Open Access Article
Open Access ArticleSpherical lignin particles: a review on their sustainability and applications
Monika
Österberg
 *a,
Mika H.
Sipponen
*a,
Mika H.
Sipponen
 ab,
Bruno D.
Mattos
ab,
Bruno D.
Mattos
 a and
Orlando J.
Rojas
a and
Orlando J.
Rojas
 ac
ac
aAalto University, School of Chemical Engineering, Department of Bioproducts and Biosystems, P.O. Box 16300, Aalto, Finland. E-mail: monika.osterberg@aalto.fi
bDepartment of Materials and Environmental Chemistry, Stockholm University, SE-106 91 Stockholm, Sweden
cDepartments of Chemical and Biological Engineering, Chemistry and Wood Science, University of British Columbia, 2360 East Mall, Vancouver, BC, Canada
First published on 6th April 2020
Abstract
There is an increased interest in renewable carbon as a source of materials, where lignin is expected to play a prominent role. This stems, partially, from new regulations aiming to achieve a cleaner and safer environment. Lignin, as a polyaromatic plant-derived biomolecule, is not only abundant but widely accessible in industrial streams. Due to recent developments in production scalability as well as promising application prospects, nanoscaled lignin particles have recently generated interest in the research and industrial communities. This review describes the main routes to prepare spherical lignin particles, highlighting aspects associated to their shape and topology as well as performance. We discuss the use of spherical lignin particles as dispersants and in the formulation of coatings, adhesives and composites, focusing on the advantages of the spherical shape and nanoscaled size. The state of the particles is furthermore compared in terms of their applicability in dry and wet forms. Finally, we discuss the sustainability, stability and degradation of lignin particles, which are issues that are critically important for any prospective use.
Introduction
Lignin is a polyphenolic biomolecule found in woody parts of trees and other vascular plants. The monomeric lignin precursors, corresponding structural units and the most common covalent linkages in polymeric lignin are shown in Fig. 1. During both pulping and biofuel production, lignin is removed as an unwanted side stream. The efficient valorization of lignin has been hampered by its complex and varying chemical structure partly arising from chemical reactions during these pulping1–3 and biomass pretreatment processes.4 In effect, cleavage of the inter-unit linkages of native lignin (Fig. 1c–h) and associated formation of new covalent bonds leads to technical lignins with complex and incompletely elucidated structures. Lignin nanoparticles (LNPs) or colloidal lignin particles (CLPs), as they are also referred to, have attracted recent increased attention. Some reports even envision that lignin nanoparticles will have a strong influence on promoting lignin valorization, akin to the development of synthetic nanoparticles in the polymer industry.5 Indeed, as will be discussed in this review, the formation of spherical, well-defined particles removes many of the obstacles that have hindered efficient and broad utilization of lignin.There are two main drivers in the application of lignin particles. One is the need to valorize lignin as an industrial stream. The second one is to replace synthetic particles with a biobased alternative. Some of the possibilities for lignin particles involve large volume applications, like adhesives, composites and emulsion stabilizers, i.e. applications already proven for other forms of lignin, but where spherical particles could give an advantage. Focusing on the size and shape of the particles introduce totally novel lignin applications, including drug delivery6–11 and enzyme immobilization12,13 for biocatalysis.
Usually, the approach to use LNPs is described as sustainable or “green” solely based on the fact that a synthetic material can be replaced by a renewable alternative. However, in this review we intend to address the sustainability and greenness of LNP production and applications in more depth from the viewpoint of the most central ones of the 12 principles of green chemistry14 and by assessing some environmental prospects. Waste prevention is without doubt one of the most important of the green chemistry principles. Lignin valorization can be considered “green” as it utilizes a renewable feedstock that is currently available in excess, hence reducing the amount of waste. Furthermore, isolating lignin for applications instead of burning it for energy, during the recovery of chemicals in the kraft pulping process, would contribute to carbon fixation in materials, and conform to the principles of the circular economy. One of the breakthroughs in the production of LNPs was the finding that no chemical derivatization of lignin is required, provided that there is a suitable organic solvent system. The solvent selection is a compelling engineering challenge that encompasses for instance safety considerations and economical recycling process design. Nevertheless, one needs to be critical as to when the energy input and solvents used for the production of LNPs is compensated by the added value of the products. Other issues that are not always addressed are (1) to what extent the processes are preventing the formation of waste, especially wastewater, (2) what is the atom efficiency of the process, (3) are all materials incorporated in the product, and (4) to what extent could LNPs be designed for degradation?
Lignin particles have in general been found non-toxic (in reasonable concentrations) by in vitro6,11,15,16 or in vivo11 toxicological assessment, but this has to be re-assessed every time the particles are modified or compounded with other materials.17 In contrast, the eco-toxicity and degradability of the particles have received no attention unlike that of non-particulate lignins.18 Chemical products should be designed so they do not pollute the environment, hence, when their function is complete, they should break down to non-harmful products. In this scenario, a more comprehensive discussion on the environmental fate, in terms of stability and degradability, of lignin and lignin particles is required to amplify successful applications of such particles as greener solutions for current challenges or to improve the sustainability of already consolidated processes or products.
In this review, we describe the importance of the morphology of particles in general, describe the main methods for producing spherical lignin particles and review the applications in which they have been used. The sustainability of lignin particles, their production and uses are briefly assessed with respect to carbon balance and life cycle assessment. Additionally, we discuss the fate and stability of lignin particles during storage and application, comparing their degradation rates and destabilization mechanisms with those from crude lignins. We will close with our perspectives for future research needed to advance fundamental understanding and usability of these low-cost particulate materials in applications that support implementation of sustainable circular bio-economy.
Importance of particle morphology on performance
Within materials sciences, particles with controlled morphological features allow gaining control on properties that are otherwise difficult to achieve with irregular, heterogeneous geometries. Relevant to particulate systems, properties such as packing density, color, strength and transport (mass, heat) can be cited. Improved or new functions can be achieved from multiple components integrated in the form of particles.19 Typically, nano- and micro-particle isolation is less energy demanding compared to the workup of chemical synthesis. This is a direct result of surface energy, especially if the given production method involves high reaction enthalpies (e.g. dissolution, covalent bond formation, reduction, etc.).19 Thus, small, chemically engineerable particles can be used prominently, for example as pigments for coatings, as polymer fillers, or for reinforcement, in surface finishing or as substrate in reaction media.Two morphological considerations are implicit in this review, namely, shape and surface topology. Contrary to the irregular crude lignin that is typically available from a variety of sources, as a dried powder of particles with irregular size and shape, we here focus on recent developments to produce regular spherical lignin particles with uniform size and bearing smooth surfaces. This is critically important since shape regularity is shown to be a determining factor in colloidal behavior, for example, from the point of view of flow, agglomeration and packing.20 Moreover, the effect of shape and its relation with functions have been a standing issue that is recognized for its importance in physics, materials science and other fields, including biology.21 Nevertheless, related aspects are far from being fully understood. For example, the relationship between shape and function, especially at the nanoscale, still requires attention.
From the engineering perspective, an advantage of spherical particles over other shapes is the fact that there are no sharp edges, which may break or wear off during the processing. This is particularly true in operations where, for example, finely divided catalyst particles are employed.22 On the other hand, size and size regularity matter for the efficient ordering of objects, which can be in closely packed states, where necks and pores exist. Thus, a two-component system can be assumed for any prediction, e.g. the solid and void phases, allowing theoretical and experimental approximations. For monodisperse perfect spheres, the highest packing density comprises approximately 74% of the volume. A random packing of equal spheres, however, generally has a density around 64%. Numerical simulation results show that the upper bound order of random packing densities of basic monodisperse 3D objects is cube (0.78) > ellipsoid (0.74) > cylinder (0.72) > spherocylinder (0.69) > tetrahedron (0.68) > cone (0.67) > sphere (0.64).20
Similarly as the morphologies of molecules affect the binding forces that hold them together and their ability to form crystals, also the forces between particles are affected by their size and shape, which again will affect the properties of particle assemblies. Another aspect, especially relevant to those working in the area of colloids and surfaces, is the recognized need to control interfacial interactions, which is well described for spherical particles.23 However, this aspect becomes more complex in the presence of rough surfaces.24–26 Roughness features create a distribution of interaction energies, where local inter-particle interaction potentials may be shifted from those predicted under the assumption of smooth surfaces. In general, the double-layer potential is, on average, reduced by surface roughness because the interfacial separation is effectively larger. Hence, particles with rough surfaces are more prone to aggregate due to attractive van der Waals interactions and the particles may be in a primary minimum preventing dispersion upon decrease in ionic strength. However, if colloids are attached on the asperities of rough surfaces, both theoretical and experimental results show that they may be released upon lowering of the ionic strength, demonstrating the importance of surface heterogeneity on colloidal stability.27 Likewise, the idea of matching the shapes of colloid particles to maximize the strength of depletion interactions stems from considerations of entropy effects. For example, small particles or polymers can be excluded from the spaces between larger colloid particles. This exclusion increases the osmotic pressure outside—and so provides an effective attraction between—the larger particles.
The effect of shape of particles is best illustrated if one considers it as a parameter affecting the toxicity in vitro and in vivo.28–30 High aspect ratio particles can more easily penetrate a biological cell, increasing both cyto- and eco-toxicity. Hence, spherical particles are in general considered to have the lowest toxicity level. Relevant to this review is to highlight the advantages inherent to spherical particles: they facilitate the most efficient utilization if used as a substrate for reactions or as a catalyst support. A practical challenge is therefore to afford particles with a smallest suitable diameter. In operations wherein the particles are employed in a fixed bed, spherical shapes provide a means of obtaining uniform packing, thus preventing a variation in pressure drop through the column. If variations in pressure drop occur, channeling results and a large portion of the bed is by-passed, thereby decreasing the effectiveness of the system. These aspects have been relevant in some of the recent reports on the use of lignin particles in multifunctional membranes, anti-oxidative microfiltration, patterning and 3D structuring.31 The following section describes the processes most typically used to produce spherical lignin particles.
Processes to produce spherical lignin particles
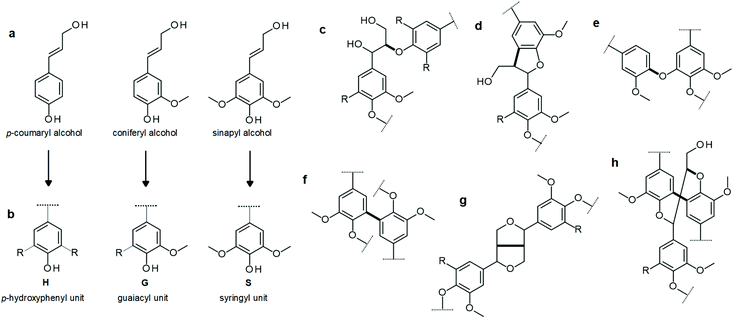
There is a multitude of methods reported on preparation of lignin particles and the main ones are summarized in Table 1. Unfortunately, the nomenclature can be confusing. Just as the produced particles are called lignin nanoparticles, colloidal lignin particles, lignin nanospheres etc., the same method has been described differently in the literature. One reason for this confusion is that there is no consensus about the mechanism behind the particle formation. In this review we attempt to clarify discrepancies in nomenclature and instead of using the specific nomenclatures adopted so far, we refer to the given work depending on the underlying particle formation method. As described above, compared to amorphous and irregular powders, spherical lignin particles can often add advantages in applications. Whereas irregular lignin powders may be sufficient for certain uses e.g. as fillers,32–34 a better control over the particle morphology is preferable for high-performance applications, such as in Pickering stabilization. As a consequence, we will focus on methods leading to spherical particles but also correlate to results obtained using as-produced, irregular powders. The methods to prepare spherical lignin particles can roughly be divided into dry and wet methods (Fig. 2). These are reviewed in the following section.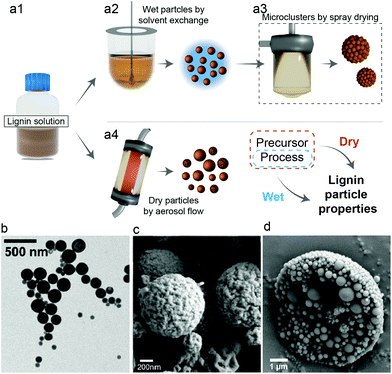 | ||
| Fig. 2 The most common processes involved in the formation of spherical lignin particles. (a1) Lignin solutions can be converted into spherical particles by (a2) controlled solvent exchange resulting in aqueous colloidal lignin particles dispersions that can be used as such or, if needed, be further spray dried to obtain (a3) lignin particle microclusters. (a4) Lignin solutions can be atomized into micro/nano scaled droplets and dried under, fluidized, controlled atmospheres. These processes yield (b) spherical smooth35 (c) wrinkled36 or (d) superstructured37 particles. Reproduced with permission from ref. 34, 35 and 36. Copyright © 2016 and 2018, Royal Society of Chemistry. | ||
| Method | Lignin type | Solvent | Antisolvent | Dry/wet | Spherical particles | Demonstration of application | Ref. |
|---|---|---|---|---|---|---|---|
| THF, tetrahydrofuran, DMF = N,N-dimethylformamide, EG = ethyleneglycol, KL = kraft lignin, AL = alkalilignin, OSL = organosolv lignin. | |||||||
| Solvent shifting | AL, KL, others | THF | H2O | Wet | Yes | Drug delivery and antiproliferation of cancer cells, UV blocking | 7, 8, 35, 42 and 43 |
| KL | THF/H2O | H2O | Wet/dry | Yes | Glue, composite with PLA, Pickering emulsion | 37 and 44 | |
| KL | Acetone/H2O | H2O | Wet | Yes | Pickering emulsion, enzyme immobilization, drug delivery, composite films with functionality (anti UV, antioxidant) | 12 and 45–47 | |
| AL | Ethanol | H2O | Wet | Yes | Drug delivery | 9 | |
| Acidification | KL | EG | HCl | Wet | — | Drug delivery | 48 |
| KL | NaOH (aq) | HNO3 | Wet | — | 48 | ||
| KL | EG | HNO3 | Antimicrobial (silver infused particles) | 49 and 50 | |||
| AL | EG | HCl | Wet | Composites, adhesives | 51 and 52 | ||
| Reverse micelle formation | Dioxane | Cyclohexane | Wet | Yes | Composites | 53 | |
| Aerosol flow | KL/HTT, OSL | DMF, acetone | None | Dry | Yes | Pickering emulsions, particulate membranes and anti-oxidative microfiltration | 38, 40, 54 and 55 |
| Aerosol + ice segregation | DMSO | H2O | Dry | Yes | Coatings | 56 | |
| Mechanical treatment | AL | H2O | Dry | — | — | 57 | |
| KL | H2O | Dry | — | Polymer blends | 58 | ||
| CO2 precipitation | KL | DMF | CO2 | Wet | — | UV absorption | 59 |
Dry particles
Dry lignin particles are formed simply from a well-controlled drying of dilute lignin solutions. Uncontrolled drying, e.g. oven-drying of a lignin solution, leads to the formation of aggregated, irregular, and heterogeneous micron-sized powder. A few strategies have been presented for the preparation of dry lignin particles with a variety of controlled surface topologies and particle size. Briefly, such methods involve the controlled evaporation of atomized droplets of lignin solution,36,38 or the post-drying of dispersed particles formed by emulsion or solvent exchange techniques.37,39 The latter are described in more detail in the next section related to wet particles.Ago et al.38 introduced the use of an aerosol flow reactor for the production of dried lignin particles, in a wide range of size fractions and from different sources. They showed that spherical particles could be produced from a broad variety of lignin types, like alkali, kraft or organosolv lignins, provided that a suitable solvent is used. Unless the lignin stream is already sourced as a solution, the process involves dissolution followed by atomization of the solution into droplets, which are suspended and carried by a carrier gas (for example, air or nitrogen) through a heated laminar flow system.
