Open Access Article
Open Access ArticleReporting the unreported: the reliability and comparability of the literature on organic solvent nanofiltration†
Hai Anh
Le Phuong
 ab,
Christopher F.
Blanford
ab,
Christopher F.
Blanford
 *bc and
Gyorgy
Szekely
*bc and
Gyorgy
Szekely
 *ad
*ad
aDepartment of Chemical Engineering & Analytical Science, School of Engineering, The University of Manchester, The Mill, Sackville street, Manchester, M13 9PL, United Kingdom. E-mail: gyorgy.szekely@manchester.ac.uk
bDepartment of Materials, School of Natural Sciences, The University of Manchester, Oxford Road, Manchester, M13 9PL, United Kingdom
cManchester Institute of Biotechnology, The University of Manchester, 131 Princess Street, Manchester, M1 7DN, United Kingdom. E-mail: christopher.blanford@manchester.ac.uk; Tel: +44 (0)1613068915
dAdvanced Membranes and Porous Materials Center, Physical Science and Engineering Division (PSE), King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Saudi Arabia. E-mail: gyorgy.szekely@kaust.edu.sa; Tel: +966128082769 Web: http://www.szekelygroup.com
First published on 6th May 2020
Abstract
Organic solvent nanofiltration (OSN) is an energy-efficient separation technique that has the potential to improve environmental sustainability in many industrial sectors, including food processing, biorefineries, and in the production of pharmaceuticals, fine chemicals and petrochemicals. Some issues, however, hinder the pace of development of this sustainable separation method that could ultimately provide green manufacturing strategies. These issues include lack of clear experimental designs, explicit experimental protocols, comparable performance data and long-term performance tests of membranes at industrially relevant solute concentrations in OSN studies. Here, we report on a survey of the OSN research community and on a critical assessment of 177 journal papers published from 2015 to 2019 to determine how the scientific value and industrial impact of OSN studies can be improved. Based on the results of our survey and literature analysis, we crafted a series of best-practice recommendations for researchers reporting data on membrane fabrication, membrane materials characterization and filtration performance, process integration and fundamental studies.
1. Organic solvent nanofiltration: quo vadis?
Organic solvents play a vital role as auxiliaries to chemical processes in the pharmaceutical, paint, oil, agricultural and food industries.1 Most industrial-scale chemical syntheses occur in organic solvents, and even solvent-free reactions using ball mills or microwave-assisted reactors require organic solvents for purification. The separation of these organic solvents is therefore inevitable during chemical processes that utilize conventional methods, such as distillation, evaporation, crystallization and liquid–liquid extraction. Most of these methods require elevated temperatures and are therefore energy intensive. In fact, separation processes account for 10 to 15% of the world's energy consumption; of this energy used in separation processes, 80% is associated with distillation and evaporation methods.2 The development of energy-efficient separation techniques is therefore essential for achieving the goal of environmentally sustainable manufacturing.One such technique is organic solvent nanofiltration (OSN), which separates solutes in the range of 100 to 1000 g mol−1 at a molecular level in organic media. OSN is a pressure-driven, non-thermal method in which separation is achieved mainly by size exclusion. As such, OSN can be considered an energy-efficient alternative to conventional separation methods, such as distillation and evaporation, with the potential to decrease energy consumption by 90% compared with these traditional methods.3 OSN, therefore, has the potential to improve the environmental sustainability in many industrial sectors. Applications of OSN include solvent recovery, dewaxing processes, catalyst recovery, biomass fractionations and biorefineries.
In 2014, a critical sustainability assessment led by Livingston suggested that OSN could become one of the best separation methods for processing organic solvents.4 The group identified advantages of OSN, including its low energy requirements, low solid waste generation, low labor intensity, simple scale-up through modularity, tolerance to harsh chemical environments, low operating temperature, milder operating pressure compared with reverse osmosis, straightforward solvent exchange from high- to low-boiling point solvents and simultaneous removal of solutes from various chemical classes. They also identified some hurdles that could hinder the development of OSN (Table 1).
| Hurdles | Solution | Status | |
|---|---|---|---|
| Membrane fabrication | Large amounts of wastewater containing toxic polar aprotic solvents | Trap toxic solvents with resin or adsorbents and reuse treated wastewater | ✓ |
| Toxic solvents and chemicals | Substitute toxic solvents with greener solvents | ✓ | |
| Avoid or minimize the amount of toxic solvents | ✓ | ||
| Petroleum-based polymers | Select renewable, biodegradable materials over petroleum-based polymers for membrane fabrication | ✓ | |
| Chemical waste from crosslinking procedures | Minimize the volume of toxic crosslinking agents | ✓ | |
| Minimize chemical waste by crosslinking the membrane during the membrane formation process (i.e., in a coagulation bath) | ✓ | ||
| Perform dry crosslinking | ✓ | ||
| Select chemically stable materials that do not require crosslinking | ✓ | ||
| Process development | Time-consuming screening | Implement quality by design or design of experiments | ✓ |
| Low rejection and yield | Develop tighter membranes | ✗ | |
| Focus on improved selectivity rather than permeance | ✗ | ||
| Implement membrane cascades | ✓ | ||
| Small molecular weight difference between solutes | Enlarge solutes | ✓ | |
| Excessive solvent consumption | Implement solvent recovery | ✓ | |
| Insufficient purity | Integrate processes: couple OSN with existing technologies (i.e., adsorption) | ✓ | |
| Scale-up | Mass transfer and pressure drop | Dedicate more research efforts to understanding the fundamentals (i.e., transport mechanisms) of OSN | ✗ |
| Dedicate more research efforts to understanding the process aspects of OSN | ✗ | ||
| Limited number of commercial membranes | Compile available membrane data | ✗ | |
| Expensive modules | Expect replacement with greater demand | ✗ | |
| Limited information in the literature | Design application-based studies on the commercial scale | ✗ | |
| Data reporting | Inability to compare existing membrane data | Adopt a standardized testing system and protocol | ✓ |
| Insufficient experimental reporting to reproduce membrane fabrication and performance characterization measurements | Create a minimum standard for data reporting in publications | ✗ |
As indicated by more than 400 papers published about OSN since 2015 (Fig. S1†), interest in this separation method has been steadily growing among academic and industrial researchers. Many of these publications describe how new materials or processes resolve some of the hurdles identified by Livingston et al., but these studies’ lack of clear experimental designs, explicit experimental protocols, comparable performance data and long-term performance tests of membranes at industrially relevant solute concentrations hinders the pace of development of this sustainable separation method that could ultimately provide greener manufacturing strategies.
Researchers who work on OSN concede that the basis on which to compare the results of their studies has not yet been established. Here, we seek to understand the reliability and comparability of published results on OSN by mining recent studies of OSN and by conducting a survey of OSN researchers (the OSN community). The anonymous survey (ESI†) was distributed to participants of the OSN2019 conference in Enschede, the Netherlands, in October 2019 and to other academic and industrial researchers whose research focuses on OSN in November and December 2019. We received 70 survey responses from undergraduate, postgraduate, faculty and industrial researchers.
