpose of aqueous biphasic hydroformylation. Since this has never been attempted before, both these systems were first scaled up in differently sized batch autoclaves in order to understand their performance in terms of chemo-/regioselectivity and conversion. Further, their reaction profile was determined to be the benchmark for the residence time in the subsequent continuous process. During continuous operations, the influence of various parameters (such as CD concentration and stirrer speed) on the catalytic activity was determined. Eventually, the effective recycling of the catalytic systems (supported by leaching data) with the optimized parameters was conducted via a long-term experiment, revealing the potential of CDs for such applications.
2. Results and discussion
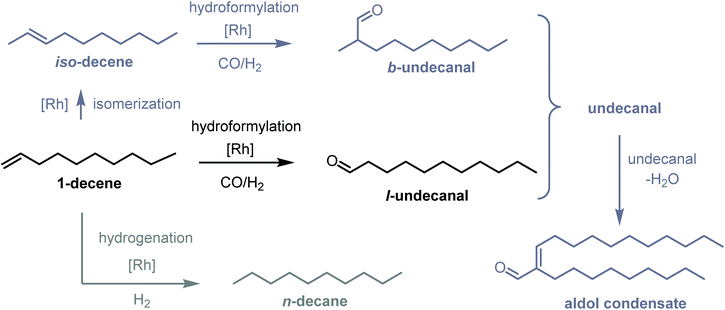
Based on the studies by Monflier et al.,16,19–22 1-decene was chosen as the model substrate for CD-based hydroformylation. The reaction network is shown in Fig. 2. The main product from the hydroformylation is undecanal, which is referred to as l-undecanal (linear undecanal). 1-Decene isomers may occur as byproducts and are referred to as iso-decene (isomerized decene). The other byproducts are n-decane (which results from the hydrogenation of 1-decene or iso-decene) and the branched hydroformylation product, namely, 2-methyldecanal (which can arise from the hydroformylation of 1-decene or iso-decene). Further, 2-methyldecanal is the representative of all the branched aldehydes since it is formed as a byproduct in the highest amount. These branched aldehydes are categorized as b-undecanal (branched undecanal). All the undecanal isomers are categorized into undecanal (l-undecanal + b-undecanal). The remaining byproducts are aldol condensates, which can result from the condensation reaction of two aldehydes. All these products are summarized, and 2-nonyl-tridec-2-enal is considered to be the representative of the aldol condensates.In this work, two catalyst systems have been investigated, since they yielded worthwhile results in earlier publications with regard to high product chemo-/regioselectivity. The already published results are listed in Table 2.18,20,24 Both these systems use water as the solvent, Rh(acac)(CO)2 as the precursor, and RAME-β-CDs (Fig. 1) as the mass transfer agent. The only difference is the choice of phosphine ligand, namely, monodentate TPPTS and bidentate sulfoxantphos.
2.1. Scaled up batch experiments
Scaled up experiments were first performed since the original reactions were carried out in 50 ml high-pressure autoclaves. Therefore, two types of high-pressure autoclaves were used for the experiments (Fig. 3). A high-pressure autoclave with a total volume of 300 ml was used as the first reference as well as for the scaling up tests. For a facilitating comparability between the experiments, the gas-to-liquid volume ratio was maintained as per the original experiments performed by Monflier et al.19 and Leclercq et al.,20 yielding a volume of 100 ml for the reaction solution. In this work, this reactor is also referred to as the 300 ml high-pressure autoclave. Another reactor is built into the continuous miniplant process and was used in the batch or continuous mode. This reactor had a total volume of 1000 ml and is correspondingly referred to subsequently. To maintain the gas-to-liquid volume ratio constant, the liquid volume was set to 330 ml.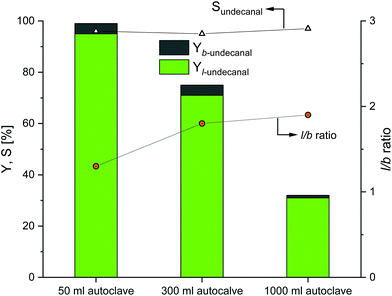 | ||