Common solvents include water, alcohols, acetone and DMF, depending on the lignin type, thus allowing the synthesis to occur, in a temperature range that also depends on the solvent, from 25 to 150 °C.40 After formation, the particles are cooled in an air stream and subsequently fractionated in a Berner-type low-pressure impactor with multiple collection stages. Fractions with narrow particle distribution from 230 nm to 1.9 μm can be directly collected. The lignin type affected both particle charge and wettability, with reported contact angles of water ranging from 57° for particles formed from kraft lignin to 69° for those from organosolv lignin (Fig. 3). The cost to produce dried lignin particles can be reduced by the utilization of selected solvents, i.e. acetone, that favor the aerosol formation and droplet consolidation under conditions of low temperature and short time. This would make such process scalable and cost-effective, with an estimated selling price of 1015 US$ per metric ton.41 Recently, acetone-extracted lignin from hydrothermally treated wood was converted into lignin nanoparticles via the aerosol flow reactor. The authors experimentally demonstrated the possibility of achieving lower time and temperature for particle formation. Additionally to that, the surface charge of the particles from biorefinery lignin differed from those obtained from kraft lignin (Fig. 3a).40
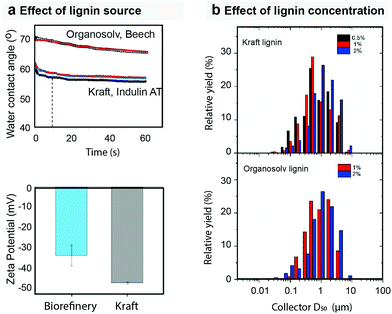 | ||
| Fig. 3 Factors affecting the formation of dry lignin particles. Process parameters (solvent, reactor conditions, flow, etc.) and source (botanical and extraction process) affect the final properties of the lignin particles. (a) The source of lignin typically affects the surface energy38 and charge40 of the obtained particles, whereas (b) the concentration of the starting solution affects the size and size distribution over a wide range of sizes.38 Reproduced with permission from ref. 37 and 41. Copyright © 2016 and 2020, American Chemical Society. | ||
A variation of the aerosol flow process was discussed by Kämäräinen et al.36 for the preparation of wrinkled kraft lignin particles. From a different selection of solvents and with the use of a blowing agent, it was possible to engineer the drying phase of the atomized droplets to induce drying stresses exceeding the critical buckling stresses of the partially dried lignin droplets. Ammonium carbonate was used as blowing agent, and ammonium hydroxide as solvent. An early-formed rigid shell around the droplets buckles toward the concave side, thus leading to particles with high roughness. Smooth particles were obtained without the use of the blowing agent, which indicate the need of a blowing agent for the formation of wrinkled lignin particles with a higher surface area.
Dried particles can also be obtained by freeze-drying39 or spray-drying37 lignin particle dispersions previously prepared by either emulsion templating or solvent exchange. Sameni et al.39 prepared non-aggregated dried lignin microspheres after freeze-drying a diluted suspension, at 0.2 mg mL−1. Although such process was efficient in obtaining dried particles, freeze-drying is a time-demanding and relatively expensive process, causing freezing gradients, and thus hindering the large-scale production of dried particles and therefore their wide application.
On the other hand, Lintinen et al.37 demonstrated an efficient and scalable method to obtain dried particles from colloidal lignin suspensions. After the formation of the lignin particles by solvent exchange, a regular spray-drier can be used to process the concentrated aqueous LNP dispersion. The process led to the formation of superstructured particle microclusters (from 5 to 80 μm), which are partially redispersible or may as well be used in their higher hierarchical architecture in e.g. delivery platforms where nanoparticles may be too mobile.60 Such microclusters can be redispersed by either tip or bath ultrasound or high-shear homogenization (Ultra-Turrax). Dry lignin nanoparticles have also been formed by evaporating solvents from a reverse micelle dispersion of lignin,53 and by using a so-called ice segregation method, which involves freezing lignin solutions on cold surfaces.56,61
Wet particles
Various precipitation methods exist for the fabrication of nano- and microscaled lignin particles,9,10,35,42,48,57,62–66 but not all of them produce spherical lignin particles in colloidally stable aqueous dispersions. The process of dissolving lignin in (aqueous) organic solvent and precipitating in a non-solvent (typically water) is commonly referred to as solvent shifting, solvent exchange or nanoprecipitation. Regardless of the precipitation scheme, lignin has an intrinsic tendency for assembly into spherical particles that minimize the surface area in contact with the non-solvent phase. However, there are some prerequisites to obtaining stable dispersions of individual particles. For example, although acidification of an alkaline lignin solution leads to precipitation, the precipitation continues to an irregular network structure and inevitable sedimentation, since acidification protonates the charged groups on lignin, preventing electrostatic stabilization of the particles. It is important to note that this differs from the nanoprecipitation methods for preparation of LNP dispersions that can remain colloidally stable for months.The morphology and colloidal stability of the lignin particles also depend on the type of lignin raw material, properties of solvent, and process parameters, such as the rate of addition of the non-solvent, or if the lignin solution is added to the water, the end-point solvent concentration, and pH. The molecules associate via non-covalent interactions including hydrogen bonding, and pi-stacking as well as hydrophobic interactions and others. Some papers discuss pi–pi interactions as the main driving force for the aggregation process that leads to particle formation.35,67 Combining molecular dynamics simulations and atomic force microscopy it was recently shown that the hydrophilic groups of enzymatic hydrolysis lignin interact with water and the hydrophobic skeleton with the organic solvent during lignin dissolution in organic solvent–water binary mixtures. When the ratio of water to organic solvent becomes high enough, lignin self-assembles into spherical particles. Interestingly, the authors found that spherical particles were also formed when the water concentration in acetone was decreased below 0.2%.68
Except for this study, we can only speculate on the impacts of different structural motifs on particle morphology. One viewpoint is the morphology that lignin molecules adopt when precipitated from solution. Motifs with different bonding segments such as β-aryl ether versus biphenyl ether probably behave differently because of the different distances between the interconnected aromatic units.
Another point of interest is the molecular weight, which has been found to determine the pattern of formation of spherical lignin particles.9 In general, it appears that precipitation of molecules with large molecular weight (and low water-solubility) initiate the process and act as nuclei for the growing particles. The last lignin molecules to precipitate and coat the particle surfaces are the low molecular weight fragments that are often enriched with hydrophilic functionalities such as carboxylic acid or sulfonic acid groups. Increasing the molecular weight by enzymatic crosslinking of the lignin molecules has been shown to lead to smaller particle size.44 In another study, ethanol and sulfuric acid were used for lignin dissolution and the authors found that this enabled cleavage of β-O-4 and β–β linkages resulting in condensed lignin with higher molecular weight.69 In line with Mattinen et al.44 they also found that a smaller particle size was obtained. This phenomenon was explained to be due to increased hydrophobic interactions. It is presently unclear whether differences in molecular weight distribution and structural motifs of lignin influence particle properties such as colloidal stability or porosity that was recently determined for the first time.70 However, many of the above points would benefit from more detailed experimental evidence, and ideally be supported by molecular dynamics simulations.
The effect of pH on the formation of lignin particles has received surprisingly little attention despite the fact that many technical lignins contain carboxylic acid groups in addition to the acidic phenolic hydroxyl groups. Leskinen et al. adjusted the pH of the water used as non-solvent in the preparation of colloidal lignin particles from aqueous THF solution of softwood kraft lignin. They found that the particle diameter decreases almost linearly when pH of the resulting lignin particle dispersion increases from 3 to 6.71 A similar trend was confirmed to take place when the pH of aqueous acetone solution of softwood kraft lignin was adjusted prior to the nanoprecipitation step with deionized water. This trend is expected, since deprotonation of the carboxylic groups leads to increased surface charge and electrostatic stabilization of the particles (Fig. 4).
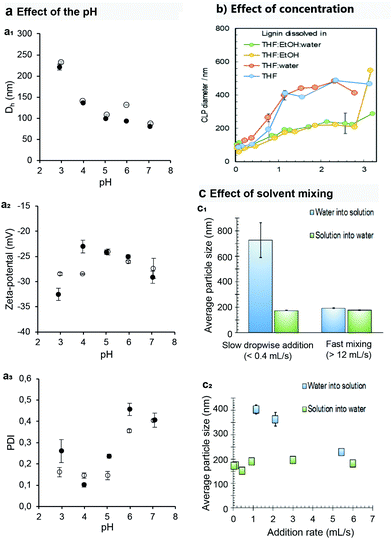 | ||
Fig. 4 Typically the process parameters affect the formation and properties of the wet lignin particles. (a) pH of the final solution has a clear effect on (a1) the size, (a2) surface charge and (a3) dispersivity of the particle population. (b) The lignin concentration and solvent composition during the solvent exchange have a decisive effect on the particle size.37 (c) The (c1) order and (c2) rate of solvent mixing during the formation process affect the particle size, being tools for engineering the size of the colloids.71 The order (solvent into water or water into solvent) has a more pronounced effect at lower addition rates. Panels in “a” are unpublished results from the authors using softwood kraft lignin dissolved in acetone water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in wt%) mixture, adjusted to target pH using 1 M hydrochloric acid or 1 M sodium hydroxide, and precipitated by rapidly adding deionized water into the vigorously stirred lignin solution. The final pH of the dispersion (CLP concentration 0.2 wt%) was measured and reported. Reproduced with permission from ref. 36 and 67. Copyright © 2018, Royal Society of Chemistry; and Copyright © 2016, Walter de Gruyter GmbH. 1 in wt%) mixture, adjusted to target pH using 1 M hydrochloric acid or 1 M sodium hydroxide, and precipitated by rapidly adding deionized water into the vigorously stirred lignin solution. The final pH of the dispersion (CLP concentration 0.2 wt%) was measured and reported. Reproduced with permission from ref. 36 and 67. Copyright © 2018, Royal Society of Chemistry; and Copyright © 2016, Walter de Gruyter GmbH. | ||
Another important aspect to consider when comparing different solvent systems is the concentration of lignin before precipitation and also the concentration of lignin particles in the final dispersion (Fig. 4b). Aggregation of particles or formation of interlinked particle networks occur if the concentration of lignin is too high. For instance, the concentration threshold for wheat straw soda lignin prior to the precipitation step was 6 g L−1 when using aqueous ethanol as solvent,9 while typically concentrations exceeding 40 g L−1 still enable the formation of freely dispersed particles from softwood kraft lignin in aqueous THF solvent system.47,71 Addition of ethanol as a co-solvent in aqueous THF has been shown to increase the applicable concentration range, giving particles with hydrodynamic diameter below 300 nm in final dispersion concentrations of up to 3 wt%.37
The wet particles are typically formed in aqueous medium, have a hydrophilic surface, and are electrostatically stabilized by the weak carboxylic groups present in the lignin. Thus, they are sensitive to the electrolyte concentration and pH of the aqueous media. The surface charge furthermore enables easy surface modification by adsorption of oppositely charged polymers or proteins to the particles. The carboxylic groups additionally offer sites for covalent modification of the surface chemistry. These various surface modifications will be discussed more with respect to applications in the following sections. However, one aspect that deserves more studies is the intra-particle porosity of the wet particles.70 Thus far, the particles have been used for entrapment and adsorption of many different active substances, as reviewed,17 and their release properties have provided indirect information of the packing and porosity of the particles.9,10,72,73 In general, the accumulated results indicate that LNPs from the solvent-exchange method are penetrable to water, which explains a rather rapid release of water-soluble small molecules,9,10 while bulkier compounds are released at lower rates.72,73
Applications
In the following section the most common applications that have been reported for spherical lignin particles are reviewed and whenever possible, the achieved properties and functionalities are compared to the results obtained using crude, irregular lignin.Dispersants
The most obvious application for spherical lignin particles and the one that was first reported is the use of the particles as dispersants. Dispersants are very practical engineering additives that play a key role across a wide range of economically important industrial segments. “Dispersant” is a term that can be generically used to describe surfactants, plasticizers or emulsifiers, depending on the field of application. Healthcare, food, civil construction and agriculture greatly benefit from dispersants that enable the mixing of immiscible liquid phases, and enhance the stability of particle suspensions. Lowering the interfacial tension between immiscible liquids, as well as increasing the repulsive forces between suspended particles are physical phenomena expected from dispersants. Such dispersive forces prevent settling and aggregation of phases, thus improving technical properties of the multiphase system such as rheology, lifetime and function. Amphiphilic molecules are the most common industrial dispersants; however, more recently, solid nanoparticles have been used to stabilize immiscible liquid phases by the formation of so-called Pickering-emulsions.74 Pickering emulsions are in general more stable against coalescence than emulsions stabilized using soluble surfactants. The majority of the industrially used dispersants are synthetic, raising concerns over their shortcomings in sustainability. There is a dual drawback in their use, namely their synthesis from non-renewable precursors and the lack of biodegradability. Therefore, there has been a wave of efforts toward the development of biobased, or more sustainable dispersants.Biomolecules, especially lignin, have a powerful dispersive capacity. Within its complex molecular structure, lignin possesses both hydrophobic and hydrophilic moieties that results in an amphiphilic character that can be engineered based, mostly, on the plant source and extraction process. Lignosulfonates (LS) are successful examples of lignin derivatives used as dispersants mostly in cement,75 but also in other minerals,76 coal,77 pesticides and dyes.78 The addition of low loadings of LS in cement mixtures greatly improves the rheological properties of the system, thus allowing the utilization of more concentrated dispersions with less water in their preparation. The major drawback of LS is that it is obtained as a side product from the sulfite pulping process that accounts only for ca. 2% of the total worldwide production of chemical pulp. Hence, there has been many attempts to use readily available kraft lignin, which accounts for 90% of the production capacity of technical lignins,79 as dispersants. However, in contrast to lignosulphonates, kraft lignin has a poor solubility in neutral water and several chemical modification efforts have been presented to increase the water solubility of kraft lignin. Some of the recent examples being sulfomethylation,80 sulfonation,81 carboxymethylation82 and a few other such as oxidations.83 Applying chemical routes to derivatize lignin, however, stands against the principles of green chemistry, generating chemical waste, sometimes using high-energy intensive processes, and decreasing the biodegradability of the final materials.