Our analysis of the results of this community survey identified three major problems in the literature (Fig. S2†). First, around two-thirds of those surveyed stated that published data are difficult to compare due to various test systems in use. Second, three-fifths of survey respondents noted the paucity of information on the long-term stability of membranes. Third, more than half of the respondents observed no or unclear evidence of reproducibility in datasets.
Additionally, more than one third of the survey participants acknowledged that experimental descriptions reported in the OSN literature are incomplete and that filtration and characterization tests performed on membranes are insufficient. Also, one-fifth of respondents reported that there is a lack of information in these publications on how datasets are obtained. The survey participants also provided other observations in a free-text box on the survey. In this box, respondents mentioned that researchers have limited experience in studying OSN; they cherry-pick the data; they provide limited or no information on filtration under industrially relevant conditions; they do not benchmark against commercially produced reference membranes; and they do not provide information on outliers in their datasets. Fewer than 10% of the participants reported that they have no concerns about the OSN literature. Taken together, these survey results suggest that researchers have considerable doubts about reliability and comparability in the OSN literature, a cause for great concern among researchers in the OSN community.
We rigorously mined the recent OSN literature to determine if and to what extent the problems identified by our survey respondents exist given these concerning results. Until now, no such data mining of the OSN literature has been conducted. Our results then allowed us to shape guidelines for conducting research on OSN to ensure that published reports are scientifically sound, reproducible and useful. With guidelines in hand, we aim to alleviate the slow progress of the field, and eventually help the chemical industries to get closer applying this sustainable technology.
2. Benchmarking to allow cross-comparison
The ability of OSN membranes to separate solutes in organic solvents depends on many inter-related factors, including the difference in molecular weight between the solutes (the “size gap”), the hydrodynamics of the solution, the equipment design, the process configuration, the operating parameters, the physicochemical properties of the solutes and solvents, and the interactions between the solutes, solvents and membrane.5,6 Likely because of this multitude of convoluted factors, no standardized protocols for OSN measurements and benchmarking have yet been established. With the exception of case studies, parameters are likely selected mainly based on the researcher's personal preference or the cost of the experiments. As a consequence, a substantial part of the OSN literature reports isolated data that cannot be directly compared with other datasets, which hinders the development of research in this field.2.1. Experimental configuration
Our literature survey revealed that two filtration configurations are used in the majority of OSN separation studies: dead-end (57%), in which the feed is applied perpendicularly to the membrane's surface, and cross-flow (40%), in which the flow of the feed is tangential to the membrane's surface (Fig. S7†). Vacuum filtration, solar-assisted evaporation and liquid–liquid phase separation are seldom used to determine the performance of OSN membranes because they have less relevance to industrial processes.The benefits of dead-end configuration include low capital investment, simple operation and a small equipment footprint. However, the dead-end configuration is frequently operated with a gradually decreasing retentate volume, which results in an increasing solute concentration over time. The dead-end configuration can be used to test the feasibility of potential membrane materials and to compare the relative performance of membranes – what we classified as “materials-focused” research in our literature analysis. On the other hand, we defined “process-focused” research as publications that assess the applicability of OSN in case studies. The literature analysis is consistent, 68% of materials-focused OSN publications used a dead-end configuration whereas only 23% of process-focused OSN publications used this configuration.
Separation data from dead-end filtration should be considered only as a rough performance estimate. The small volume of the dead-end configuration hinders continuous long-term performance tests of membranes and intrinsically transient concentrations mean that obtaining steady-state data from these experiments is challenging. Consequently, translating membrane performance data obtained through dead-end filtration to industrial process implementations becomes difficult.
In contrast, membrane screening with a cross-flow configuration allows the permeate to be recycled to the feed tank, resulting in a constant feed concentration and constant volume. Long-term filtration performance of membranes can therefore be assessed under steady-state conditions. In addition, the tangential flow geometry applies the shear force along the membrane, thus reducing the accumulation of the solute on the membrane's surface and polarization of the concentration.7 The conditions in a cross-flow configuration compared with those in a dead-end configuration are also more similar to the conditions of spiral-wound membrane module (SWMM) setups,8 which makes cross-flow performance data more reliable than dead-end performance data when process calculations are scaled up. On the other hand, cross-flow configurations take more space and cost 10 to 20 times more than dead-end filtration units.
2.2. Solutes
Many solutes are currently used by OSN researchers to characterize the separation performance of membranes and to determine their molecular weight cut-off (MWCO) (Fig. 1). The fraction of solute that remains in the retentate increases with molecular weight, and MWCO is defined as the molecular weight of the solute at which 90% is rejected by the membrane. Among various solutes, dyes are most frequently selected, accounting for almost half of all solutes used in publications (Fig. 1). Dyes are popular because they are available commercially with molecular weights covering most of the nanofiltration range. They also have distinct absorption spectra, making them easily analyzed by simple UV-vis spectrophotometry. However, dyes absorb over a wide range of wavelengths, which typically limits the number of dyes that can be applied to a single MWCO measurement, unless chromatographic analysis is employed.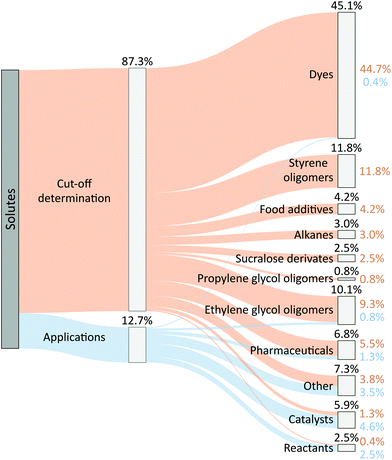 | ||
| Fig. 1 Sankey diagram of solutes used in published OSN research. Both percentages were calculated based on the total number of solutes used in the literature (N = 237). The ESI† presents additional information. | ||
Other solutes with disparate structures and chemical functionalities used for cut-off curve determination are pharmaceuticals (6.8%) and food additives such as lecithin (4.2%). Given the differences in structure and function, the strength of the solutes’ interactions with the solvent and membrane material can vary significantly. Also, these solutes often only cover a few rejection-molecular weight points, which results in less precise cut-off curve determination.
Around one in ten papers avoided difficulties with determining the cut-off curve by measuring rejection curves using homologous styrene oligomers (11.8%) or oligo(ethylene glycols) (OEGs, 10.1%). The identical chemical frameworks of the homologues (i.e., similar shape, structure, and functional groups) allow for more uniform membrane–solute, solute–solute and solvent–solute interactions than do mixtures of dissimilar solutes. Consequently, the use of homologues is recommended to minimize chemical variance and secondary interactions in the system.9
Each common homologue series has its own advantages and disadvantages. Alkanes, used as molecular-weight standards in 3.0% of the papers surveyed, have low solubility in polar solvents and few commercial suppliers, particularly for markers above ca. 400 g mol−1 (ca. C30). Styrene oligomers, on the other hand, are commercially available in a wide molecular weight range (up to 1000 g mol−1), but pure styrene oligomers with low molecular weights and low polydispersity are expensive. OEGs are a greener and more economical alternative to styrene oligomers because they are readily biodegradable, can be produced from sustainable sources such as sugars and lignocellulosic biomass,10 and they are less expensive and widely commercially available. However, OEGs are insoluble in most non-polar solvents and, due to the flexible linear chains, OEGs provide lower rejections for a given molecular weight compared with styrene oligomers. Branched OEGs provide higher rejections due to their bulky nature,11 but their solubility is still limited to polar solvents. The use of homologues of propylene glycols (PGs) was proposed to address the limitations of OEGs, including poor rejection and low solubility in non-polar solvents.9 Like OEGs, PGs are also inexpensive and are commercially available, but they have been used in fewer than 1% of the papers surveyed.