Fig. 4 Results of the CD-mediated aqueous biphasic hydroformylation of 1-decene using TPPTS depending on the reactor size. Here, the 50 ml autoclave data were adapted from Hapiot et al.21 Conditions: 1. 50 ml autoclave:21 Rh(acac)(CO)2: 0.041 mmol, TPPTS: 0.21 mmol, CDs: 0.48 mmol, water: 11.5 ml, 1-decene: 20.35 mmol, T = 80 °C, p(CO/H2) = 50 bar, n = 1500 min−1, no preformation. 2. 300 and 1000 ml autoclaves: preforming: p(CO/H2) = 20 bar, nCO/nH2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, T = 80 °C, t = 2 h; reaction: p(CO/H2) = 40 bar, nCO/nH2 = 1 1, T = 80 °C, t = 2 h; reaction: p(CO/H2) = 40 bar, nCO/nH2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, T = 80 °C, t = 6 h, mwater/msubstrate = 4 1, T = 80 °C, t = 6 h, mwater/msubstrate = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, nsubstrate/nRh = 496, nCD/nRh = 12, nP/nRh = 5, n = 800 min−1. 1, nsubstrate/nRh = 496, nCD/nRh = 12, nP/nRh = 5, n = 800 min−1. | ||
| Entry | Olefin | CD | Ligand | Selectivityc [%] | l/b |
|---|---|---|---|---|---|
Experimental conditions: Rh(acac)(CO)2: 0.04 mmol; TPPTS/sulfoxantphos: 0.21 mmol; CD: 0.48 mmol; water: 11.5 ml; olefin: 20.35 mmol; n-undecanal (internal standard): 2.03 mmol; p(CO/H2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 50 bar.a T = 80 °C, t = 6 h.b T = 120 °C, t = 24 h.c Chemoselectivity toward undecanal. 1): 50 bar.a T = 80 °C, t = 6 h.b T = 120 °C, t = 24 h.c Chemoselectivity toward undecanal. |
|||||
| 1a | 1-Decene | RAME-β-CD | TPPTS | 98 | 1.7 |
| 2b | 1-Decene | RAME-β-CD | Sulfoxantphos | >99 | 26 |
Evidently, as the reactor size increased, the catalytic activity as compared to the original 50 ml experiments decreased. Nearly full conversion could be achieved in the 50 ml autoclaves as compared to the 300 ml autoclaves; the conversion decreases to 75% and eventually to 33% when compared to the 1000 ml autoclaves. The chemoselectivity of all the three experiments are in the same range (over 95%), which indicates that the Rh/TPPTS catalyst system works as expected for all the experiments. Further, with respect to regioselectivity, the l/b ratio reached a value close to 1.8 for all the experiments.
As these experiments were conducted to demonstrate the influences of different parameters as barriers for the potential transfer into a continuous process, the obtained results revealed that a transfer is generally possible when the selectivities remain constant. However, in a continuous process, lower conversions have to be expected due to the potential mass transfer limitations due to an increase in the size of the autoclaves. Since this is a three-phase gas–liquid–liquid system, there may be limitations at the gas–liquid phase interface and/or at the liquid–liquid phase interface, which is supported by the investigations of Sieffert et al.; they suggested that the reaction does not take place in the bulk water phase, but it occurs at the aqueous phase/interface layer.28 Therefore, there would be stronger dependence between the phase interface and reaction rate and consequently the conversion rate. With scaling up and an increase in volume, the amount of substance gets proportionally scaled, but the phase interface does not. The reaction profiles for the 300 and 1000 ml systems are provided in the ESI,† revealing the potential undecanal yield for different residence times in the miniplant reactor.