Spherical lignin particles are envisioned to overcome current challenges in the use of lignin derivatives from non-sulfite pulping process. Lignin from virtually any source can be assembled into particles, as discussed earlier, either by controlled solvent shifting or atomization. Up to date, from the best of our knowledge, there is no report on the utilization of spherical lignin particles as dispersants for cement or other mineral compositions. One reason for this may be their relatively higher production cost when compared to the direct utilization of lignosulfonates, or the fact that lignin particles have not yet been available in large quantities. However, recently, there has been significant advances in process engineering that is leading to large scale production of lignin particles at lower cost,41,71 which will open opportunities for the utilization of technical lignin-based particles in large scale applications. A great advantage of lignin particles over their macromolecular counterpart is that they are readily dispersible in water, thus not being limited by solubility. Lignin particles, if not modified, possess negative surface charge (ζ potential ca. −30 mV depending on pH, lignin source and particle preparation method). Cationic lignin particles (ζ potential ca. +30 mV) can be easily prepared by adsorption of synthetic (PDADMAC)35,37 or natural polycations (chitosan)45 or cationized lignin.47 Such versatility enables the use of spherical lignin particles as dispersants for mineral particles with either negative or positive charges. It has been noted that the size of the macromolecular lignin dispersant (discussed as molecular weight) has a strong effect on its dispersive action, in which bigger molecules are more efficient due to steric effects. We envision that the charged lignin nanoparticles could lead to intensified dispersive forces driven by a combination of electrostatic and steric repulsion. Even if considered big in relation to their molecular counterpart, lignin nanoparticles are at least four orders of magnitude smaller than the mineral particles that are commonly used (e.g. cement), thus avoiding aggregation and particle clustering resulting from their adsorption. This may warrant their increased and efficient utilization as dispersants.
Whereas spherical lignin particles have not yet been applied as a dispersant for mineral suspensions, there has been many demonstrations of their applicability as Pickering emulsifiers to stabilize oil-in-water (O/W) emulsions.84 Such multiphase systems have potential for applications in many technical fields, but mostly in cosmetics, paints, food, and agriculture. Pickering emulsions usually display longer lifetime when compared to surfactant-based emulsions, which is due to the higher resistance against droplet coalescence induced by the particles adsorbed at the O/W interface.85,86 Indeed, by tuning the amphiphilicity and charge of the lignin nanoparticles a large variety of O/W emulsions can be stabilized. While O/W emulsions based on aliphatic or aromatic hydrocarbons have been easy to stabilize using LNPs,38,47,87–90 vegetable oils have been more challenging, even if using chemically modified lignin such as the graft copolymer of lignin and N-isopropylacrylamide as a source of nanoparticles.91 Nevertheless, food, cosmetics and biomedical materials require non-toxic oils and emulsifiers. Recently, Zou et al. showed that vegetable oil–water emulsions could be stabilized using LNPs coated with chitosan.45 The chitosan-coated LNPs were further cross-linked with sodium triphosphate forming emulsion capsules that resisted drying and re-wetting.45 In Fig. 5 examples of the use of LNPs in Pickering emulsions are shown.
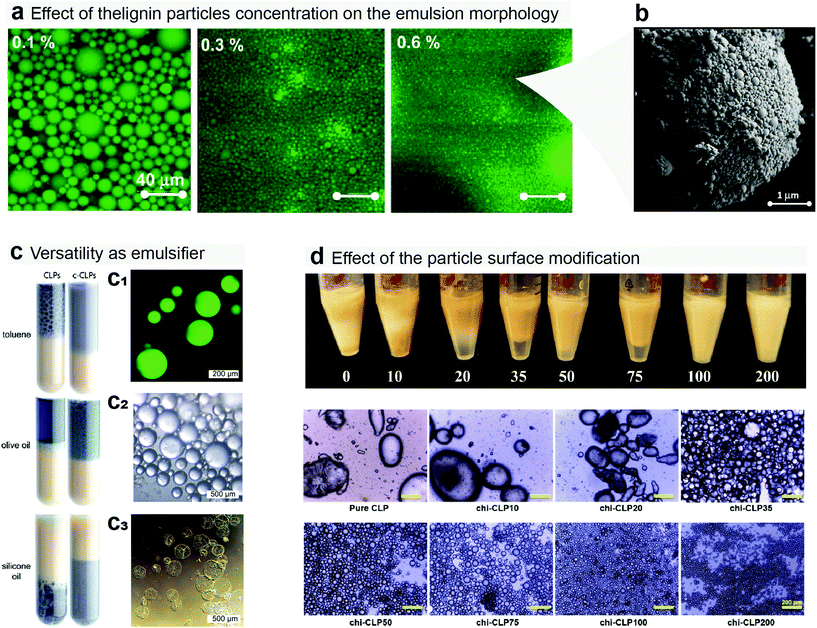 | ||
| Fig. 5 Use of lignin particles as dispersants for oil toward the formation of Pickering emulsions. (a) The concentration of lignin particles changes the morphology of the emulsion, commonly decreasing the oil droplet size with the addition of more particles.38 (b) Scanning electron microscope image showing lignin particles assembling around the oil droplet at the oil/water interface.38 (c) Lignin particles can be used to effectively disperse a wide range of oils, such as toluene, olive and silicone oil. Confocal (c1) and optical (c2) microscopy images of toluene-in-water emulsions before and after (c3) evaporation of toluene.47 (d) Surface chemical modification of the lignin particles can be further used to control their stabilizing action as demonstrated by the small droplet size obtained using chitosan coated LNPs.92 Reproduced with permission of ref. 37, 47 and 88. Copyright © 2016, American Chemical Society; Copyright © 2017, Royal Society of Chemistry; and Copyright © 2019, Zou, Sipponen, Österberg. | ||
The use of lignin particles to stabilize Pickering emulsions additionally brings, technical and environmental benefits when compared to e.g. silica93–95 or polystyrene96 particles that are currently used in Pickering emulsions. Lignin particles are, if not biodegradable, at least compostable,97,98 which is not the case of the common synthetic counterparts. Additionally, lignin possesses high antioxidant and UV-shielding properties.46,99,100 Such features enhance the long-term stability of the emulsified oil by reducing the access of light and air, therefore reducing photodegradation and oxidation of the active ingredient. Lignin-based Pickering emulsions are attractive for outdoor uses, especially for pesticide applications in agriculture where the losses of the efficiency driven by photodegradation and oxidation leads to overuse of pesticides and contamination of non-target organisms.101
Emulsions and colloids for sunscreens
Due to their UV-light absorbance, finely ground wood powder,102 various technical lignins,103–106 chemically modified lignins,107,108 and more recently spherical lignin particles43,109–111 have been evaluated in sunscreen formulations. Tan et al. showed that LNPs prepared from pine wood organosolv lignin (OL) exhibited better sunscreen performance than the non-spherical OL particles.111 Another benefit of using LNPs is that the sunscreen formulations are less brownish than those containing non-spherical lignins. Zhu et al. prepared pH-responsive sprayable hydrogels based on chitosan and nanoparticles consisting of lignin reacted with amine and carboxylic acid capped poly(ethylene glycol) (PEG).107 In this process, peptide formation catalyzed by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) is expected to result in a mixture of lignin-grafted PEG and PEG-based peptides. Kai et al. synthesized lignin–PEG methacrylate (PEGMA) copolymers via atom transfer radical polymerization (ATRP) to enhance the dispersion efficiency of lignin in the commercial creams.108 The modified lignins had only a minor increment on the SPF (sun protection factor) levels of the moisturizing cream and were less effective than the corresponding lignosulfonate supplements. Blending of the lignin copolymers at 10 wt% concentration in commercial sunscreen formulations increased the SPF of the blend. However, comparative values for lignosulfonate were not shown in the tested sunscreens.Recent investigations have specifically focused on the development of light-colored lignin-based sunscreens.112 One way to control the whiteness is to use lignin isolated under mild thermochemical conditions.112 Another approach assessed recently is to fractionate lignin based on solubility in organic solvents, and acetylation of the fractions.113 Compared to these solvent-intensive processes, morphology control through particle size and shape modifications seems more environmentally benign. Zhang et al. spray-dried lignin from aqueous dispersion to obtain spherical microparticles that were then formulated in sunscreens.106 Despite the pursuit of developing white sunscreens, the consumer preference for white versus brownish cream may differ. Gutiérrez-Hernández et al. suggested that LNPs could be matched to the skin tone of the person using the sunscreen formulation.114
Lignin has been combined with photoactive metal oxide nanoparticles to quench photocatalytic activity of titanium dioxide NPs115–117 and to broaden the UV-blocking spectrum of zinc oxide NPs in sunscreen formulations.114 The excellent ability of LNPs to stabilize emulsions is an additional advantage of their use in sunscreens.38,45,47,89 A challenge for further work is to develop all-natural sunscreen formulations in which lignin provides multi-functionality such as emulsion stability, sun protection, anti-oxidant, and antimicrobial activity.
Adhesives
There are very few reports on the use of LNPs in technical adhesives. Recently Yang et al.51 added 5–10 wt% lignin micro- and nanoparticles to a phenol-formaldehyde resin and found that low concentrations of lignin particles could increase the shear strength of wood lap joints when using the mixtures under dry conditions, but moisture resistance was not studied. However, already 10 wt% of lignin particles decreased the joint strength. They used LNPs prepared by acidification, and the irregularity of the particles may be one reason that higher amounts of lignin could not be used. Unfortunately, there are no systematic studies on the use of well-defined spherical LNPs in technical adhesives. The spherical shape may enable higher lignin concentrations without detrimental effect on viscosity of the resins. Chemical modification, increasing the reactivity of the LNPs, could also enable higher lignin contents without loss in adhesive properties.
Coatings and paints
The purpose of applying a coating is to protect the substrate, introduce or enhance functional properties, or to ameliorate decorative or technical properties. According to Mathiazhagan and Joseph, technical requirements for functional coatings include: (i) durability (abrasion, pH, ions and other solutes, temperature, moisture, etc.) (ii) reproducibility (iii) easy application and cost-effectiveness (iv) tailored surface morphology and (v) environmental friendliness.131 Spherical lignin particles seem beneficial regarding many of the above requirements, since lignin can provide surfaces with UV-protection,132 corrosion inhibition,133,134 antimicrobial activity,129,130 removable/self-healable,135 as well as fire-retardant136 and drug release properties,137 making lignin attractive for functional coating purposes.Biocompatible and biodegradable coating materials are gaining importance in response to increasing consumer awareness to sustainable materials that help tackle environmental pollution. Particulate coatings have in general some intrinsic benefits over solution coatings, such as the ability to achieve water-resistant and breathable coating layer that is important in e.g. technical textile applications.138 The possibility to apply particulate coating from aqueous dispersion of spherical lignin particles cuts off the emission of volatile organic compounds (VOCs), contributing to the waste prevention goal.