The MWCO of the same membrane can vary greatly with solvent.12 Depending on the solvent, the tortuosity of the membranes and the form of oligomer solutes could change. In good solvents, the chains are more likely to be extended while in poor solvents, the oligomers will be shaped to minimize the solvent–polymer contact area. The solvation of membranes and solutes also influences the effective pore and solute size, thereby altering the solute's rejection.13 In addition, the relative affinity between solute–solvent and solute–membrane has been shown to influence the rejection.14 Therefore, MWCO must not be used for comparisons of performance using data obtained from a different solute–solvent–membrane system.
2.3. Solvents
In addition to solute rejection, solvents also influence the permeability of membranes through viscosity effects and through solvent–membrane affinity.15 In the papers surveyed, membrane performance data (Fig. 2) was collected with only a single solvent nearly two-fifths of the time (39%). Filtration tests were most frequently run in alcohols (39.0%) and polar aprotic solvents (24.1%). Almost all alcohols and esters are categorized as green solvents according to the expanded GlaxoSmithKline (GSK) solvent selection guide,16 but most polar aprotic solvents fall into the red category with the exception of DMSO (7.2%) and acetonitrile (17.6%), which fall into the amber category (Fig. S10†). Moreover, only 5.6% of tested polar aprotic solvents were greener solvents, including 2-methyltetrahydrofuran, propylene carbonate, and dimethyl carbonate. With the increasing focus on green chemistry and the increasing number of reactions that use green solvents over conventional toxic solvents, there is a need to test membranes in green polar aprotic solvents as well.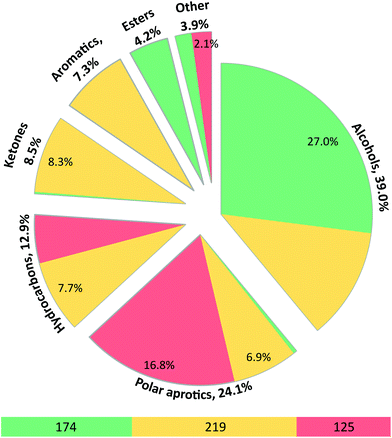 | ||
| Fig. 2 Types of organic solvents commonly used in published OSN studies. Green, yellow and red indicate the solvent types from GlaxoSmithKline's solvent selection guide in the same category. Percentages are based on the number of solvents used (N = 518) in all publications surveyed. The ESI† presents the values for the number of solvents used categorized by type. | ||
2.4. Recommendation for a standardized protocol
Many experimental data sets from published OSN studies remain isolated because there is currently no consensus on a standardized protocol to allow cross-comparisons of membranes. The wide variety of solvent–solute systems selected by the OSN community means that finding a suitable membrane for a selected application requires rescreening all prospective membranes, which is tedious and requires time, energy and materials.Recently, a selectivity figure of merit (SFM) was defined to allow compilation and comparison of membrane performance data in a single plot.17 Although a great initiative, this SFM is limited to operating conditions in which concentration polarization is absent (i.e., diluted solutions) and to membrane materials that swell. A standardized protocol remains necessary for effective comparisons of all membrane data.
The membrane research community has accepted a standardized protocol for reverse osmosis (RO) systems18 that fixes the concentration of sodium chloride in water and all operating parameters. Although the solute–solvent system in OSN is more complex than in RO, defining model systems and providing performance data at fixed operating conditions are essential to allow the cross-comparison of membranes.
There have been initiatives to develop a standardized membrane testing procedure, mainly focusing on the solute–solvent system. Livingston et al. proposed that membranes be tested in four different solvents, including non-polar, polar protic, mild polar aprotic and strong polar aprotic solvents using a series of oligomers. Testing membranes using three similarly sized solutes with different physicochemical characteristics was also advised. The recommendation, however, included general considerations and did not specify exact solutes, solvents or fixed operating conditions.
Wessling et al.,12 on the other hand, proposed a well-defined test system: six solvents, both protic and aprotic, with a range of polarity and falling outside the red solvent category (Table S1†) plus two series of oligomers and a compound that is relevant to the petrochemical, food and polymer industry. They reported that comparable performance results were obtained in a round-robin test between five independent laboratories, even though these labs used systems with different geometries and different analytical methods. Their proposed test system is a viable first step towards a standardized procedure.
Neither of the proposed protocols standardized the operating conditions, which is equally important as selection of the solute–solvent system. For example, most polymeric membranes compact under pressure and lower permeance values are obtained at higher operating pressures. Performance parameters are also affected by the viscosity of solutions and hence the operating temperature and solute concentration. Therefore, fixing the operating conditions is also essential to a standardized protocol. In response, Table 2 presents the minimum and ideal process conditions for membrane testing. The most frequently employed parameters are selected as base conditions. Table 2 was prepared for papers that develop new membranes and is less relevant to “process-focused” papers that perform filtration for a case study. For “process-focused” publications we have prepared a checklist (ESI†).