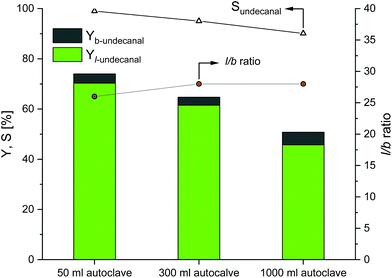 | ||
Fig. 5 Results of the CD-mediated aqueous biphasic hydroformylation of 1-decene using sulfoxantphos depending on the reactor size. Here, the 50 ml autoclave data were adapted from earlier studies.20 Conditions: 1. 50 ml autoclave:20 Rh(acac)(CO)2: 0.04 mmol, sulfoxantphos: 0.21 mmol, CD: 0.48 mmol, water: 11.5 ml, olefin: 20.35 mmol, n-undecanal (internal standard): 2.03 mmol, p(CO/H2) = 50 bar, T = 120 °C. 2. 300 ml autoclave: preforming: p(CO/H2) = 20 bar, nCO/nH2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, T = 120 °C, t = 12 h; reaction: p(CO/H2) = 40 bar, nCO/nH2 = 1 1, T = 120 °C, t = 12 h; reaction: p(CO/H2) = 40 bar, nCO/nH2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, T = 120 °C, t = 6 h, mw/mS = 4 1, T = 120 °C, t = 6 h, mw/mS = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, nS/ncat = 496, nCD/ncat = 12, nP/ncat = 5, n = 800 min−1. 3. 1000 ml autoclave: preforming: p(CO/H2) = 20 bar, nCO/nH2 = 1 1, nS/ncat = 496, nCD/ncat = 12, nP/ncat = 5, n = 800 min−1. 3. 1000 ml autoclave: preforming: p(CO/H2) = 20 bar, nCO/nH2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, T = 120 °C, t = 12 h; reaction: p(CO/H2) = 40 bar, nCO/nH2 = 1 1, T = 120 °C, t = 12 h; reaction: p(CO/H2) = 40 bar, nCO/nH2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, T = 120 °C, τ = 6 h, mw/mS = 4 1, T = 120 °C, τ = 6 h, mw/mS = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, cCat = 0.006 mol%, nCD/nCat = 12, n = 800 min−1. 1, cCat = 0.006 mol%, nCD/nCat = 12, n = 800 min−1. | ||
2.2. Continuous miniplant experiments
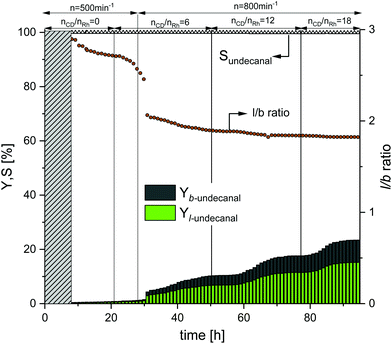
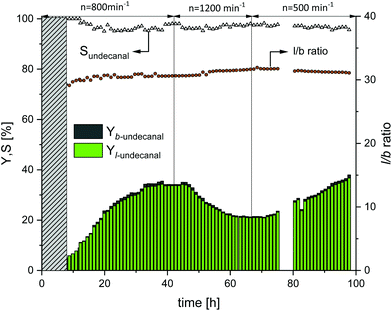
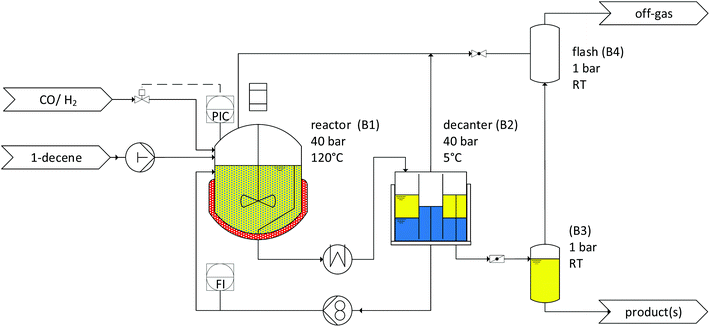
The proofs of concept for the successful scaling up of the Rh/TPPTS and Rh/sulfoxantphos catalyst systems have been shown in earlier batch experiments. As regio- and chemoselectivity are nearly constant for these experiments, the catalyst systems are transferred for the first time into a continuous process (Fig. 10). In this study, the long-term stability of catalytic systems in combination with CD, as well as influences of parameters such as CD concentration and stirrer speed in the reactor, were investigated.Initially, the regioselectivity (l/b ratio) was ∼2.8. After the first addition of CDs, the ratio rapidly dropped to 2.0 and then slowly reached a value within 1.8–1.9, which was the ratio determined for TPPTS in the batch experiments. The further addition of CDs had no influence on the ratio. The chemoselectivity (∼99%) was calculated over the entire duration of the experiment and therefore was higher than that in the batch experiments (Fig. 4). In Table 2, the catalyst leaching results for this experiment are listed. The samples taken from the product stream showed the total leaching (L) of the ligand of 1.2% and L value of rhodium of 0.8% after 94 h.