There are only a few works involving spherical lignin particles in coatings or films. Yi et al. synthesized multilayer composite microcapsules by using Pickering emulsion templates and demonstrated their application in a self-healing coating for steel (Fig. 6b).139 Cusola et al. experimented and simulated evaporation-induced assembly of spherical lignin particles into 30–40 μm thick coatings on silica wafers (Fig. 6a).55 They observed stratification upon drying of the polydisperse particles. Microscaled particles sedimented into the bottom layer, while <300 nm colloidal particles formed the top layer upon evaporation of water from the dispersion. Drying at higher temperatures increased heterogeneity of particle size distribution across the film thickness – a phenomenon that the authors explained by kinetic constraint for arrangement of the particles at higher evaporation rates. Zikeli et al. isolated lignin from sawdust for the preparation of LNPs that were successively used for coating of beech wood.140 Spherical LNPs that originated from spruce sawdust and contained aromatic extractive compounds exhibited better protection against overall color change during accelerated weathering compared to the LNPs from hardwood sawdust. The authors observed disintegration of the spherical particles after one to five days exposure to UV irradiation.
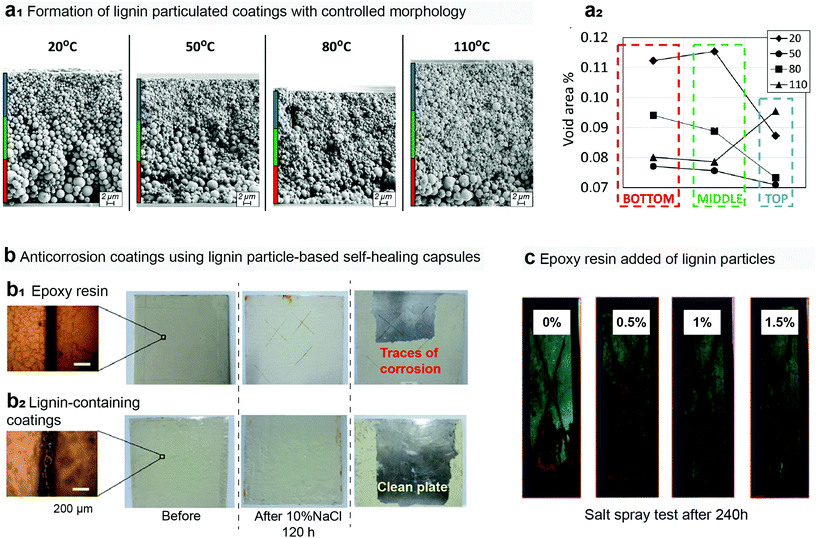 | ||
| Fig. 6 Utilization of lignin particles in coatings of paints toward given functionalities. (a1) The morphology of the lignin particle coatings can be manipulated by controlling the drying step, in which (a2) the packing of the spherical lignin over a given substrate is influenced by the dispersivity of the system and drying rate.55 (b) Anticorrosion films were obtained using microcapsules prepared from a lignin particle stabilized emulsion. The coating displays self-healing properties and advanced corrosion protection when compared to their neat epoxy counterparts.139 Brine accelerating and (c) salt spray test methods have showed the high performance of lignin particles against corrosion. (c) A simple addition of lignin particles to epoxy films already warrants anticorrosion features to the coating.133 Reproduced with permission of ref. 55, 135, and 129. Copyright © 2018, American Chemical Society; Copyright © 2015, Royal Society of Chemistry; and Copyright © 2018, Royal Society of Chemistry. | ||
Rahman et al. studied synthesis, characterization and corrosion protection performance of acid-precipitated lignin nanoparticles (Fig. 6c).133 These non-spherical lignin particles were blended with epoxy resin and the resulting nanocomposite was used to coat carbon steel. Potentiodynamic polarization and electrical impedance spectroscopy measurements showed that the nanocomposite coating inhibited corrosion of carbon steel better than the sole epoxy resin. There are a few other studies on composite coatings that contain non-spherical lignin.141–143 The combination of cellulose nanocrystals (CNC) and lignin produced smooth and homogeneous nanocomposite coatings on quartz surfaces.141 In another composite approach, incorporation of lignin in siloxane–poly(methyl methacrylate) coating increased hydrophobicity, scratch resistance, and thermal stability as well as provided corrosion resistance to carbon steel in saline.143
Overall, lignin-based particulate coatings are relatively rare in the current literature, and future work should demonstrate their properties compared to technically and commercially relevant benchmarks. For example, multilayer coatings are typical in industrial products.131 Furthermore, strong adhesion of the coating on the substrate is in most cases essential to ensure stability under application conditions.144 Commercial formulations contain curing agents that “harden” the coating and strengthen its adhesion on the substrate. Stabilization of coatings based on lignin particles is very likely needed as well. Cusola et al. demonstrated this by using wet strength agents to stabilize spherical lignin particle membranes against disintegration in water.31
Fundamental understanding of adhesion, adsorption and other relevant interfacial phenomena relay on studies using well-defined thin films and surface sensitive techniques like atomic force microscopy and quartz crystal microbalance. These methods have been used to understand, for example, direct surface forces between lignin surfaces,145 or enzyme146 or protein147 adsorption to lignin. However, during the solvent shifting method for the production of stable lignin particle dispersions, lignin will assemble with hydrophilic moieties concentrated at the surface, leading to different surface chemistry compared to lignin model films used so far. Hence, more research is needed also to develop methodology to control packing density and uniformity of model films made of spherical lignin particles in order to investigate how particulate films differ from films prepared from dissolved lignin.
Composites
With the societal demand for eco-friendly and renewable materials there has been an increasing interest to use lignin in various composite materials. As summarized in a recent review on lignin valorization,148 lignin can enhance the mechanical and thermal stability of polymer blends or composite, act as adsorbing agent, or as reactive component in various resins, act as UV blocker, antimicrobial agent or flame retardant.148 However, it has been observed that the use of raw, irregular lignin powders hinders high loads in composites, mostly because of the natural poor compatibility but also due to the uneven distribution of particles with varying size and morphology. Spherical lignin particles with controlled surface chemistry could overcome these issues, thus allowing their broader use in composite applications and being very promising although the reported results are rather contradictory. The main reason is that the geometry, morphology and surface chemistry display a crucial effect on miscibility with the matrix, and to date there are very few reports on spherical well-defined particles.In a study comparing mechanical and thermal properties of particlulate composites from polylactic acid (PLA) and lignin with PLA and tannin, Anwer et al.149 found that said properties decreased with increasing amount of either tannin or lignin particles. Although the authors did not use spherical lignin particles, it is interesting to notice that they found that the droplet size of lignin within the PLA matrix had an effect on the mechanical properties, with smaller droplets having less pronounced detrimental effect. This would suggest that the easily dispersable spherical lignin particles with diameters of a few hundred nanometers or less would probably enhance further the mechanical and thermal performance of lignin-containing PLA composites. Indeed Yang et al.150 used LNPs prepared using the acidolysis method48 to reinforce PLA composites. They found that the lignin particles induced nucleation when added at 1 wt% in melt-extruded samples, leading to enhanced mechanical properties, but already an addition of 3 wt% led to aggregation and to a decrease in mechanical properties. The acidolysis method leads to irregular particles, which may be the reason for the aggregation and negative impact already at very low addition levels. Del Saz-Orozco et al.151 reported reinforcement of phenolic foams using lignin particles and found over 100% increase in both compressive strength and modulus. However, they used calcium lignosulphonate microparticles.
Several authors have reported increased antioxidant and UV shielding properties by the addition of lignin particles to various polymer matrixes (Fig. 7c and d).46,152–155 Most probably due to the spherical structure of the LNPs used in their studies, both Farooq et al.46 and Tian et al.154 were able to add higher amounts of lignin particles without loss in the mechanical properties when compared to previous reports. The hydrogen bonding ability between lignin and the matrices (PVA or cellulose nanofibrils) was an additional feature inducing high mechanical strength. In a recent study LNPs were also shown to reduce thermal conductivity, stability and flame retardancy to boron nitride PVA composites (Fig. 7a).156
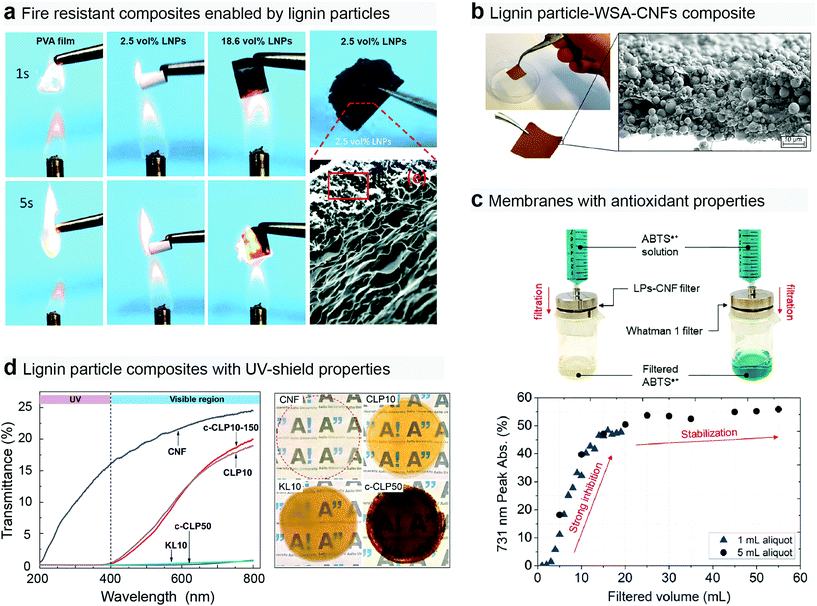 | ||
| Fig. 7 Composites containing lignin particles displaying a series of important technical properties. (a) PVA films with added lignin particles display anti-fire resistance.156 Composites with cellulose nanofibrils, lignin particles and a small fractions of wet-strength agent (polyamidoamine-epichlorohydrin resin) formed to (b) water resistant and (c) antioxidant membranes.31 (d) Lignin particles were also shown to form very strong UV-shielding films together with cellulose nanofibrils.46 Reproduced with permission of ref. 152, 30, and 46. Copyright © 2019, Royal Society of Chemistry; and Copyright © 2018 and 2019, American Chemical Society. | ||
The particle-matrix interactions are crucial to achieve an even distribution of the particles. Frequently, chemical modification of the particles is required for such endeavor. This was demonstrated by He et al. who showed that esterification and etherification of lignin nanoparticles using citric acid enables an even distribution of up to 10 wt% of the particles in a PLA matrix to form transparent and active films.155 Lignin nanoparticles have also been chemically modified to achieve specific properties. Setälä et al. prepared LNPs from tall oil fatty acid ester of kraft lignin and bound these to cellulose fibers achieving composites with antimicrobial properties.157
UV shielding, antioxidant and antimicrobial properties gained by the use of mostly nano but also submicron lignin particles argue for their use in composite materials, especially for packaging applications. However, the enhanced mechanical performance reported justifies the use of purely spherical particles instead of their irregular counterpart. The next steps should be focused on the formulation optimization as far as the choice of particle production method and interfacial compatibilization. The hydrophilic particles obtained by solvent shifting seem promising to combine with hydrophilic matrixes like cellulose or PVA, while dry particles, or surface modified ones may be a better choice to combine with PLA or other melt extruded matrices. Since chemical modification of the particles adds both cost and environmental burden, the need for modification should be carefully evaluated.