| Minimum conditions | Ideal additional conditions | |
|---|---|---|
| Membrane stability (Dissolution test) | Qualitative (visual) in the following solvents: | Quantitative with weight loss measurement in solvents listed under minimum conditions. |
| Alcohols: methanol, ethanol | Any additional solvents, such as p-cymene, p-xylene | |
| Ketones: acetone | ||
| Esters: ethyl acetate | ||
| Hydrocarbons: n-heptane | ||
| Dipolar aprotics: DMF, DMSO, acetonitrile, 2-methyltetrahydrofuran | ||
| Aromatics: toluene, p-cymene, p-xylene | ||
| Pressure (bar) | 10 | 20, 30, 40 |
| Temperature (°C) | 20 | 40, 60, 80 |
| Agitation | 100 L h−1 (CF), 500 rpm (DE) | — |
| Solvents | Alcohols: ethanol | Alcohols: methanol, isopropanol |
| Ketones: acetone | Ketones: methyl ethyl ketone | |
| Esters: ethyl acetate | Dipolar aprotics: DMF, DMSO, acetonitrile, 2-methyltetrahydrofuran | |
| Hydrocarbons: n-heptane | Aromatics: toluene, p-cymene, p-xylene | |
| Solutes | Styrene oligomer | Lecithin, catalyst, pharmaceutical |
| Concentration | 1 g L−1 | Up to 100 g L−1 depending on solute solubility |
| Number of replicates | 3 | More than 3 |
3. The pillars of reliable OSN reports
Sufficiently detailed reporting of conditions for membrane fabrication, operating parameters used for performance characterization and data presentation is crucial for reliability, reproducibility and comparability of published OSN results.3.1. Reporting reliable protocols for membrane fabrication
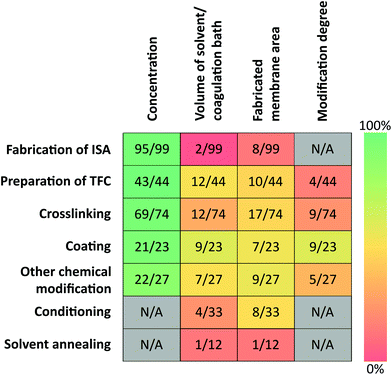
The fabrication of the desired membrane can involve several steps. These include the cast of integrally skinned asymmetric membranes (ISAs) via phase inversion, the formation of thin active layer through interfacial polymerization and other post-treatments such as crosslinking, coating, surface-grafting or solvent annealing. The polymer concentration, the degree of chemical modification, the amount of crosslinker, monomers, grafting agents, other reagents and solvent needed to produce a membrane of certain size is indispensable for the reproducibility and comparability of fabricated membranes. For example, a membrane with the same crosslinking degree cannot be fabricated unless the amount of crosslinker required per membrane area or mass is specified. A thin film composite (TFC) membrane with the same thickness cannot be reproduced unless the amount of monomer solution required per membrane area is provided. In addition, these information allows calculating green metrics, such as E-factor (eqn (S5)†), process mass intensity (eqn (S6)†) and atom economy (eqn (S7)†) which allows the comparison of membrane fabrication processes in terms of sustainability.Currently a meaningful green comparison of the fabrication processes is not possible due to the limited presented information found in the literature. Concentration of dope solutions, monomers for TFCs, cross-linkers, grafting agents and reagents were reported in more than 90% of the cases. However, the fabricated membrane area and the amount of solvent used for the fabrication process were less reported. Fewer than one tenth of publications employing ISA provided information on the area of fabricated membrane and only 2% reported the amount of coagulation bath needed for the fabrication (Fig. 3).
Although qualitative information on chemical modification of membranes were frequently provided, quantitative data on the efficiency of crosslinking and polyamide formation were limited. Only 12% of papers that employ crosslinking report the efficiency of the reaction while less than tenth of TFC papers provided quantitative information on the polyamide formation.
Detailed information on conditions, on the material resources and on the chemical modification efficiency is inevitable for (i) producing membranes with same physicochemical characteristics and for (ii) calculating green metrics accurately thereby allowing the comparison of fabrication processes. Therefore, we have prepared a checklist for essential information on the membrane fabrication protocol (ESI†).
3.2. Reliable reports on the characterization of membranes
We have identified 13 process parameters that affect the membrane performance (Table 3). The results of membrane separation experiments can be interpreted correctly and independently reproduced only if all the applicable parameters are reported. Reporting these parameters also allows the work to be further exploited for transport modelling, machine learning or process design and scale-up.| Information | MD | P | F |
|---|---|---|---|
| Filtration conditions affecting membrane performance: | |||
| Membrane–solvent–solute system | ++ | ++ | ++ |
| Pressure | ++ | ++ | ++ |
| Temperature | ++ | ++ | ++ |
| Solute concentration | ++ | ++ | ++ |
| Membrane area | ++ | ++ | ++ |
| Filtration time | ++ | ++ | ++ |
| Configuration type | ++ | ++ | ++ |
| Agitation: cross-flow velocity/stirring speed | ++ | ++ | ++ |
| Feed flow rate | + | + | + |
| System volume | + | + | + |
| Operating mode (continuous/batch) | ++ | ++ | — |
| Conditioning time required to achieve steady-state | ++ | ++ | ++ |
| Any interruption of filtration | ++ | ++ | — |
| Flux decline during the pre-compaction or conditioning period | ++ | o | o |
| Performance data obtained at stable conditions (if a steady-state cannot be reached justification must be made) | ++ | ++ | ++ |
| Standard deviation of membrane performance (obtained at least from three membrane coupons, source must be provided in all cases): | |||
| Originated from different membrane coupons (either from different dope or from the same membrane sheet). Source must be defined. | ++ | ++ | ++ |
| Originated from deviation in performance | o | o | o |
| Performance parameters (all values should be summarized in a table at least in the supporting information): | |||
| Flux/permeance and rejection of a single solute-solvent system | ++ | ++ | ++ |
| Flux/permeance and rejection of multiple solute-solvent systems | + | + | + |
| Pure solvent flux/permeance | + | o | o |
| MWCO | + | o | o |
| Materials characterization (Table S2†) | ++ | — | o |
| Membrane screening based on a standardized method (Table 2) | + | o | o |
| Membrane screening for application (i.e., case study) | + | ++ | + |
| Filtration at industrial concentration | + | ++ | o |
| Green considerations (greener solvent, chemical, reduced steps, etc.) | + | + | + |
| Green metric analysis if green-focused (E-factor, atom efficiency, energy calculations) | + | + | — |
Fig. 4 compares the parameters that the OSN community reported as essential with the actual parameters that appeared in the literature that was surveyed. Configuration, operating pressure and solute concentration were reported in more than 90% of cases. Around 4 in 5 papers reported the membrane area which is in alignment with the responses from our survey. Some researchers may have found the report of area unnecessary, probably because the flux or permeance calculations include the effective membrane area. However, due to possible defects or inhomogeneity of membrane materials, the upscaling of membranes could lead to the deviation of performance as high as 23% when compared to lab-scale area.19 Therefore, membrane area is also essential part of a reliable OSN report.
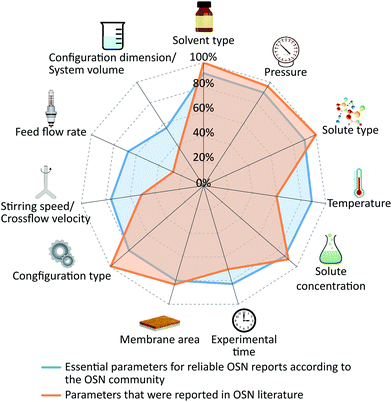 | ||
| Fig. 4 Essential parameters for reproducible OSN reports based on responses from survey participants (blue) and percentages of parameters that were reported in the OSN literature (orange). The ESI† presents the percentages of responses from survey participants and reported parameters in the OSN literature. | ||
Only 57% of published papers reported the operating temperature even though 9 in 10 survey participants listed operating temperature as one of the most important parameters to report. More than half of the respondents reported that parameters that provide information on hydrodynamics should be provided, whereas cross-flow velocity and stirring speed remained underreported in the surveyed literature with only 51% of the papers listing those parameters and the flow rate of the feed was provided in less than 30% of publications. More than 60% of the papers did not report the system volume.