| t [h] | P [ppm] | L ligand [%] | L ligand [% h−1] | Rh [ppm] | L Rh [%] | L Rh [% h−1] |
|---|---|---|---|---|---|---|
| 0–39 | 3 | 0.64 | 0.0165 | 1 | 0.22 | 0.0057 |
| 39–87 | 2 | 0.48 | 0.0102 | 2 | 0.50 | 0.0106 |
| L(94 h) | — | 1.20 | 0.0128 | — | 0.80 | 0.0086 |
In Table 3, the catalyst leaching results are represented. In the beginning, the leaching values of rhodium and ligand were slightly higher. This is most likely due to the startup procedure and lower average residence time in the decanter. Further loss corresponded approximately to the value determined for the entire operating time. Over the operating time of 93 h and with an average leaching of the ligand of 0.0122% h−1, the total ligand leaching added to 1.14%. The average loss of rhodium was 0.0059% h−1, and therefore, the total rhodium leaching added to 0.55%.
| t [h] | P [ppm] | L ligand [%] | L ligand [% h−1] | Rh [ppm] | L Rh [%] | L Rh [% h−1] |
|---|---|---|---|---|---|---|
| 0–5 | 4 | 0.09 | 0.0170 | 2 | 0.07 | 0.0128 |
| 5–22 | 4 | 0.24 | 0.0147 | 1 | 0.09 | 0.0055 |
| 22–45 | 3 | 0.30 | 0.0129 | 1 | 0.13 | 0.0056 |
| 45–93 | 3 | 0.50 | 0.0105 | 1 | 0.26 | 0.0055 |
| L(93 h) | — | 1.14 | 0.0122 | — | 0.55 | 0.0059 |
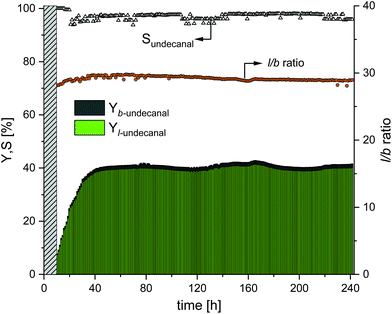
In Table 4, the catalyst leaching results are presented for the entire time of operation. In general, no significant deviation in leaching was observed, which supports the evidence that CDs are very efficient as mass transfer agents. Over the operating time of 241 h, the average leaching of ligand was 0.0098% h−1; therefore, the total leaching of the ligand added to 2.17%. The average loss of rhodium was 0.0027% h−1, and therefore, the total rhodium leaching added to 0.59%. The long-term stability of the system could, therefore, be demonstrated in this experiment. No makeup stream was needed to maintain the catalytic activity over the entire course of the experiment. During the experiment, a white solid deposit was observed in the decanter through a viewing window. This was noticed for the first time after ∼70 h of operation, and this deposit increased over the test period. The formation of this solid could be attributed to an increase in the CD concentration at the beginning in the sulfoxantphos system (nCD/nRh = 18 instead of nCD/nCat = 12 as compared to the batch experiments) and not to the long operating time itself. Solubility problems, therefore, appear to occur, which further exacerbate with an increase in the CD concentration, which could become problematic during continuous operation and should not be considered.