Other applications
The application of spherical lignin particles in biomedical applications has been recently reviewed17 and will hence not be further discussed here. Some other interesting applications of LNPs are as templates for carriers and for enzyme immobilization12,13,158,159 polymer composites and blends,160 precursors for carbonaceous materials161–163 and metal–organic particles,164 agglomeration agents to facilitate virus removal in water purification,165 and hybrid lignin–fatty acid capsules as phase-change materials for thermal energy storage.70 These examples exemplify that spherical lignin particles may find use in a plethora of versatile and even unexpected applications.The stability in applications and rate of degradation of lignin particles
A series of factors affect the stability of lignin during application and its rate of degradation upon use, storage and disposal. Destabilization or degradation can derive from, but are not limited to, variations in salinity or pH conditions of the environment, light, microorganisms (mostly through enzymatic activity) and exposure to solvents and heat (Fig. 8a). Unless any modification is done during the lignin particle preparation (e.g. bulk or surface functionalization),47,147,166 the physicochemical features (e.g. molecular mass, surface energy, solvation, thermal stability) of the lignin particles tend to mirror the ones from the starting lignin precursor.38,64,65,167 Therefore it is reasonable to suggest that the morphological distinction between the lignin nanoparticles and their macrostructured counterparts is the only parameter modifying their rate of degradation and stability. For instance, the rate of surface-associated degradation mechanisms (e.g. photodegradation and enzymatic depolymerization) should be proportional to the accessible surface area of the lignin particulates (colloids or irregular powder) (Fig. 8a).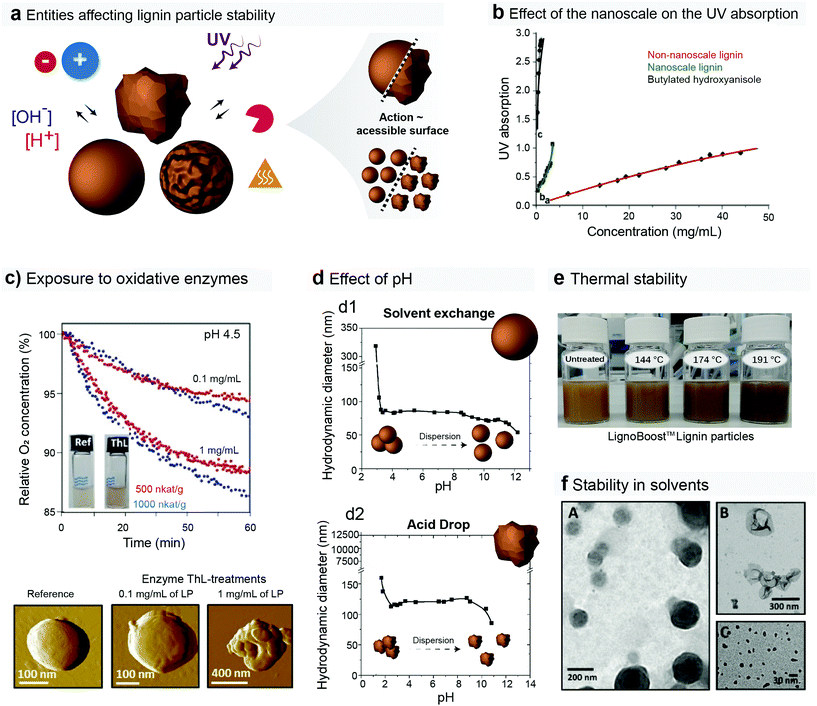 | ||
| Fig. 8 (a) Possible entities (salts, pH conditions, light, enzymes, heat and solvents) affecting the degradation rate and stability of lignin particles either in storage conditions or applications. The degradation rate of surface-associated degradation may be proportional to the accessible surface area of the lignin particulates. (b) Effect of the nanoscale on the light absorption of the lignin particles.168 (c) Oxidation of lignin particles induced by enzymes.169 (d) Effect of pH on the stability, as far as hydrodynamic diameter, of irregular and spherical-shaped lignin nanoparticles.49 (e) Effect of thermal treatments on the lignin particles dispersions properties.71 (f) Stability of unmodified and cross-linked lignin particles upon contact with THF.169 Reproduced with permission of ref. 163, 164, 49, and 67. Copyright © 2012 and 2018, Elsevier Ltd; Copyright © 2016, American Chemical Society; and Copyright © 2016, Walter de Gruyter GmbH. | ||
Regardless of the type of molecular structures (as affected by plant source and processing conditions), lignin is composed by chromophore species (phenols) in abundance. Such characteristic is, in fact, what puts lignin among the best biobased light-absorbing compounds. This has been harnessed in many applications where light needs to be filtered or blocked, such as the applications in sunscreens discussed in earlier sections of this review. However, such intense light absorption triggers photodegradation of lignin170 and lignin-containing materials.171 When comparing the absorbance at 700 nm of nano-scaled and macroscopic lignin powders it has been shown that the upper boundaries for light absorption are similar; however, this maximum value is achieved at remarkably lower concentration (12-fold lower) when lignin is used at the nano-scale (Fig. 8b).46,154 It is likely, although not yet scientifically proven, that such behavior does also intensify the photodegradation mechanisms of lignin, especially as light can easily penetrate micrometers into particulated, macroscopic materials60 thus exposing the whole core of the nanoparticles to light. However, even with the potentially higher degradation rate, lignin nanoparticles are highly efficient to act as light barriers even at low fraction, creating a positive relationship between function and degradation over time.
Fungi is the most intense group of living organisms degrading lignin. Several bacteria can also decompose lignin, but to a lesser extent. Fungi excrete enzymes (e.g. peroxidases and laccases) that are able to modify and partially depolymerize the lignin macromolecule to further decompose its primary units. Although not yet fully understood, this subject has been discussed in different contexts.172–174 Surprisingly, although this has been a discipline of study for biomass-based materials (e.g. wood),175 biodegradation of lignin particles by fungi has not been addressed in depth in the available literature. Fungal enzymes have been, however, exploited toward the controlled surface modification of the lignin particles (Fig. 8c). Mattinen et al. observed a 13% decrease in the dissolved oxygen concentration when lignin particles were treated with laccases, thus indicating evident oxidation of the lignin particles by the enzyme.169 This suggests that enzymes could severely damage the lignin colloids if they interact for a longer time. In fact, enzymatic oxidation is the first stage for degradation of lignin in soil and therefore is key for composting efforts.176 Also relevant for the fate of lignin particles in soil is the fact that they may possess the same strong chelating properties commonly observed in polyphenolic molecules.177,178 This may warrant their low mobility in soil due to ionic binding with alkali metals and especially alkaline earth metals (e.g. Na+, K+, Mg2+, Ca2+) that are abundant in several types of soil, leading to aggregation and retention of the lignin nanoparticles in the organic and the surface layers of the soil.
The colloidal stability of lignin particles is heavily influenced by the conditions of the surrounding media, such as pH and ionic strength. A few recent studies have investigated the effects of pH and salt concentration on the hydrodynamic diameter and surface charge of the lignin particles when suspended in aqueous media. The formation of particle aggregates at pH below 3 is a consensus, regardless of particle source, shape or size. The protonation of the carboxyl groups present in the chemical structure of lignin takes place predominantly at pH 3–6, while phenolic hydroxyls in many lignin model compounds have pKa values in the range of 7–10,179 both thus providing surface charge on the particles that promotes the formation of repulsive electrical double layers. Such phenomenon has been investigated by tracking the zeta potential profiles of the lignin particles as a function of pH. The surface charge of the lignin particles gets closer to the isoelectric point under acidic conditions.35,47,49 Therefore, only above pH 3 the lignin particles are stabilized through electrical double layer repulsion. Interestingly, lignin particles from the same source (Indulin AT) but with different morphologies (irregular and spherical) presented different patterns in particle destabilization – particle aggregation – over pH changes (Fig. 8d). Spherical particles showed an extended region for particle stability with a sharper aggregation at pH ∼3. Irregular lignin nanoparticles showed stability, with respect to size, from pH 3 to 9, with a remarkable decrease in size at pH 10 (Fig. 8d2). This is likely to be arising from fragmentation of the particles or even their partial dissolution.49 Sipponen et al. showed that spherical particles prepared from soda lignin display a sharp onset of aggregation below pH 3, and that particle size decreases slightly during 4 h incubation at pH 7–10.9 In another study, Lievonen et al. showed that lignin particles are extremely stable in slightly acidic to neutral pH as, as their size and monodispersity in such conditions remained constant for over 60 days.35 Carboxylic acid groups appear to be central also for the formation and dissolution of the particles. Figueiredo et al. synthesized carboxylated kraft lignin using succinic anhydride and found that excessive succinylation rendered lignin water-soluble, while a lower extent of succinylation allowed for peptide grafting on LNPs in water.8
Like for pH, the ionic strength of the dispersing media interferes with the stability of the kraft (LignoBoost) lignin particles. Lievonen et al. showed a significant shift to less negative zeta potential of the lignin particles upon addition of NaCl (from −60 to −10 mV); however, the dispersion was still stable at 500 mM NaCl and the particle size remained unchanged after seven days.35 Particle aggregation took place at 1 M NaCl with average particle size increasing from 300 nm to 1.3 μm seven days after the preparation. The addition of salts changes the interparticle interactions due to the accumulation of counterions around the particles, thus reducing the thickness of the electrical double layer (predicted by DLVO theory) and favoring the attractive van der Waals forces that may eventually lead to particle aggregation. Similar observations were made from the aggregation behavior of Indulin AT lignin nanoparticles upon addition of NaCl.49 Such nanoparticles retained their colloidal stability up to 300 mM NaCl added, after which an exponential increase was observed reaching diameters of ∼2 μm. The slightly distinct thresholds for destabilization may arise either from differences in surface charge or size of the lignin particles.
A few cross-linking or coating strategies have been carried out toward higher stability of the lignin particles for specific applications, e.g. soft tissue adhesion, emulsion stabilization, and for use in physiological conditions. Enzymes have been used to cross-link protein coated lignin particles.180 With a combination of β-casein coating followed by crosslinking with transglutaminase, Mattinen et al. have shown remarkably high long-term stability (25 days) at pH below 4, conditions under which lignin particles usually start to aggregate.44 On another instance, Leskinen et al. have taken advantage of the protein corona formation on lignin particles to tailor their surface properties to meet requirements of the potential applications in advanced biomaterials.147 Relevant for stability purposes, the authors have shown that the addition of poly-L-lysine onto lignin particles reverses their zeta potential from negative (−40 mV) to positive (+40 mV); however, at a concentration range from 0.04 to 0.2 g protein by g of particles intense particle aggregation occurs due to neutralization of the surface charges. The size of the lignin particles starts to increase significantly after the coating with gelatin. The threshold for destabilization is at 0.1 g of gelatin by g of particles, at which point the zeta potential of the particles drops from −40 to −10 eV, thus being close to the isoelectric point of the system.
Coating of lignin particles was also carried out with synthetic polymeric polyelectrolytes, such as poly(diallyldimethylammonium chloride) (PDAC),47,49 aiming at extending their properties to broaden the applications. Charge reversal is observed for PDAC-coated lignin particles. Additionally, the PDAC coating increased the stability of the lignin particles against dissolution at pH over 10 due to the formation of a stable shell of polyelectrolyte–lignin complex around the particles.49 PDAC-coated particles heavily aggregate at such high pH, going from 100 nm to 10 μm of diameter. The PDAC-coated nanoparticles aggregate at lower molarity of NaCl (at least one order of magnitude lower) when compared to native lignin particles. Sipponen et al. have taken a different, greener, approach to tune the surface properties of lignin nanoparticles and therefore to manipulate their performance and stability in applications.47 Colloidally stable cationic dispersions were obtained with adsorption of only 40 mg g−1 of cationic lignin (Catlig) onto the lignin particles. The zeta potential was reversed from −30 to +30 mV using such strategy, and the size remained ca. 300 nm after the destabilization region – Catlig![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LNPs ratio 10 to 40 mg g−1 – is overcome. With positively-charged LNPs, the authors could prepare efficiently stabilized toluene-in-water emulsions, with an additional feature related to emulsion breakage stimulated by pH (ca. pH 6) that is not possible when using anionic lignin particles.
LNPs ratio 10 to 40 mg g−1 – is overcome. With positively-charged LNPs, the authors could prepare efficiently stabilized toluene-in-water emulsions, with an additional feature related to emulsion breakage stimulated by pH (ca. pH 6) that is not possible when using anionic lignin particles.
Exposure of the lignin particles to heat and solvents can affect their stability or performance in applications. Nair et al. have demonstrated that the conversion of lignin macromolecules into nanoparticles does not significantly change their thermogravimetric profiles, with a maximum of thermal degradation taking place at 350 °C.58 This is a key observation for the utilization of lignin particles in polymeric composites prepared using any heat-centered molding process (e.g. extrusion, hot press, injection). In another effort, thermal treatments (up to ∼200 °C) of aqueous lignin particle dispersions were performed in order to increase the stability of the particles in organic solvents.71 The treated particles showed reduced solubility in lignin solvent systems, such as THF and methyl ethyl ketone (MEK)–water. However, under harsher treatment conditions the particles were heavily modified displaying darker color and sulfuric malodorous odors.
Higher solvent stability of the lignin particles can also be achieved through enzymatic cross-linking of the particle surfaces. Mattinen et al. performed intraparticle cross-linking reaction on the lignin particle surfaces using laccases from Trametes hirsuta and Melanocarpus albomyces fungi. With particle size and surface charges roughly unchanged, the cross-linked particles presented significantly higher resistance to organic solvents after dispersion and incubation. It is important to note that for some applications, especially as template for advances materials,159 the control over solubility of lignin particles in organic solvents is a key feature for their utilization. Additionally, it is still not known what the effects of cross-linking on biodegradability and recyclability of lignin particles are. These are paramount features for creating a robust platform of sustainable materials.