Reliable OSN reports provide performance data that are obtained under steady-state conditions. Conditioning membranes under experimental conditions prior to measuring performance data is crucial because polymeric membranes suffer from compaction under pressure, resulting in a flux decline of around 40%.20,21
In addition, some solutes adsorb on the membrane's surface, which may result in artificial rejection of the solute if the system is not stabilized.22 Although adsorption of solutes is frequently associated with dyes as indicated by the appearance of the membrane after filtration, other solutes could also adsorb on the membrane's surface.23 Therefore, irrespective of the solutes used, measurements should be taken under steady-state conditions to avoid reporting of misleading membrane performance data. In some instances, achieving the steady state is not possible without interruption of the filtration process (e.g., opening and refilling the dead-end cell). Nevertheless, justifications should be presented when equilibrium cannot be achieved and, in these cases, the experimental conditions, such as compaction time, pressure and temperature, are indispensable to the reader. Interruptions of the filtration process must be also reported and the flux decline should be described to provide an estimate of membrane performance for a longer filtration time. Among the 70% of surveyed papers that report membrane conditions, fewer than half reported the compaction conditions (46%) and only around a quarter (28%) presented the flux decline.
Information on the reproducibility and uncertainty of results is also important. The sample standard deviation for membrane screening data should be provided and it should be based on at least three independent replicates.24 Replicates for long processes, in particular continuous operation, are not necessary given that the membrane screening proved the reliability of the membrane performance. New/prospective membrane materials cannot be used in an application if a similar performance to that reported in a previous paper cannot be obtained. Scaling-up processes are not feasible if membranes cannot be reproduced. Multiple measurements with different membrane coupons are indispensable because the variation in the flux of the same membrane material could be as high as 33% even under the same experimental conditions due to possible leakages and defects in the membrane material.24
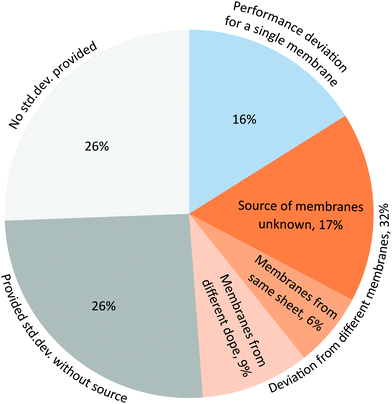
Despite the need for reliable estimates of uncertainty, around a quarter of the OSN papers surveyed provided performance data as individual data points (Fig. 5). Less than half of the papers (46%) provided partial information on the standard deviation of membrane performance and less than one-third (28%) provided standard deviations for all membrane performance data (Fig. S12†). Around 1 in 4 papers provided standard deviations on graphs and figures but did not report whether the error was obtained from replicates or from repeat experiments (Fig. 5). One-third of reported standard deviations (33%) originated from the deviation in the performance of a single membrane calculated from several flux and rejection measurements of the membrane at different time intervals, rather than from repeats across several runs or with different membranes. Information on performance deviation can provide some information on the uncertainty of performance measurements, but it can provide no insight into reproducibility and reliability.
In the surveyed literature, about two-thirds of the reported standard deviations (67%) were calculated from two or three different membrane coupons, of which about one fifth (19%) originated from the same membrane sheet and about one third (28%) came from separate dope solutions. The type of replicate was not specified in the remaining papers. Standard deviations that originated from different membrane coupons were mostly obtained from membrane samples that were connected in parallel (39%) rather than from independent measurements (20%). Connecting membrane coupons in parallel provides a quicker experiment and allows obtaining performance data under the same experimental conditions. Independent measurements on the other hand also provide information on the reliability of experimental set-up.
There is generally a lack of agreement on the number of solutes and solvents in which a membrane should be tested (Fig. S6†). More than half of the survey respondents stated that rejection data for a single solute and the permeance value for a single solvent and for a single solution are sufficient to characterize membrane performance. However, around 2 in 5 participants reported that providing rejection data for multiple solutes and permeance values for multiple solvents is necessary. There is also no clear agreement on the MWCO of membranes and proof of flux decline either, with only 49% and 40% of the survey participants identifying those parameters as essential to report, respectively.
One of the main reasons for the lack of agreement is that the minimum performance data that needs to be reported also depends on the purpose of the research. The focal point of the published papers on OSN can be divided into three main topics: (i) membrane development reports that involve developing new materials, optimizing fabrication processes and screening new membranes for application-based solutes; (ii) process reports that include case studies, applications and descriptions that aid process design; and (iii) fundamental studies that aim to describe separation and transport mechanisms through application of various models.
Papers that describe the use of commercial membranes for a specific separation may not need to report chemical stability tests or a thorough MWCO characterization. A process development study may focus on a specific industrial case study, and therefore varying some of the operating parameters and conditions may be unnecessary. Therefore, creating a universal protocol that requires reporting of minimum performance data for all OSN research is challenging; judging which performance data are necessary to report is the joint responsibility of authors and reviewers. However, rejection and permeance data are the minimum result requirements that must be included in any OSN publication.
Although the minimum essential parameters for OSN reports are publication dependent, 96% of the survey participants agreed that a guideline on the report of experimental data and results would be beneficial for the community. Therefore, we provided a table of guidelines (Table 3) and crafted checklists for the minimum essential performance characterization parameters classified by the focal point of papers (ESI†).
There is also a lack of consensus on the minimum materials characterization results that are necessary in OSN publications. Only half of the survey respondents stated that materials characterization is necessary to report to ensure reproducibility and comparability of membrane performance. Around one-quarter of the survey participants used the free text box to state that minimum materials characterization results strongly depend on the focus of the work and on the type of membrane.
Characterization of new or modified membrane materials is essential to allow synthesis, structure and performance to be inter-related; to assess membranes’ suitability for a particular operating environment; and to ensure that the materials’ characteristics are uniform. OSN membranes are used in solvents and under pressure, but almost all materials testing is done away from the solvent and under atmospheric pressure or vacuum. Nonetheless, certain techniques are essential when reporting research on new membrane (Table S2†).
4. The gap between academic and industrial significance
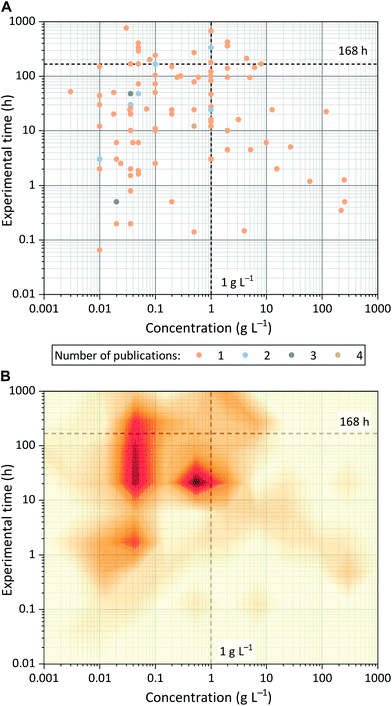
Most OSN publications do not provide information on the scalability of new membrane materials or on their performance using industrially relevant solutions and conditions. As a result, there is a gap between academic results and industrial requirements that needs to be bridged.This gap is the result of the limited use of cross-flow configurations (40% of the literature analyzed), the lack of filtration studies on SWMMs (2%), and the lack of industrially relevant concentrations. Most OSN papers (80%) report filtrations at concentrations equal to or below 1 g L−1 (Fig. 6) despite the fact that industrially relevant concentrations are usually one- to two-fold higher.25 Concentration polarization, fouling, self-assembly and increases in viscosity, which can result in changes in membrane separation performance, or in severe cases the failure of the filtration, cannot be neglected. Promising new membrane materials may fail when it comes to real, industrial conditions.