| t [h] | P [ppm] | L ligand [%] | L ligand [% h−1] | Rh [ppm] | L Rh [%] | L Rh [% h−1] |
|---|---|---|---|---|---|---|
| 0–8 | 2 | 0.07 | 0.0098 | 0.3 | 0.01 | 0.0022 |
| 8–19 | 2 | 0.10 | 0.0098 | 0.3 | 0.02 | 0.0026 |
| 19–30 | 2 | 0.10 | 0.0098 | 0.3 | 0.03 | 0.0027 |
| 30–43 | 2 | 0.12 | 0.0098 | 0.4 | 0.03 | 0.0031 |
| 43–64 | 2 | 0.20 | 0.0098 | 0.3 | 0.05 | 0.0026 |
| 64–95 | 2 | 0.30 | 0.0098 | 0.3 | 0.07 | 0.0025 |
| 95–122 | 2 | 0.26 | 0.0098 | 0.3 | 0.06 | 0.0023 |
| 122–168 | 2 | 0.45 | 0.0098 | 0.4 | 0.14 | 0.0032 |
| 168–184 | 2 | 0.15 | 0.0098 | 0.4 | 0.05 | 0.0033 |
| 184–221 | 2 | 0.36 | 0.0098 | 0.3 | 0.08 | 0.0023 |
| L(240 h) | — | 2.17 | 0.0098 | — | 0.59 | 0.0027 |
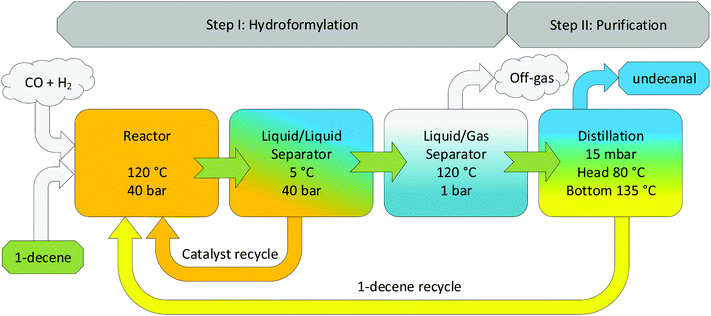
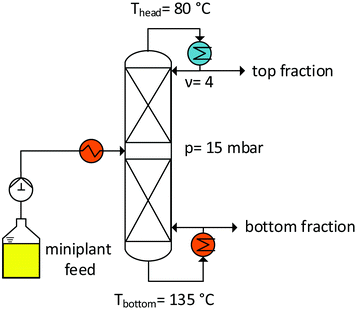
To prove that this concept is feasible, a distillation column (Fig. 11) was setup, as described in the Experimental section. In particular, vacuum distillation (temperature: <150 °C) was chosen to suppress the isomerization reactions of the substrate, but also for efficient product and substrate separation. The results of continuous distillation over 8 h are shown in the ESI.† A distillate stream (top fraction) with a composition of ∼94.2 wt% 1-decene and 5.8 wt% decane/decene isomers can be formulated. The regioselectivity (l/b ratio) of decene in the feed and at the top of the column remains constant at a value of ∼6 wt%. Therefore, the isomer portion of the feed, when completely separated, is decisive for determining the quality of the distillate stream. Therefore, the general feasibility of the process concept is evident.
3. Experimental section
3.1. Chemicals
In Table 5, all the chemicals used in this work are listed. All the solvents and substrates were degassed before the reaction, but not purified further. The purity of each component was tested via gas chromatography.| Chemicals | Manufacture | Purity [%] |
|---|---|---|
| 1-Decene | Acros organics | 99 |
| Argon | Messer Industriegase | — |
| Rh(acac)(CO)2 | Umicore | — |
| Triphenylphosphine-3,3′,3′′-trisulfonic acid trisodium salt | Oxea GmbH | — |
| Sulfoxantphos | MOLISA | — |
| Di-n-butylether | Acros organics | 99.5 |
| Isopropanol | VWR international | 98 |
| CO/H2 | Messer Industriegase | 99.9 |
| Bi-distilled water | — | — |
3.2. Analytics
A gas chromatograph from Agilent Technologies (Type 7890A) with an HP-5 column (30 m × 0.32 mm × 0.25 m) and a flame ionization detector (FID) was used for gas chromatographic measurements of the offline samples from the batch and continuous experiments. n-Dodecane was used as the standard and isopropanol, the solvent. For measuring the online samples for continuous experiments, a gas chromatograph (Type 7890A, Agilent Technologies) was used for the gas chromatographic measurements. This system was equipped with a FID. An HP-5 (30 m × 0.25 mm × 0.25 m) was used as the separation column. A sample volume of 1 μl was injected with a split ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 with a constant supply of helium as the carrier gas.
50 with a constant supply of helium as the carrier gas.
The contents of rhodium and phosphorus in the liquid phases were quantified via inductively coupled plasma emission spectroscopy (ICP-OES, Thermo Elemental Iris Intrepid). The catalyst leaching rates for rhodium and phosphorus were calculated on the basis of the total amount of the related product fraction, the appropriate mass fraction (ppm) contained, and the initial mass of rhodium and phosphorus at the start of the miniplant. For example, 1% h−1 of leaching represents a loss of 1% of the initial catalyst amount via product flow in 1 h.