Sustainability aspects
In its simplest form, valorization of lignin has been shown to produce environmental benefits.181 For example, a cradle-to-gate analysis of the production of bleached fibers indicates that introducing lignin extraction in the kraft process, the most common process available in industry to produce wood pulp for papermaking, resulted in global warming potential of 602 kg CO2eq per air dry metric ton (ADmt) pulp as compared to 722 kg CO2eq per ADmt pulp for the reference scenario of the kraft mill with no lignin extraction. Moreover, if the so-called “system expansion” is considered, for example, considering the displacement of petroleum derived phenolic resins, the global warming potential is further reduced to 234 kg CO2eq.181 More specifically, the system expansion calculations included credits by displacing grid electricity and petroleum derived phenolic resins. Thereby, significant greenhouse gas emission reductions were determined. However, lignin particles possess even further advantages, and it is possible to achieve even better environmental metrics and sustainability scores, for example, in such applications as emulsion stabilizers, UV protection products, chelating agents, carbon nanofillers (other than carbon black), and for composite reinforcement, all of which are economically attractive.41 Finally, these possibilities for substitution are of relatively limited market size but, as introduced in this review, other potential applications of larger scales can be considered for lignin particles, such as in thermoplastics, advanced resin composites, foams, bactericides, and others. It is apparent that, while LCA studies are available to assess the environmental impacts of lignocellulosic biorefineries, around the cellulosic and hemicellulose fractions, LCA studies on lignin, and especially lignin nanoparticles, are far less common. A first attempt in this regards was recently made by Koch et al.182 They reported life cycle assessment calculations on a lignin nanoparticle biorefinery using hot ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixture to dissolve lignin and precipitating the lignin particles in water at pH 2, thus combining solvent and pH shifting. They found that one of the hot spots was the thermal energy needed for ethanol recovery, which is in accordance to the techno economical assessment of another solvent shifting process that also found that solvent recovery was the most energy demanding step during lignin particle production.183 That calculation was made for THF
water mixture to dissolve lignin and precipitating the lignin particles in water at pH 2, thus combining solvent and pH shifting. They found that one of the hot spots was the thermal energy needed for ethanol recovery, which is in accordance to the techno economical assessment of another solvent shifting process that also found that solvent recovery was the most energy demanding step during lignin particle production.183 That calculation was made for THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol
ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water system. However, recently Lourençon et al. found that using acetone
water system. However, recently Lourençon et al. found that using acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water as solvent in the atomization process the reactor did not need to be heated for solvent evaporation and lignin concentration up to 5% could be used.40 Although the authors concentrated on the economic savings, it can be concluded that the right choice of solvent will have a decisive effect on energy consumption and an impact on the overall sustainability of the processes. With regards to eutrophication, ethanol leakage into fresh water was found to be a hot spot.182 There is clearly a need for more LCA regarding lignin nanoparticle production. Various particle production methods should be compared and, most importantly, the applications should be included in the cradle-to-gate investigations.
water as solvent in the atomization process the reactor did not need to be heated for solvent evaporation and lignin concentration up to 5% could be used.40 Although the authors concentrated on the economic savings, it can be concluded that the right choice of solvent will have a decisive effect on energy consumption and an impact on the overall sustainability of the processes. With regards to eutrophication, ethanol leakage into fresh water was found to be a hot spot.182 There is clearly a need for more LCA regarding lignin nanoparticle production. Various particle production methods should be compared and, most importantly, the applications should be included in the cradle-to-gate investigations.
Conclusions and prospects
From the literature reviewed above it is clear that spherical lignin particles with well-defined surface chemistry and morphology have a great potential in many applications. They can improve the properties of renewable and biodegradable composites, be part of greener adhesives and decrease the need for synthetic emulsifiers, to name a few applications. Even reports on experiments using crude lignin often conclude that the size of particles and dispersability are crucial for good performance, suggesting that spherical lignin particles with narrow size distributions in nanometer range and controlled surface chemistry would have an advantage over irregular lignin. Good spreading on a surface, even distribution in media, or high affinity to e.g. biological cells are important factors that can be achieved by controlling the surface chemistry of the particles. With dry particles the surface energy can be controlled by the right choice of lignin source and the surface topography of the particles using a blowing agent. The solvent shifting method, on the other hand, results in hydrophilic particles with excellent dispersability in aqueous media. They can be modified by adsorption or grafting of molecules on the surface to tune the interactions with composite matrix, surfaces, cells etc.In many of the applications the lignin particle production method does not matter, both dry and wet particles work well. However, to minimize the consumption of solvents and energy it would be beneficial to assess what type of particles would be more beneficial. In applications that require large volumes of particles the production process needs to be scalable. The techno-economical feasibility of production processes has been evaluated for both dry41 and wet approaches183 and both are scalable. For the wet approach, the most energy consuming steps are the drying of particles and recycling of the solvents. However, demonstrations of producing large quantities (kg) of particles have so far been made only using the wet approach and a continuous process has also been developed.184
Most reported methods rely on dissolving lignin that has been precipitated from the pulping process, sometimes further purified, and dried. These intermediate steps add substantial energy and chemical consumption and it would be ideal if the black liquor could be used as such as raw material or alternatively if the lignin particles could be produced directly from biorefinery residues. It has been shown that sequentially isolated lignin fractions from the same feedstock differ in their structural features,185,186 and that these fractions lead to different properties of the spherical lignin particles.186 However, there is a lack of correlation of particle properties with lignin properties from various sources and after different extent of purification. When precipitated from spent pulping liquors, lignin often contains impurities such as polysaccharides, rosin acids, and other extractives from wood. These impurities may affect the properties of the lignin nanoparticles, but more studies are needed to elucidate such relationship. Different solvents have been used with the solvent shifting process but not much knowledge is yet available related to how the choice of solvent system affects the properties. There would be a demand for fundamental research into these issues, utilizing e.g. molecular modelling or surface force measurements to gain a better understanding of the interactions and process–property relationship for the particles.
In applications where dry particles are needed, like in the production of carbonized materials or composites together with nonpolar matrixes, it is probably better to apply the dry approach of particle production to avoid the energy needed to remove the excess of water from wet particles. Also for producing highly hydrophobic particles, this may be the approach of choice. Although hydrophobic molecules may be adsorbed onto the surface of lignin nanoparticles in aqueous media, successful hydrophobization will evitable lead to phase separation. However, for applications in aqueous media, like emulsion stabilization, drug delivery or composites with cellulose fibers or fibrils or other hydrophilic polymers, the easy surface modification by adsorption and low energy consumption of the process speaks for the choice of the wet nanoprecipitation method for these applications.
To guide the decision making during process development and application of spherical lignin particles, one aspect that needs more focus is the LCA and sustainability metrics. Especially considering both the effect of chemical modification during the production as well as the impact of using lignin particles instead of current, non-renewable components, in the applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was a part of the Academy of Finland's Flagship Programme under Projects No. 318890 and 318891 (Competence Center for Materials Bioeconomy, FinnCERES). O. J. R. also acknowledges support by the European Research Council under the European Union's Horizon 2020 research and innovation program (ERC Advanced Grant Agreement No. 788489, “BioElCell”), the Canada Excellence Research Chair initiative and the Canada Foundation for Innovation (CFI).References
- F. S. Chakar and A. J. Ragauskas, Ind. Crops Prod., 2004, 20, 131–141 CrossRef CAS.
- J. H. Lora and W. G. Glasser, J. Polym. Environ., 2002, 10, 39–48 CrossRef CAS.
- J. Gierer, Wood Sci. Technol., 1980, 14, 241–266 CrossRef CAS.
- M. H. Sipponen, J. Rahikainen, T. Leskinen, V. Pihlajaniemi, M.-L. Mattinen, H. Lange, C. Crestini and M. M. O. Österberg, Nord. Pulp Pap. Res. J., 2017, 32, 550–571 CAS.
- W. Zhao, B. Simmons, S. Singh, A. Ragauskas and G. Cheng, Green Chem., 2016, 18, 5693–5700 RSC.
- P. Figueiredo, K. Lintinen, A. Kiriazis, V. Hynninen, Z. Liu, T. Bauleth-Ramos, A. Rahikkala, A. Correia, T. Kohout, B. Sarmento, J. Yli-Kauhaluoma, J. Hirvonen, O. Ikkala, M. A. Kostiainen and H. A. Santos, Biomaterials, 2017, 121, 97–108 CrossRef CAS PubMed.
- P. Figueiredo, C. Ferro, M. Kemell, Z. Liu, A. Kiriazis, K. Lintinen, H. F. Florindo, J. Yli-Kauhaluoma, J. Hirvonen, M. A. Kostiainen and H. A. Santos, Nanomedicine, 2017, 12, 2581 CrossRef CAS PubMed.
- P. Figueiredo, M. H. Sipponen, K. Lintinen, A. Correia, A. Kiriazis, J. Yli-Kauhaluoma, M. Österberg, A. George, J. Hirvonen, M. A. Kostiainen and H. A. Santos, Small, 2019, 15, 1901427 CrossRef PubMed.
- M. H. Sipponen, H. Lange, M. Ago and C. Crestini, ACS Sustainable Chem. Eng., 2018, 6, 9342–9351 CrossRef CAS PubMed.
- Y. Li, X. Qiu, Y. Qian, W. Xiong and D. Yang, Chem. Eng. J., 2017, 327, 1176–1183 CrossRef CAS.
- L. Dai, R. Liu, L. Q. Hu, Z. F. Zou and C. L. Si, ACS Sustainable Chem. Eng., 2017, 5, 8241–8249 CrossRef CAS.
- M. H. Sipponen, M. Farooq, J. Koivisto, A. Pellis, J. Seitsonen and M. Österberg, Nat. Commun., 2018, 9, 2300 CrossRef PubMed.
- E. Capecchi, D. Piccinino, I. Delfino, P. Bollella, R. Antiochia and R. Saladino, Nanomaterials, 2018, 8, 438 CrossRef PubMed.
- P. Anastas and N. Eghbali, Green Chem., 2010, 39, 301–312 CAS.
- M. Tortora, F. Cavalieri, P. Mosesso, F. Ciaffardini, F. Melone and C. Crestini, Biomacromolecules, 2014, 15, 1634–1643 CrossRef CAS PubMed.
- S. Rai, B. K. Singh, P. Bhartiya, A. Singh, H. Kumar, P. K. Dutta and G. K. Mehrotra, J. Lumin., 2017, 190, 492–503 CrossRef CAS.
- M. H. Sipponen, H. Lange, C. Crestini, A. Henn and M. Österberg, ChemSusChem, 2019, 12, 2039–2054 CrossRef CAS PubMed.
- F. J. Ruiz-Dueñas and Á. T. Martínez, Microb. Biotechnol., 2009, 2, 164–177 CrossRef PubMed.
- W. J. Stark, P. R. Stoessel, W. Wohlleben and A. Hafner, Chem. Soc. Rev., 2015, 44, 5793–5805 RSC.
- S. Li, J. Zhao, P. Lu and Y. Xie, Chin. Sci. Bull., 2010, 55, 114–119 CrossRef.
- A. Albanese, P. S. Tang and W. C. W. Chan, Annu. Rev. Biomed. Eng., 2012, 14, 1–16 CrossRef CAS PubMed.
- A. T. Bell, Science, 2003, 299, 1688–1691 CrossRef CAS PubMed.
- J. C. Fröberg, O. J. Rojas and P. M. Claesson, Int. J. Miner. Process., 1999, 56, 1–30 CrossRef.
- L. Suresh and J. Y. Walz, J. Colloid Interface Sci., 1996, 183, 199–213 CrossRef CAS.
- L. Suresh and J. Y. Walz, J. Colloid Interface Sci., 1997, 196, 177–190 CrossRef CAS PubMed.
- S. Y. Shulepov and G. Frens, J. Colloid Interface Sci., 1996, 182, 388–394 CrossRef CAS.
- C. Shen, L.-P. Wang, B. Li, Y. Huang and Y. Jin, Vadose Zone J., 2012, 11(1) DOI:10.2136/vzj2011.0057.
- S. Sharifi, S. Behzadi, S. Laurent, M. L. Forrest, P. Stroeve and M. Mahmoudi, Chem. Soc. Rev., 2012, 41, 2323–2343 RSC.
- A. E. Nel, L. Mädler, D. Velegol, T. Xia, E. M. V. Hoek, P. Somasundaran, F. Klaessig, V. Castranova and M. Thompson, Nat. Mater., 2009, 8, 543–557 CrossRef CAS PubMed.
- X. Zhao, S. Ng, B. C. Heng, J. Guo, L. Ma, T. T. Y. Tan, K. W. Ng and S. C. J. Loo, Arch. Toxicol., 2013, 87, 1037–1052 CrossRef CAS PubMed.
- O. Cusola, O. J. Rojas and M. B. Roncero, ACS Appl. Mater. Interfaces, 2019, 11, 45226–45236 CrossRef CAS PubMed.
- S. Baumberger, C. Lapierre, B. Monties and G. Della-Valle, Polym. Degrad. Stab., 1998, 59, 273–277 CrossRef CAS.
- B. Košíková, A. Gregorová, A. Osvald and J. Krajčovičová, J. Appl. Polym. Sci., 2007, 103, 1226–1231 CrossRef.
- O. Gordobil, R. Delucis, I. Egüés and J. Labidi, Ind. Crops Prod., 2015, 72, 46–53 CrossRef CAS.
- M. Lievonen, J. J. Valle-Delgado, M.-L. Mattinen, E.-L. Hult, K. Lintinen, M. A. Kostiainen, A. Paananen, G. R. Szilvay, H. Setälä and M. Österberg, Green Chem., 2016, 18, 1416–1422 RSC.
- T. Kämäräinen, M. Ago, J. Seitsonen, J. Raula, E. I. Kauppinen, J. Ruokolainen and O. J. Rojas, Soft Matter, 2018, 14, 3387–3396 RSC.
- K. Lintinen, Y. Xiao, R. Bangalore Ashok, T. Leskinen, E. Sakarinen, M. Sipponen, F. Muhammad, P. Oinas, M. Österberg and M. Kostiainen, Green Chem., 2018, 20, 843–850 RSC.
- M. Ago, S. Huan, M. Borghei, J. Raula, E. I. Kauppinen and O. J. Rojas, ACS Appl. Mater. Interfaces, 2016, 8, 23302–23310 CrossRef CAS PubMed.
- J. Sameni, S. Krigstin, S. A. Jaffer and M. Sain, Ind. Crops Prod., 2018, 117, 58–65 CrossRef CAS.
- T. V. Lourençon, L. G. Greca, D. Tarasov, M. Borrega, T. Tamminen, O. J. Rojas and M. Y. Balakshin, ACS Sustainable Chem. Eng., 2020, 8, 1230–1239 CrossRef.
- C. Abbati de Assis, L. G. Greca, M. Ago, M. Y. Balakshin, H. Jameel, R. Gonzalez and O. J. Rojas, ACS Sustainable Chem. Eng., 2018, 6, 11853–11868 CrossRef CAS PubMed.