The lack of information on long-term filtration performance widens the gap between academic results and industrial requirements. In practice, membranes must maintain their performance for as long as possible to reduce the need for replacement and therefore to decrease the capital cost and the environmental burden. Yet, around one-third of the surveyed OSN papers did not specify the filtration time, and around half of these papers reported tests that lasted less than 24 h. Among the papers that reported the experimental time, 42% provided data on filtration tests that lasted more than one day and only 8% provided data on filtration tests lasted more than one week (Fig. S14†). Even if extended tests are impossible or impractical, rejection and permeance data should be collected over a period of several days to ensure constant performance and steady-state operations for at least one membrane. Some papers (3%) reported extremely high permeance up to 236 L m−2 h−1 bar−1 but the back-calculation from the permeate volume reveals that the duration of the experiment was less than 15 minutes. These data can be misleading because some membranes go through a significant compaction at the start of their use.
More research should focus on the up-scaling of membrane materials. Industries frequently must process large amounts of solvents annually. On average, pharmaceutical industries process 22 kg of solvents to produce 1 kg of active pharmaceutical ingredients.26 ExxonMobil handles up to 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 m3 lubrication oil daily for dewaxing processes alone.2 In industrial processes, SWMMs are the most frequently used OSN. SWMMs are complex products consisting of several membrane leaves (flat sheet membranes) that are separated by spacers and wound around a central collection pipe. While the hydrodynamics of flat-sheet membranes depends on the operating conditions and the configuration type only, the hydrodynamics of SWMMs also depends on several other factors, including the number and geometry of membrane leaves, the spacer type and the spacer geometry. The performance of SWMMs will therefore inherently differ from the corresponding flat sheet membrane. The number of new, high-performance membrane materials, prepared as flat sheets, is rapidly increasing, but their technology transfer into SWMMs has not kept pace over the years. The literature on both SWMM fabrication and application is scarce. Consequently, there is a need for more research on turning flat sheet membranes into SWMMs as well as on process development using SWMMs. Only 2% of the OSN literature reported on SWMMs, which indicates that not many membrane materials are actually tested beyond the laboratory scale.
500 m3 lubrication oil daily for dewaxing processes alone.2 In industrial processes, SWMMs are the most frequently used OSN. SWMMs are complex products consisting of several membrane leaves (flat sheet membranes) that are separated by spacers and wound around a central collection pipe. While the hydrodynamics of flat-sheet membranes depends on the operating conditions and the configuration type only, the hydrodynamics of SWMMs also depends on several other factors, including the number and geometry of membrane leaves, the spacer type and the spacer geometry. The performance of SWMMs will therefore inherently differ from the corresponding flat sheet membrane. The number of new, high-performance membrane materials, prepared as flat sheets, is rapidly increasing, but their technology transfer into SWMMs has not kept pace over the years. The literature on both SWMM fabrication and application is scarce. Consequently, there is a need for more research on turning flat sheet membranes into SWMMs as well as on process development using SWMMs. Only 2% of the OSN literature reported on SWMMs, which indicates that not many membrane materials are actually tested beyond the laboratory scale.
A more balanced growth of studies on membrane development, process development and fundamental properties is essential to bridge the gap between laboratories and industries. For example, novel membrane materials cannot contribute to the advancement of use of OSN in industries unless they are tested for potential applications. Prediction of performance parameters and the development of process designs will be difficult unless the transport mechanism of OSN is fully understood.
Nearly three-quarters of publications focus on the membrane development (71.2%, Fig. 7). Among these papers, the majority report on the fabrication of new materials that provide improved separation performance in a chemically challenging environment. However, these publications rarely provide information on the membrane's potential applicability in processes, with only 6.3% of paper on membrane development reporting on screenings for applications. Among the process-focused papers, 40% reported on in-house-fabricated membranes that were used for processes, such as solvent recovery,27 crystallization,28 or a particular reaction;27 though these papers were from just five research groups and reported on only a few materials. In addition, many papers on membrane development do not provide sufficient information on membrane characteristics that would assist in understanding the separation mechanisms in new membranes. Despite that there are an excessive number of new materials most potential membranes may not be tested beyond laboratory experiments.
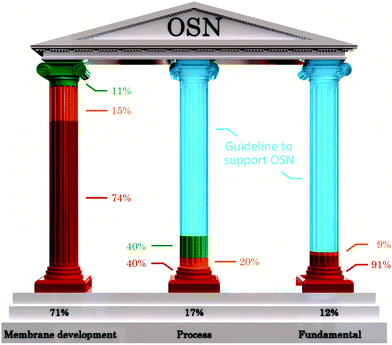 | ||
| Fig. 7 The development of OSN is supported by three main pillars: (i) membrane development, including development of new materials, optimizing fabrication processes and screening new membranes; (ii) process development, including case studies and applications; and (iii) fundamental studies, including understanding the separation and transport mechanisms. The relative height of each pillar the number of studies in the OSN literature. The development of the three main pillars is currently disproportionate. With guidelines (Table 3) and checklists provided in the ESI† there would be a more balanced growth. The number of papers analyzed was 177. Green: sustainability focused literature, orange: sustainability was not the focus but some green aspects were improved, and red: no sustainability considerations. | ||
The potential of OSN has been demonstrated in several processes, either as a stand-alone process or in combination with conventional techniques. Examples of such processes include catalyst recycling, purification of pharmaceuticals and nucleotides, and isolation of natural products. However, the papers in the OSN literature that focus on process development account for only 17.4% of the publications surveyed.
One of the greatest hurdles to advancing the use of OSN is the lack of knowledge about the separation mechanism due to the complex interactions between solutes, solvents and the membrane. Fundamental understanding of the transport phenomena and the separation mechanism in OSN systems would allow the rational design of new materials and the optimization of operating conditions to achieve maximum productivity and selectivity in existing membranes. Despite its importance, only 11.8% of analyzed papers in the OSN literature focus on the fundamental studies.