3.3. Experimental setup
A 1000 ml high-pressure autoclave (Büchi) was used to perform further scaling up experiments. The reactor was stable up to 60 bar, including a blade stirrer and baffles. The procedure for carrying out the experiments is the same as that for the 300 ml high-pressure autoclave. The reactor was also used as a continuous stirred-tank reactor (CSTR) for performing continuous miniplant experiments.
4. Conclusion
The Rh-catalyzed aqueous biphasic hydroformylation of 1-decene was successfully conducted only using water as the environmentally friendly solvent and RAME-β-CD as the green mass transfer agent. Initially, two catalyst systems comprising of Rh/TPPTS and Rh/sulfoxantphos were scaled up from a 50 ml autoclave to a 1000 ml high-pressure autoclave to evaluate the feasibility of these phase systems for a potential continuous process application. Herein, both these systems performed efficiently in terms of chemoselectivity (>95%) toward the aldehyde product. However, these experiments reveal that for both these catalyst systems, the reaction rates slowed down in the larger autoclaves; therefore, conversion decreased from 99% to 30% (Rh/TPPTS) and from 70% to 50% (Rh/sulfoxantphos). However, as the chemoselectivity remained high for both these systems, they were tested in two continuous experiments (each over 90 h). These experiments showed that the reaction activity could be drastically influenced by the stirrer speed and nCD/nRh values inside the reactor. The Rh/sulfoxantphos catalyst system not only performed better in terms of aldehyde yield (45% as compared to that the Rh/TPPTS system, i.e., 30%), but also afforded higher regioselectivities (l/b ratios), i.e., 27, as compared to the value of 1.7; therefore, this system was chosen for the long-term experiment. For a total operation time of 240 h in a continuous miniplant and that of more than 200 h for steady-state operation, the leaching of catalyst was determined to be extremely low, affording ligand loss of 0.0098% h−1 and rhodium loss of 0.0027% h−1. As compared to a commercialized hydroformylation process (Shell) and other recently proposed processes (SILP/CXL and Aqueous TMS) in the literature, a CD-based system does not yield the highest turnover rates in continuous operations, but one of the highest chemo- and regioselectivities and lowest rhodium leaching rates (Table 6). Further, the CD process necessitates very mild conditions (120 °C and 40 bar) as compared to other processes.| Parameter | CDsa (Rh) | SILP/CXLb (Rh)30 | Aqueous TMS (Rh)26 | Shell (Co)31 |
|---|---|---|---|---|
| a Referring to the experiment shown in Fig. 8. b SILP/CXL: supported ionic liquid phase + carbon-dioxide-expanded liquids; TOS: time on stream. | ||||
| T [°C] | 120 | 100 | 140 | 200 |
| p [bar] | 40 | 100 | 21 | 80 |
| S aldehyde [%] | 97 | 98 | 93 | 80 |
| l/b ratio [—] | 27 | 3 | 19 | — |
| TOF [h−1] | 19 | 500 | 1500 | 20 |
| TOS [h] | 240 | 40 | 21 | — |
| Rh loss [ppm h−1] | 0.01 | 0.005 | 0.7 | — |
Reaction rate improvements such as stirrer speed, stirrer type, phase ratios, and CD concentrations are still under investigation and will be discussed in an additional publication in more detail. On the other hand, process optimization was successfully described by the continuous distillation of the product mixture (undecanal, 1-decene, and iso-decene) and recovering nonconverted 1-decene substrate with 94% purity. With the possibility of continuous distillation of the nonconverted substrate, a new process concept was proposed by implementing a second recycling loop into the process. Therefore, combining an aqueous biphasic hydroformylation process using methylated β-CDs for higher olefins with extremely low catalyst leaching and high chemo- and regioselectivities toward the corresponding aldehyde product can be achieved.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
Gefördert durch die Deutsche Forschungsgemeinschaft (DFG) – TRR 63 “Integrierte chemische Prozesse in flüssigen Mehrphasensystemen” (Teilprojekt D3) – 56091768. Authors would like to thank Umicore AG &Co. KG for donating the rhodium precursor Rh(acac)(CO2) and Oxea GmbH for donating the ligand NaTPPTS.References
- R. Franke, D. Selent and A. Börner, Chem. Rev., 2012, 112, 5675–5732 CrossRef CAS PubMed.