- Y. Qian, Y. Deng, X. Qiu, H. Li and D. Yang, Green Chem., 2014, 16, 2156–2163 RSC.
- S. X. Li, M. F. Li, J. Bian, X. F. Wu, F. Peng and M. G. Ma, Int. J. Biol. Macromol., 2019, 132, 836–843 CrossRef CAS PubMed.
- M.-L. Mattinen, G. Riviere, A. Henn, R. Nugroho, T. Leskinen, O. Nivala, J. Valle-Delgado, M. Kostiainen and M. Österberg, Nanomaterials, 2018, 8, 1001 CrossRef PubMed.
- T. Zou, M. H. Sipponen and M. Österberg, Front. Chem., 2019, 7, 2300 Search PubMed.
- M. Farooq, T. Zou, G. Riviere, M. H. Sipponen and M. Österberg, Biomacromolecules, 2019, 20, 693–704 CrossRef CAS PubMed.
- M. H. Sipponen, M. Smyth, T. Leskinen, L.-S. Johansson and M. Österberg, Green Chem., 2017, 19, 5831–5840 RSC.
- C. Frangville, M. Rutkevičius, A. P. Richter, O. D. Velev, S. D. Stoyanov and V. N. Paunov, ChemPhysChem, 2012, 13, 4235–4243 CrossRef CAS PubMed.
- A. P. Richter, B. Bharti, H. B. Armstrong, J. S. Brown, D. Plemmons, V. N. Paunov, S. D. Stoyanov and O. D. Velev, Langmuir, 2016, 32, 6468–6477 CrossRef CAS PubMed.
- A. P. Richter, J. S. Brown, B. Bharti, A. Wang, S. Gangwal, K. Houck, E. A. Cohen Hubal, V. N. Paunov, S. D. Stoyanov and O. D. Velev, Nat. Nanotechnol., 2015, 10, 817–823 CrossRef CAS PubMed.
- W. Yang, M. Rallini, M. Natali, J. Kenny, P. Ma, W. Dong, L. Torre and D. Puglia, Mater. Des., 2019, 161, 55–63 CrossRef CAS.
- W. Yang, F. Dominici, E. Fortunati, J. M. Kenny and D. Puglia, Ind. Crops Prod., 2015, 77, 833–844 CrossRef CAS.
- Y. Zhou, Y. Qian, S. Wu, X. Zhong, J. Huang and X. Qiu, Holzforschung DOI:10.1515/hf-2019-0091.
- M. Ago, B. L. Tardy, L. Wang, J. Guo, A. Khakalo and O. J. Rojas, MRS Bull., 2017, 42, 371–378 CrossRef CAS.
- O. Cusola, S. Kivistö, S. Vierros, P. Batys, M. Ago, B. L. Tardy, L. G. Greca, M. B. Roncero, M. Sammalkorpi and O. J. Rojas, Langmuir, 2018, 34, 5759–5771 CrossRef CAS PubMed.
- P. K. Mishra and R. Wimmer, Ultrason. Sonochem., 2017, 35, 45–50 CrossRef CAS PubMed.
- I. A. Gilca, V. I. Popa and C. Crestini, Ultrason. Sonochem., 2015, 23, 369–375 CrossRef CAS PubMed.
- S. S. Nair, S. Sharma, Y. Pu, Q. Sun, S. Pan, J. Y. Zhu, Y. Deng and A. J. Ragauskas, ChemSusChem, 2014, 7, 3513–3520 CrossRef CAS PubMed.
- A. A. Myint, H. W. Lee, B. Seo, W. S. Son, J. Yoon, T. J. Yoon, H. J. Park, J. Yu, J. Yoon and Y. W. Lee, Green Chem., 2016, 18, 2129–2146 RSC.
- B. D. Mattos, L. G. Greca, B. L. Tardy, W. L. E. Magalhães and O. J. Rojas, Small, 2018, 14, 1801256 CrossRef PubMed.
- P. K. Mishra and A. Ekielski, Colloids Interfaces, 2019, 3, 52 CrossRef CAS.
- M. B. Agustin, P. A. Penttilä, M. Lahtinen and K. S. Mikkonen, ACS Sustainable Chem. Eng., 2019, 7, 19925–19934 CrossRef CAS.
- Y. Pang, S. Wang, X. Qiu, Y. Luo, H. Lou and J. Huang, J. Agric. Food Chem., 2017, 65, 11011–11019 CrossRef CAS PubMed.
- H. Li, Y. Deng, H. Wu, Y. Ren, X. Qiu, D. Zheng and C. Li, Holzforschung, 2016, 70, 725–731 CAS.
- H. Li, Y. Deng, B. Liu, Y. Ren, J. Liang, Y. Qian, X. Qiu, C. Li and D. Zheng, ACS Sustainable Chem. Eng., 2016, 4, 1946–1953 CrossRef CAS.
- M. Yang, W. Zhao, S. Singh, B. Simmons and G. Cheng, Nanoscale Adv., 2019, 1, 299–304 RSC.
- F. Xiong, Y. Han, S. Wang, G. Li, T. Qin, Y. Chen and F. Chu, Ind. Crops Prod., 2017, 100, 146–152 CrossRef CAS.
- J. Wang, Y. Qian, L. Li and X. Qiu, ChemSusChem, 2020 DOI:10.1002/cssc.201903132.
- Z.-H. Liu, N. Hao, S. Shinde, M. L. Olson, S. Bhagia, J. R. Dunlap, K. C. Kao, X. Kang, A. J. Ragauskas and J. S. Yuan, ACS Sustainable Chem. Eng., 2019, 7, 2634–2647 CrossRef CAS.
- M. H. Sipponen, A. Henn, P. Penttilä and M. Österberg, Chem. Eng. J., 2020, 393, 124711 CrossRef CAS.
- T. Leskinen, M. Smyth, Y. Xiao, K. Lintinen, M.-L. Mattinen, M. A. Kostiainen, P. Oinas and M. Österberg, Nord. Pulp Pap. Res. J., 2017, 32, 586–596 CAS.
- Z. Liu, R. Qie, W. Li, N. Hong, Y. Li, C. Li, R. Wang, Y. Shi, X. Guo and X. Jia, New J. Chem., 2017, 41, 3190–3195 RSC.
- Y. Deng, H. Zhao, Y. Qian, L. Lü, B. Wang and X. Qiu, Ind. Crops Prod., 2016, 87, 191–197 CrossRef CAS.
- Y. Chevalier and M. A. Bolzinger, Colloids Surf., A, 2013, 439, 23–34 CrossRef CAS.
- F. M. Ernsberger and W. G. France, Ind. Eng. Chem., 1945, 37, 598–600 CrossRef CAS.
- D. Liu and Y. Peng, Fuel, 2015, 142, 235–242 CrossRef CAS.
- D. Yang, X. Qiu, M. Zhou and H. Lou, Energy Convers. Manage., 2007, 48, 2433–2438 CrossRef CAS.
- D. Yang, H. Li, Y. Qin, R. Zhong, M. Bai and X. Q. Qiu, J. Dispersion Sci. Technol., 2015, 36, 532–539 CrossRef CAS.
- T. Li and S. Takkellapati, Biofuels, Bioprod. Biorefin., 2018, 12, 756–787 CrossRef CAS PubMed.
- M. K. R. Konduri and P. Fatehi, ACS Sustainable Chem. Eng., 2015, 3, 1172–1182 CrossRef CAS.
- X. Ouyang, L. Ke, X. Qiu, Y. Guo and Y. Pang, J. Dispersion Sci. Technol., 2009, 30, 1–6 CrossRef CAS.
- M. K. Konduri, F. Kong and P. Fatehi, Eur. Polym. J., 2015, 70, 371–383 CrossRef CAS.
- J. Chen, A. Eraghi Kazzaz, N. AlipoorMazandarani, Z. Hosseinpour Feizi and P. Fatehi, Molecules, 2018, 23, 868 CrossRef PubMed.
- L. Bai, L. G. Greca, W. Xiang, J. Lehtonen, S. Huan, R. W. N. Nugroho, B. L. Tardy and O. J. Rojas, Langmuir, 2019, 35, 571–588 CrossRef CAS PubMed.
- H. Fan and A. Striolo, Soft Matter, 2012, 8, 9533–9538 RSC.
- A. B. Pawar, M. Caggioni, R. Ergun, R. W. Hartel and P. T. Spicer, Soft Matter, 2011, 7, 7710–7716 RSC.
- Z. Wei, Y. Yang, R. Yang and C. Wang, Green Chem., 2012, 14, 3230–3236 RSC.
- Y. Yang, Z. Wei, C. Wang and Z. Tong, Chem. Commun., 2013, 49, 7144–7146 RSC.
- T. E. Nypelö, C. A. Carrillo and O. J. Rojas, Soft Matter, 2015, 11, 2046–2054 RSC.
- M. R. V. Bertolo, L. B. Brenelli de Paiva, V. M. Nascimento, C. A. Gandin, M. O. Neto, C. E. Driemeier and S. C. Rabelo, Ind. Crops Prod., 2019, 140, 111591 CrossRef CAS.
- L. Dai, Y. Li, F. Kong, K. Liu, C. Si and Y. Ni, ACS Sustainable Chem. Eng., 2019, 7, 13497–13504 CrossRef CAS.
- T. Zou, M. H. Sipponen and M. Österberg, Front. Chem., 2019, 7, 2300 Search PubMed.
- A. Sadeghpour, F. Pirolt and O. Glatter, Langmuir, 2013, 29, 6004–6012 CrossRef CAS PubMed.
- K. Lebdioua, A. Aimable, M. Cerbelaud, A. Videcoq and C. Peyratout, J. Colloid Interface Sci., 2018, 520, 127–133 CrossRef CAS PubMed.
- V. O. Ikem, A. Menner and A. Bismarck, Angew. Chem., Int. Ed., 2009, 48, 632 CrossRef CAS.
- B. P. Binks and S. O. Lumsdon, Langmuir, 2001, 17, 4540–4547 CrossRef CAS.
- T. Iwata, Angew. Chem., Int. Ed., 2015, 54, 3210–3215 CrossRef CAS PubMed.
- M. Tuomela, M. Vikman, A. Hatakka and M. Itävaara, Bioresour. Technol., 2000, 72, 169–183 CrossRef CAS.
- J. D. Nguyen, B. S. Matsuura and C. R. J. Stephenson, J. Am. Chem. Soc., 2014, 136, 1218–1221 CrossRef CAS PubMed.
- D. Ye, S. Li, X. Lu, X. Zhang and O. J. Rojas, ACS Sustainable Chem. Eng., 2016, 4, 5248–5257 CrossRef CAS.
- B. D. Mattos, B. L. Tardy, W. L. E. Magalhães and O. J. Rojas, J. Controlled Release, 2017, 262, 139–150 CrossRef CAS PubMed.
- S. C. Lee, S. H. Lee and K. Won, Biotechnol. Bioprocess Eng., 2019, 24, 258–263 CrossRef CAS.
- Y. Qian, X. Qiu and S. Zhu, Green Chem., 2015, 17, 320–324 RSC.
- Y. Qian, X. Qiu and S. Zhu, ACS Sustainable Chem. Eng., 2016, 4, 4029–4035 CrossRef CAS.
- X. Qiu, Y. Li, Y. Qian, J. Wang and S. Zhu, ACS Appl. Bio Mater., 2018, 1, 1276–1285 CrossRef CAS.
- H. Zhang, F. Chen, X. Liu and S. Fu, ACS Sustainable Chem. Eng., 2018, 6, 12532–12540 CrossRef CAS.
- W. Zhu, J. Lu and L. Dai, Part. Part. Syst. Charact., 2018, 35, 1–8 CAS.
- D. Kai, Y. K. Chua, L. Jiang, C. Owh, S. Y. Chan and X. J. Loh, RSC Adv., 2016, 6, 86420–86427 RSC.
- Y. Qian, X. Zhong, Y. Li and X. Qiu, Ind. Crops Prod., 2017, 101, 54–60 CrossRef CAS.
- B. Wang, D. Sun, H. M. Wang, T. Q. Yuan and R. C. Sun, ACS Sustainable Chem. Eng., 2019, 7, 2658–2666 CrossRef CAS.
- S. Tan, D. Liu, Y. Qian, J. Wang, J. Huang, C. Yi, X. Qiu and Y. Qin, Holzforschung, 2019, 73, 485–491 CAS.
- S. C. Lee, T. M. T. Tran, J. W. Choi and K. Won, Int. J. Biol. Macromol., 2019, 122, 549–554 CrossRef CAS PubMed.
- H. Zhang, X. Liu, S. Fu and Y. Chen, Int. J. Biol. Macromol., 2019, 133, 86–92 CrossRef CAS PubMed.
- J. M. Gutiérrez-Hernández, A. Escalante, R. N. Murillo-Vázquez, E. Delgado, F. J. González and G. Toríz, J. Photochem. Photobiol., B, 2016, 163, 156–161 CrossRef PubMed.
- Y. Li, D. Yang, S. Lu, X. Qiu, Y. Qian and P. Li, ACS Sustainable Chem. Eng., 2019, 7, 6234–6242 CrossRef CAS.
- M. Morsella, M. Giammatteo, L. Arrizza, L. Tonucci, M. Bressan and N. D'Alessandro, RSC Adv., 2015, 5, 57453–57461 RSC.
- J. Yu, L. Li, Y. Qian, H. Lou, D. Yang and X. Qiu, Ind. Eng. Chem. Res., 2018, 57, 15740–15748 CrossRef CAS.
- B. Danielson and R. Simonson, J. Adhes. Sci. Technol., 1998, 12, 923–939 CrossRef CAS.