In addition to a more balanced growth of studies on membrane development, process development and fundamental properties, close collaboration between academics and industrialists would also help to bridge the existing gap. Input on the requirements of industries would allow novel membrane materials and membrane processes to be designed for specific demands.29
5. Towards green membrane technologies
There is room for improvement of the sustainability of membranes. Since the 2014 sustainability assessment of OSN,4 around 70 papers have been published to address the environmental effects of membrane fabrication and OSN processes.Among the membrane development papers, 15% proposed a greener fabrication process although the focal point of these studies was not on improvement of the sustainability of membranes. Around one-tenth of the membrane development publications (11%) focused on greening the fabrication process. Among these publications more than half replaced toxic solvents with greener alternatives (54%), such as PolarClean,30 Cyrene,31 ionic liquids,32 γ-valerolactone,33 methyl lactate,34 or water35 in the preparation of membranes. Around one third of the green-focused membrane development papers (35%) minimized solvent waste. Wastewater from the phase inversion process was treated and re-used for further membrane fabrication in these surveyed studies.21
Solvent waste was also minimized by reducing the number of fabrication steps.36,37 More than 20% of green-focused membrane development papers (23%) reported fabrication of membranes from renewable and biodegradable materials despite the great challenge of designing biodegradable but robust membranes. These examples include the fabrication of OSN membranes from cellulose and from sodium alginate.38,39 Although these potentially biodegradable, solvent-resistant membranes were fabricated from renewable materials, the degradability of these materials has yet to be demonstrated. In addition, around one-fifth of the OSN publications employed greener chemicals for the chemical modification of membranes.40
Progress towards sustainable membranes is undeniable. Yet, only a fraction of OSN papers were aimed at reporting green membranes whereas the majority (74%) of membrane development publications did not assess the sustainability of the fabrication process. Most reported fabrication methods (71%) still require pre- or post-treatment to improve the solvent stability with a median of two steps to fabricate the desired membranes on top of a non-woven support or glass plate. Among the papers that report on the fabrication of crosslinked membranes, around 90% employed wet crosslinking, thereby generating more solvent waste.
With more stringent environmental regulations expected in the future, greener membrane fabrication processes are not only needed, but the whole life cycle of membrane fabrication, use and replacement should be considered to limit their environmental impact. More sustainability considerations will be required in membrane fabrication processes in the future, such as selecting solvent-resistant membrane materials that do not require crosslinking,38 choosing dry crosslinking over wet crosslinking, and adopting greener solvents and chemicals40 for the membrane fabrication and chemical modification processes.
Less than half (40%) of OSN process development papers focus on mitigating the environmental issues of a separation technique. One-fifth of the process publications reported on improved sustainability of a procedure by exploiting the potential of OSN as a green technology. More than 80% of these green-focused process publications employed OSN to minimize chemical waste, of which 51% used membranes for catalyst recycling, 44% incorporated OSN as a solvent recovery process, and 5% employed OSN for the recycling of reagents. In addition, more than 20% of these green-focused process papers employed OSN for the valorization of renewable materials, including biomass, lignocellulose, and agricultural and fish waste. However, only around one-tenth (12%) of the sustainability-focused process papers reported green metrics and/or techno-economic analysis to demonstrate the improved energy efficiency, and reduced carbon footprint of the process as a result of OSN implementation. It is crucial to provide some green metrics analysis for the comparison of the processes with and without OSN.
Fundamental studies focus on understanding the separation and transport mechanisms in conventional solvent–solute systems that are commonly used by OSN researchers. There is still limited fundamental information on conventional OSN systems and even less on green solvent–solute systems, accounting for one-tenth of the papers analyzed in the fundamental studies group. Nevertheless, with an increasing interest in green membranes and sustainable processes, we expect a shift towards understanding the transport mechanisms of green solvents and solutes as they pass through the membrane.
6. Conclusions and the way forward
The interest in OSN is increasing steadily, yet a recent survey distributed among the OSN community has shown that there are some general issues within the OSN literature that need to be addressed to improve the scientific value and industrial impact of these reports. The survey responses suggested that the literature on OSN should be carefully mined to reveal if and to what extent these issues exist.Our community survey identified three main issues within the OSN literature: (i) inability to cross-compare results due to lack of standardization, (ii) limited insight in long-term membrane performance and (iii) limited information on the reproducibility of membrane.
Many experimental data sets from OSN publication are left in isolation because there is no consensus on a standardized protocol to allow the cross-comparison of membranes. The solute–solvent–membrane system as well as operating parameters are usually selected based on personal preference and cost, therefore finding a suitable membrane for a selected application requires rescreening all prospective membranes which is resource intensive. The pathway to real-world impact is held back unless a standardized protocol is adopted.
The industrial impact of OSN is also limited due to the lack of information on the long-term stability of membranes and limited information on industrially relevant filtration. Around 20% of publications reported filtrations at industrially relevant concentrations and around a tenth performed filtration that lasted for more than a week. Only a small fraction of publications studied filtration in SWMMs. More information on SWMMs and filtration at industrially relevant conditions are essential to bridge the existing gap between academics and industries.
Reproducibility of membrane performance was also among the top issues according to our community survey. Although around 3 in 4 publications provided information on standard deviation, less than a third of them originated from membrane replicates. We have also provided evidence on misalignment between needs of the OSN community and information that are being published. Among the five most important operating conditions according to the OSN community, temperature is underreported with only 57% of surveyed papers providing the information.
The sustainability of OSN was improved by addressing the environmental issues posed by membrane fabrication and OSN processes. Three-fifth of process papers improved the sustainability while this number dropped to 27% for membrane development publications. The majority of the green focused process papers achieved sustainability through waste minimization while more than half of membrane development publications improved the sustainability of membrane by selecting greener solvents for the fabrication process. However, there are limited research on the modelling studies of ‘green’ solvent–solute–membrane systems.
Despite the recent efforts in improving sustainability, our analysis showed that currently a meaningful green comparison of OSN literature is not possible due to the limited information on the experimental conditions and lack of green analysis.
To improve the scientific value and the industrial impact of OSN publications, we advocate that the aforementioned issues should be addressed. With the guideline provided here for both authors and reviewers, we hope that reports on experimental procedures and on membrane performance will improve and that more characterization methods will be described that could aid further research.
Conflicts of interest
The authors claim no conflict of interest.Acknowledgements
The authors express their gratitude to the OSN2019 conference organizers for helping to distribute the survey on the conference website. The authors also thank everyone who completed the survey and shared their responses. Fruitful discussion with Mr Levente Cseri from the University of Manchester is gratefully acknowledged. The authors acknowledge the UK's Engineering and Physical Sciences Research Council (EPSRC) under grant code EP/N509565/1 for funding HALP's doctoral studies through the University of Manchester's doctoral training account administered by their Department of Materials. The research reported in this publication was supported by funding from King Abdullah University of Science and Technology (KAUST).Notes and references
- L. Cseri, M. Razali, P. Pogany and G. Szekely, Organic Solvents in Sustainable Synthesis and Engineering, in Green Chemistry: An inclusive Approach, ed. B. Török and T. Dransfield, Elsevier, Oxford, 2018, pp. 513–553 Search PubMed.
- R. P. Lively and D. S. Sholl, Nat. Mater., 2017, 16, 276–279 CrossRef CAS PubMed.
- D. S. Sholl and R. P. Lively, Nature, 2016, 532, 435–437 CrossRef PubMed.