- F. Hebrard and P. Kalck, Chem. Rev., 2009, 109, 4272–4282 CrossRef CAS PubMed.
- P. W. N. M. Leeuwen, Homogeneous Catalysis. Understanding the Art, Kluwer Academic Publishers, Dordrecht, 2004 Search PubMed.
- (a) H. Masui, E. Honda, S. Niitsu, M. Shoji and T. Takahashi, Int. J. Org. Chem., 2018, 08, 135–141 CrossRef CAS; (b) S. Wang, J. Zhang, F. Peng, Z. Tang and Y. Sun, Ind. Eng. Chem. Res., 2020, 59, 88–98 CrossRef CAS; (c) M. L. Abrams, J. Y. Buser, J. R. Calvin, M. D. Johnson, B. R. Jones, G. Lambertus, C. R. Landis, J. R. Martinelli, S. A. May, A. D. McFarland and J. R. Stout, Org. Process Res. Dev., 2016, 20, 901–910 CrossRef CAS; (d) S. A. May, J. Flow Chem., 2017, 7, 137–145 CrossRef CAS.
- U. Hintermair, G. Franciò and W. Leitner, Chem. Commun., 2011, 47, 3691–3701 RSC.
- Z. Xie and B. Subramaniam, ACS Sustainable Chem. Eng., 2014, 2, 2748–2757 CrossRef CAS.
- J. M. Marinkovic, A. Riisager, R. Franke, P. Wasserscheid and M. Haumann, Ind. Eng. Chem. Res., 2019, 58, 2409–2420 CrossRef CAS.
- M. Janssen, C. Müller and D. Vogt, Dalton Trans., 2010, 39, 8403–8411 RSC.
- J. Bianga, K. U. Künnemann, T. Gaide, A. J. Vorholt, T. Seidensticker, J. M. Dreimann and D. Vogt, Chem. – Eur. J., 2019, 11586–11608 CrossRef CAS PubMed.
- M. Schwarze, T. Pogrzeba, K. Seifert, T. Hamerla and R. Schomäcker, Catal. Today, 2015, 247, 55–63 CrossRef CAS.
- B. Cornils and E. G. Kuntz, J. Organomet. Chem., 1995, 502, 177–186 CrossRef CAS.
- D. J. Adams, P. J. Dyson and S. J. Tavener, Chemistry in alternative reaction media, Wiley, Chichester, West Sussex, 2005 Search PubMed.
- (a) S. K. Sharma and R. V. Jasra, Catal. Today, 2015, 247, 70–81 CrossRef CAS; (b) L. Obrecht, P. C. J. Kamer and W. Laan, Catal. Sci. Technol., 2013, 3, 541–551 RSC; (c) Y. Zhang, Z.-S. Mao and J. Chen, Ind. Eng. Chem. Res., 2001, 40, 4496–4505 CrossRef CAS.
- L. C. Matsinha, S. Siangwata, G. S. Smith and B. C. E. Makhubela, Catal. Rev. Sci. Eng., 2019, 61, 111–133 CrossRef CAS.
- (a) Cyclodextrin Fundamentals, Reactivity and Analysis, ed. S. Fourmentin, G. Crini and E. Lichtfouse, Springer International Publishing, Cham, 2018, vol. 16 Search PubMed; (b) J. Szejtli, Chem. Rev., 1998, 98, 1743–1754 CrossRef CAS.
- H. Bricout, F. Hapiot, A. Ponchel, S. Tilloy and E. Monflier, Sustainability, 2009, 1, 924–945 CrossRef CAS.
- (a) J. Yi, W. Liang, X. Wei, J. Yao, Z. Yan, D. Su, Z. Zhong, G. Gao, W. Wu and C. Yang, Chin. Chem. Lett., 2018, 29, 87–90 CrossRef CAS; (b) A. Cocq, H. Bricout, F. Djedaïni-Pilard, S. Tilloy and E. Monflier, Catalysts, 2020, 10, 56 CrossRef CAS; (c) X. Wei, W. Wu, R. Matsushita, Z. Yan, D. Zhou, J. J. Chruma, M. Nishijima, G. Fukuhara, T. Mori, Y. Inoue and C. Yang, J. Am. Chem. Soc., 2018, 140, 3959–3974 CrossRef CAS PubMed.