- M. Olivares, J. A. Guzmán, A. Natho and A. Saavedra, Wood Sci. Technol., 1988, 22, 157–165 CrossRef CAS.
- S. Kalami, M. Arefmanesh, E. Master and M. Nejad, J. Appl. Polym. Sci., 2017, 134, 1–9 CrossRef.
- T. V. Lourençon, S. Alakurtti, T. Virtanen, A. S. Jääskeläinen, T. Liitiä, M. Hughes, W. L. E. Magalhães, G. I. B. Muniz and T. Tamminen, Holzforschung, 2020, 74, 175–183 Search PubMed.
- R. J. Li, J. Gutierrez, Y. L. Chung, C. W. Frank, S. L. Billington and E. S. Sattely, Green Chem., 2018, 20, 1459–1466 RSC.
- X. Sun, Q. Lang, H. Zhang, L. Cheng, Y. Zhang, G. Pan, X. Zhao, H. Yang, Y. Zhang, H. A. Santos and W. Cui, Adv. Funct. Mater., 2017, 27, 1604617 CrossRef.
- X. Zhao, Q. Lang, L. Yildirimer, Z. Y. Lin, W. Cui, N. Annabi, K. W. Ng, M. R. Dokmeci, A. M. Ghaemmaghami and A. Khademhosseini, Adv. Healthcare Mater., 2016, 5, 108–118 CrossRef CAS PubMed.
- K. L. Spiller, S. A. Maher and A. M. Lowman, Tissue Eng., Part B, 2011, 17, 281–299 CrossRef CAS PubMed.
- R. Lev and D. Seliktar, J. R. Soc., Interface, 2018, 15, 20170380 CrossRef PubMed.
- D. Gan, W. Xing, L. Jiang, J. Fang, C. Zhao, F. Ren, L. Fang, K. Wang and X. Lu, Nat. Commun., 2019, 10, 1–10 CrossRef CAS PubMed.
- S. Rose, A. Prevoteau, P. Elzière, D. Hourdet, A. Marcellan and L. Leibler, Nature, 2014, 505, 382–385 CrossRef CAS PubMed.
- S. E. Klein, A. Alzagameem, J. Rumpf, I. Korte, J. Kreyenschmidt and M. Schulze, Coatings, 2019, 9, 494 CrossRef CAS.
- S. Erakovic, A. Jankovic, G. C. P. Tsui, C. Y. Tang, V. Miskovic-Stankovic and T. Stevanovic, Int. J. Mol. Sci., 2014, 15, 12294–12322 CrossRef PubMed.
- A. Mathiazhagan and R. Joseph, Int. J. Chem. Eng. Appl., 2011, 2, 225–237 CAS.
- H. Wang, X. Qiu, W. Liu, F. Fu and D. Yang, Ind. Eng. Chem. Res., 2017, 56, 11133–11141 CrossRef CAS.
- O. U. Rahman, S. Shi, J. Ding, D. Wang, S. Ahmad and H. Yu, New J. Chem., 2018, 42, 3415–3425 RSC.
- A. Dastpak, K. Yliniemi, M. Cecilio, D. O. Monteiro, S. Höhn, S. Virtanen, M. Lundström and B. P. Wilson, Coatings, 2018, 8, 454 CrossRef.
- C. Hao, T. Liu, S. Zhang, L. Brown, R. Li, J. Xin, T. Zhong, L. Jiang and J. Zhang, ChemSusChem, 2019, 12, 1049–1058 CrossRef CAS PubMed.
- C. Zheng, D. Li and M. Ek, Nord. Pulp Pap. Res. J., 2019, 34, 96–106 CAS.
- E. Larrañeta, M. Imízcoz, J. X. Toh, N. J. Irwin, A. Ripolin, A. Perminova, J. Domínguez-Robles, A. Rodríguez and R. F. Donnelly, ACS Sustainable Chem. Eng., 2018, 6, 9037–9046 CrossRef PubMed.
- N. Forsman, A. Lozhechnikova, A. Khakalo, L.-S. Johansson, J. Vartiainen and M. Österberg, Carbohydr. Polym., 2017, 173, 392–402 CrossRef CAS PubMed.
- H. Yi, Y. Yang, X. Gu, J. Huang and C. Wang, J. Mater. Chem. A, 2015, 3, 13749–13757 RSC.
- F. Zikeli, V. Vinciguerra, A. D. Annibale, D. Capitani, M. Romagnoli and G. S. Mugnozza, Nanomaterials, 2019, 9, 1–18 CrossRef PubMed.
- A. Hambardzumyan, L. Foulon, B. Chabbert and V. Aguié-Béghin, Biomacromolecules, 2012, 13, 4081–4088 CrossRef CAS PubMed.
- L. Dumitrescu, D. Perniu and I. Manciulea, Solid State Phenom., 2009, 151, 139–144 CAS.
- S. V. Harb, B. M. Cerrutti, S. H. Pulcinelli, C. V. Santilli and P. Hammer, Surf. Coat. Technol., 2015, 275, 9–16 CrossRef CAS.
- D. S. Rickerby, Surf. Coat. Technol., 1988, 36, 541–557 CrossRef CAS.
- S. M. Notley and M. Norgren, Langmuir, 2006, 22, 11199–11204 CrossRef CAS PubMed.
- J. L. Rahikainen, R. Martin-Sampedro, H. Heikkinen, S. Rovio, K. Marjamaa, T. Tamminen, O. J. Rojas and K. Kruus, Bioresour. Technol., 2013, 133, 270–278 CrossRef CAS PubMed.
- T. Leskinen, J. Witos, J. J. Valle-Delgado, K. Lintinen, M. Kostiainen, S. K. Wiedmer, M. Österberg and M.-L. Mattinen, Biomacromolecules, 2017, 18, 2767–2776 CrossRef CAS PubMed.
- M. N. Collins, M. Nechifor, F. Tanasă, M. Zănoagă, A. McLoughlin, M. A. Stróżyk, M. Culebras and C.-A. Teacă, Int. J. Biol. Macromol., 2019, 131, 828–849 CrossRef CAS PubMed.
- M. A. S. Anwer, H. E. Naguib, A. Celzard and V. Fierro, Composites, Part B, 2015, 82, 92–99 CrossRef CAS.
- W. Yang, E. Fortunati, F. Dominici, J. M. Kenny and D. Puglia, Eur. Polym. J., 2015, 71, 126–139 CrossRef CAS.
- B. Del Saz-Orozco, M. Oliet, M. V. Alonso, E. Rojo and F. Rodríguez, Compos. Sci. Technol., 2012, 72, 667–674 CrossRef CAS.
- T. Ju, Z. Zhang, Y. Li, X. Miao and J. Ji, RSC Adv., 2019, 9, 24915–24921 RSC.
- Y. Liu, ACS Sustainable Chem. Eng., 2018, 6, 5524–5532 CrossRef CAS.
- D. Tian, J. Hu, J. Bao, R. P. Chandra, J. N. Saddler and C. Lu, Biotechnol. Biofuels, 2017, 10, 192 CrossRef PubMed.
- X. He, F. Luzi, X. Hao, W. Yang, L. Torre, Z. Xiao, Y. Xie and D. Puglia, Int. J. Biol. Macromol., 2019, 127, 665–676 CrossRef CAS PubMed.
- X. Wang, S. Ji, X. Wang, H. Bian, L. Lin, H. Dai and H. Xiao, J. Mater. Chem. C, 2019, 7, 14159–14169 RSC.
- H. Setälä, H.-L. Alakomi, A. Paananen, G. R. Szilvay, M. Kellock, M. Lievonen, V. Liljeström, E.-L. Hult, K. Lintinen, M. Österberg and M. Kostiainen, Cellulose, 2020, 27, 273–284 CrossRef.
- D. Piccinino, E. Capecchi, L. Botta, P. Bollella, R. Antiochia, M. Crucianelli and R. Saladino, Catal. Sci. Technol., 2019, 9, 4125–4134 RSC.
- B. L. Tardy, J. J. Richardson, J. Guo, J. Lehtonen, M. Ago and O. J. Rojas, Green Chem., 2018, 20, 1335–1344 RSC.
- F. Xiong, Y. Wu, G. Li, Y. Han and F. Chu, Ind. Eng. Chem. Res., 2018, 57, 1207–1212 CrossRef CAS.
- D. Yiamsawas, S. Beckers, H. Lu, K. Landfester and F. R. Wurm, ACS Biomater. Sci. Eng., 2017, 3, 2375–2383 CrossRef CAS.
- J. Köhnke, H. Rennhofer, C. Unterweger, N. Gierlinger, J. Keckes, C. Zollfrank, O. J. Rojas and W. Gindl-Altmutter, Nanomaterials, 2018, 8(12), 1055 CrossRef PubMed.
- W. Gindl-Altmutter, J. Köhnke, C. Unterweger, N. Gierlinger, J. Keckes, J. Zalesak and O. J. Rojas, Composites, Part A, 2019, 121, 175–179 CrossRef CAS.
- K. Lintinen, M. Latikka, M. H. Sipponen, R. H. A. Ras, M. Österberg and M. A. Kostiainen, RSC Adv., 2016, 6, 31790–31796 RSC.
- G. N. Rivière, A. Korpi, M. H. Sipponen, T. Zou, M. A. Kostiainen and M. Österberg, ACS Sustainable Chem. Eng., 2020, 8, 4167–4177 CrossRef.
- P. Figueiredo, K. Lintinen, J. T. Hirvonen, M. A. Kostiainen and H. A. Santos, Prog. Mater. Sci., 2018, 93, 233–269 CrossRef CAS.
- W. Gao and P. Fatehi, Can. J. Chem. Eng., 2019, 97, 2827 CrossRef CAS.
- Q. Lu, M. Zhu, Y. Zu, W. Liu, L. Yang, Y. Zhang, X. Zhao, X. Zhang, X. Zhang and W. Li, Food Chem., 2012, 135, 63–67 CrossRef CAS.
- M.-L. Mattinen, J. J. Valle-Delgado, T. Leskinen, T. Anttila, G. Riviere, M. Sipponen, A. Paananen, K. Lintinen, M. Kostiainen and M. Österberg, Enzyme Microb. Technol., 2018, 111, 48–56 CrossRef CAS PubMed.
- A. M. McNally, E. C. Moody and K. McNeill, Photochem. Photobiol. Sci., 2005, 4, 268 RSC.
- K. Chaochanchaikul, K. Jayaraman, V. Rosarpitak and N. Sombatsompop, BioResources, 2012, 7, 38–55 CAS.
- G. Janusz, A. Pawlik, J. Sulej, U. Świderska-Burek, A. Jarosz-Wilkołazka and A. Paszczyński, FEMS Microbiol. Rev., 2017, 41, 941–962 CrossRef CAS PubMed.
- M. A. Lara, A. J. Rodríguez-Malaver, O. J. Rojas, O. Holmquist, A. M. González, J. Bullón, N. Peñaloza and E. Araujoa, Int. Biodeterior. Biodegrad., 2003, 52, 167–173 CrossRef CAS.
- R. Martin-Sampedro, E. A. Capanema, I. Hoeger, J. C. Villar and O. J. Rojas, J. Agric. Food Chem., 2011, 59, 8761–8769 CrossRef CAS PubMed.
- C. Sánchez, Biotechnol. Adv., 2009, 27, 185–194 CrossRef PubMed.
- R. Datta, A. Kelkar, D. Baraniya, A. Molaei, A. Moulick, S. R. Meena and P. Formanek, Sustainability, 2017, 9 Search PubMed.
- M. H. Sipponen, O. J. O. J. Rojas, V. Pihlajaniemi, K. Lintinen and M. Österberg, ACS Sustainable Chem. Eng., 2017, 5, 1054–1061 CrossRef CAS.
- J. Guo, B. L. Tardy, A. J. Christofferson, Y. Dai, J. J. Richardson, W. Zhu, M. Hu, Y. Ju, J. Cui, R. R. Dagastine, I. Yarovsky and F. Caruso, Nat. Nanotechnol., 2016, 11, 1105 CrossRef CAS PubMed.
- M. Ragnar, C. T. Lindgren and N.-O. Nilvebrant, J. Wood Chem. Technol., 2000, 20, 277–305 CrossRef CAS.
- A. Henn and M.-L. Mattinen, World J. Microbiol. Biotechnol., 2019, 35, 125 CrossRef PubMed.
- C. Culbertson, T. Treasure, R. Venditti, H. Jameel and R. Gonzalez, Nord. Pulp Pap. Res. J., 2016, 31, 30–40 CAS.
- D. Koch, M. Paul, S. Beisl, A. Friedl and B. Mihalyi, J. Cleaner Prod., 2020, 245, 118760 CrossRef CAS.
- R. P. Bangalore Ashok, P. Oinas, K. Lintinen, G. Sarwar, M. A. Kostiainen and M. Österberg, Green Chem., 2018, 20, 4911–4919 RSC.
- R. P. Bangalore Ashok, Y. Xiao, K. Lintinen, P. Oinas, M. A. Kostiainen and M. Österberg, Colloids Surf., A, 2020, 587, 124228 CrossRef.
- M. H. Sipponen, C. Lapierre, V. Méchin and S. Baumberger, Bioresour. Technol., 2013, 133, 522–528 CrossRef CAS PubMed.
- Z. H. Liu, N. Hao, S. Shinde, Y. Pu, X. Kang, A. J. Ragauskas and J. S. Yuan, Green Chem., 2019, 21, 245–260 RSC.
| This journal is © The Royal Society of Chemistry 2020 |