- G. Szekely, M. F. Jimenez-Solomon, P. Marchetti, J. F. Kim and A. G. Livingston, Green Chem., 2014, 16, 4440–4473 RSC.
- A. Imbrogno and A. I. Schafer, J. Membr. Sci., 2019, 585, 67–80 CrossRef CAS.
- M. Galizia and K. P. Bye, Front. Chem., 2018, 6, 511–532 CrossRef PubMed.
- B. van der Bruggen, Microfiltration, ultrafiltration, nanofiltration, reverse osmosis, and forward osmosis, in Fundamental Modelling of Membrane Systems, ed. P. Luis, Elsevier, Oxford, 2018, pp. 25–70 Search PubMed.
- C. P. Koutsou and A. J. Karabelas, J. Membr. Sci., 2012, 399–400, 60–72 CrossRef CAS.
- C. J. Davey, Z.-X. Low, R. H. Wirahan and D. A. Patterson, J. Membr. Sci., 2017, 526, 221–228 CrossRef CAS.
- C. J. Clarke, W.-C. Tu, O. Levers, A. Brohl and J. P. Hallett, Chem. Rev., 2018, 118, 747–800 CrossRef CAS PubMed.
- A. Yushkin, R. Borisov, V. Volkov and A. Volkov, Sep. Purif. Technol., 2019, 211, 108–116 CrossRef CAS.
- A. Bocking, V. Koleva, J. Wind, Y. Thiermeyer, S. Blumenschein, R. Goebel, M. Skiborowski and M. Wessling, J. Membr. Sci., 2019, 575, 271–228 CrossRef.
- J. Geens, K. Peeters, B. Van der Bruggen and C. Vandecasteele, J. Membr. Sci., 2005, 255, 255–264 CrossRef CAS.
- S. Postel, G. Spalding, M. Chirnside and M. Wessling, J. Membr. Sci., 2013, 447, 57–65 CrossRef CAS.
- M. Razali, C. Didaskalou, J. F. Kim, M. Babaei, E. Drioli, Y. M. Lee and G. Szekely, ACS Appl. Mater. Interfaces, 2017, 9, 11279–11289 CrossRef CAS PubMed.
- C. M. Alder, J. D. Hayler, R. K. Henderson, A. M. Redman, L. Shukla, L. E. Shuster and H. F. Sneddon, Green Chem., 2016, 18, 3879–3890 RSC.
- P. Marchetti, L. Peeva and A. Livingston, Annu. Rev. Chem. Biomol. Eng., 2017, 8, 473–497 CrossRef PubMed.
- T. Y. Cath, M. Elimelech, J. R. McCutcheon, R. L. McGinnis, A. Achilli, D. Anastasio, A. R. Brady, A. E. Childress, I. V. Farr, N. T. Hancock, J. Lampi, L. D. Nghiem, M. Xie and N. Y. Yip, Desalination, 2013, 312, 31–38 CrossRef CAS.
- T. Schipolowski, A. Jeżowska and G. Wozny, Desalination, 2006, 189, 71–80 CrossRef CAS.
- M. F. Jimenez-Solomon, Q. Song, K. E. Jelfs, M. Munoz-Izabel and A. G. Livingston, Nat. Mater., 2016, 15, 760–767 CrossRef CAS PubMed.
- M. Razali, J. F. Kim, M. Attfield, P. M. Budd, E. Drioli, Y. M. Lee and G. Szekely, Green Chem., 2015, 17, 5196–5205 RSC.
- S. Tsarkov, V. Khotimskiy, P. M. Budd, V. Volkov, J. Kukushkina and A. Volkov, J. Membr. Sci., 2012, 423–424, 65–72 CrossRef CAS.
- S. Postel, C. Schneider and M. Wessling, J. Membr. Sci., 2016, 497, 47–54 CrossRef CAS.
- R. Goebel, M. Schreiber, V. Koleva, M. Horn, A. Gorak and M. Skiborowski, Chem. Eng. Res. Des., 2019, 148, 271–279 CrossRef CAS.
- B. Shi, P. Marchetti, D. Peshev, S. Zhang and A. G. Livingston, J. Membr. Sci., 2017, 525, 35–47 CrossRef CAS.
- R. K. Henderson, C. Jimenez-Gonzalez, D. J. C. Constable, S. R. Alston, G. G. A. Inglis, G. Fisher, J. Sherwood, S. P. Binks and A. D. Curzons, Green Chem., 2011, 13, 854–862 RSC.
- C. Didaskalou, J. Kupai, L. Cseri, J. Barabas, E. Vass, T. Holtzl and G. Szekely, ACS Catal., 2018, 8, 7430–7438 CrossRef CAS.
- A. Vartak and A. S. Myerson, Org. Process Res. Dev., 2017, 21, 253–261 CrossRef.
- S. P. Nunes, P. Z. Culfaz-Emecen, G. Z. Ramon, T. Visser, G. H. Koops, W. Jin and M. Ulbricht, J. Membr. Sci., 2020, 598, 117761 CrossRef CAS.
- H. H. Wang, J. T. Jung, J. F. Kim, S. Kim, E. Drioli and Y. M. Lee, J. Membr. Sci., 2019, 574, 44–54 CrossRef CAS.
- T. Marino, F. Galiano, A. Molino and A. Figoli, J. Membr. Sci., 2019, 580, 224–234 CrossRef CAS.
- D. L. Kim, N. Moreno and S. P. Nunes, Polym. Chem., 2016, 7, 113–124 RSC.
- M. A. Rasool and I. F. J. Vankelecom, Green Chem., 2019, 21, 1054–1064 RSC.
- M. A. Rasool, C. Van Goethem and I. F. J. Vankelecom, Sep. Purif. Technol., 2020, 232, 115903 CrossRef.
- H. M. Tham, S. Japip, D. Hua and T.-S. Chung, ChemSusChem, 2018, 11, 2612–2619 CrossRef CAS PubMed.
- A. Hermans, E. Dom, H. Marien, G. Koeckelberghs and I. F. J. Vankelecom, J. Membr. Sci., 2015, 476, 356–363 CrossRef.
- J. H. Kim, M. Cook, S. H. Park, S. J. Moon, J. F. Kim, A. G. Livingston and Y. M. Lee, Green Chem., 2018, 20, 1887–1898 RSC.
- D. Kim, S. Livazovic, G. Falca and S. P. Nunes, ACS Sustainable Chem. Eng., 2019, 7, 5649–5659 CrossRef CAS.
- J. H. Aburabie, T. Puspasari and K.-V. Peinemann, J. Membr. Sci., 2020, 596, 117615 CrossRef CAS.
- K. Konca and P. Z. Culfaz-Emecen, J. Membr. Sci., 2019, 587, 117175 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: List of definitions, survey questions, detailed methods and results; additional results from the literature analysis; summaries of two recommended performance testing protocols; details of materials characterization methods for OSN membranes; table of essential materials characterization needed for membranes for OSN according to membrane type, reporting checklist. See DOI: 10.1039/d0gc00775g |
| This journal is © The Royal Society of Chemistry 2020 |