- F. Hapiot, S. Menuel, H. Bricout, S. Tilloy and E. Monflier, Appl. Organomet. Chem., 2015, 29, 580–587 CrossRef CAS.
- E. Monflier, S. Tilloy, G. Fremy, Y. Castanet and A. Mortreux, Tetrahedron Lett., 1995, 36, 9481–9484 CrossRef CAS http://www.sciencedirect.com/science/article/pii/004040399502073X .
- L. Leclercq, F. Hapiot, S. Tilloy, K. Ramkisoensing, J. N. H. Reek, P. W. N. M. van Leeuwen and E. Monflier, Organometallics, 2005, 24, 2070–2075 CrossRef CAS.
- F. Hapiot, L. Leclercq, N. Azaroual, S. Fourmentin, S. Tilloy and E. Monflier, Curr. Org. Synth., 2008, 5, 162–172 CrossRef CAS.
- F. Hapiot, S. Tilloy and E. Monflier, Chem. Rev., 2006, 106, 767–781 CrossRef CAS PubMed.
- (a) F. Hapiot, H. Bricout, S. Tilloy and E. Monflier, Top. Curr. Chem., 2013, 342, 49–78 CrossRef CAS PubMed; (b) H. Bricout, F. Hapiot, A. Ponchel, S. Tilloy and E. Monflier, Curr. Org. Chem., 2010, 14, 1296–1307 CrossRef CAS.
- S. Tilloy, F. Bertoux, A. Mortreux and E. Monflier, Catal. Today, 1999, 48, 245–253 CrossRef CAS.
- (a) A. Behr, A. Kämper, R. Kuhlmann, A. J. Vorholt and R. Franke, Catal. Sci. Technol., 2016, 6, 208–214 RSC; (b) M. Zagajewski, J. M. Dreimann, M. Thönes and A. Behr, Chem. Eng. Process., 2016, 99, 115–123 CrossRef CAS; (c) K. U. Künnemann, J. Bianga, R. Scheel, T. Seidensticker, J. M. Dreimann and D. Vogt, Org. Process Res. Dev., 2020, 24, 41–49 CrossRef; (d) H. Warmeling, A. Behr and A. J. Vorholt, Chem. Eng. Sci., 2016, 149, 229–248 CrossRef CAS.
- T. Gaide, J. M. Dreimann, A. Behr and A. J. Vorholt, Angew. Chem., Int. Ed., 2016, 55, 2924–2928 CrossRef CAS PubMed.
- T. Mathivet, C. Méliet, Y. Castanet, A. Mortreux, L. Caron, S. Tilloy and E. Monflier, J. Mol. Catal. A: Chem., 2001, 176, 105–116 CrossRef CAS http://www.sciencedirect.com/science/article/pii/S1381116901002291 .
- N. Sieffert and G. Wipff, Chem. – Eur. J., 2007, 13, 1978–1990 CrossRef CAS PubMed.
- (a) H. W. F. Warmeling, T. Hafki, T. von Söhnen and A. J. Vorholt, Chem. Eng. J., 2017, 326, 298–307 CrossRef CAS; (b) H. W. F. Warmeling, D. Janz, M. Peters and A. J. Vorholt, Chem. Eng. J., 2017, 330, 585–595 CrossRef CAS.
- U. Hintermair, Z. Gong, A. Serbanovic, M. J. Muldoon, C. C. Santini and D. J. Cole-Hamilton, Dalton Trans., 2010, 39, 8501–8510 RSC.
- P. B. Webb, T. E. Kunene and D. J. Cole-Hamilton, Green Chem., 2005, 7, 373 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Reaction profiles for the Rh/TPPTS and Rh/sulfoxantphos systems are shown for both 300 and 1000 ml autoclaves. Further, the composition of the distillate stream for the continuously operated distillation is shown. See DOI: 10.1039/d0gc00820f |
| This journal is © The Royal Society of Chemistry 2020 |