 Open Access Article
Open Access ArticleEndocrine activities of phthalate alternatives; assessing the safety profile of furan dicarboxylic acid esters using a panel of human cell based reporter gene assays†
Barbara M. A.
van Vugt-Lussenburg
 *a,
Daan S.
van Es
*a,
Daan S.
van Es
 *b,
Matthijs
Naderman
a,
Jerome
le Notre
b,
Frits van der
Klis
b,
Abraham
Brouwer
ac and
Bart
van der Burg
b
*b,
Matthijs
Naderman
a,
Jerome
le Notre
b,
Frits van der
Klis
b,
Abraham
Brouwer
ac and
Bart
van der Burg
b
aBioDetection Systems bv, Science Park 406, 1098XH Amsterdam, The Netherlands
bWageningen Food and Biobased Research (WFBR), Bornse Weilanden 9, 6708WG, Wageningen, The Netherlands. E-mail: daan.vanes@wur.nl
cVU University, Faculty of Sciences, Department of Animal Ecology, De Boelelaan 1085, 1081HV Amsterdam, The Netherlands
First published on 27th February 2020
Abstract
FDCA esters are highly relevant biobased alternatives for currently used benzene dicarboxylic acid esters. Despite all the developments on 2,5-FDCA applications, to the best of our knowledge thus far no toxicological data were available for 2,5-FDCA esters. In the present study we aimed to fill this gap, by using an in vitro reporter gene assay approach to compare the activity profile of commonly used phthalates to that of their furan-based counterparts. The assay selection was aimed at the detection of endocrine activity, since several phthalates are heavily scrutinised for their endocrine disrupting properties. However, to avoid missing other relevant toxicological endpoints, several assays able to detect various forms of cellular stress were also included in the panel. The results showed that the (ortho)benzene dicarboxylic acid esters were predominantly active on several of the endocrine assays. In comparison, six of the seven furan dicarboxylic acid based diesters tested here showed no activity in any of the 13 assays used. Only the isobutyl derivative DIBF showed moderate estrogenic activity on one assay, compared to much more pronounced activities on four assays for the ortho-phthalate analogue. Overall, the results presented in this paper are a strong indication that 2,5-FDCA based diesters in general are not only technically viable alternatives to phthalates, but also offer significant toxicological benefits, which supports a non-regrettable substitution.
Introduction
Global climate change and the depletion of finite feedstocks are strong drivers for the developing transition from a mainly fossil fuels and feedstocks based linear economy to a more sustainable circular one. As a consequence, the industrial production of chemicals and materials will increasingly be based on renewable energy and feedstocks such as wind- and solar energy and biomass. True circular end-of life options for materials require the possibility to depolymerise the material to its constituent chemical building blocks, followed by purification, and rebuilding the materials to the required specifications. Hence polyesters, which can be efficiently depolymerised by hydrolysis, are materials with a very high circular potential.1–3 Furthermore, by changing to biobased monomers, increasing greenhouse gas emissions and the use of finite fossil resources can be eliminated altogether.Currently, the dominant industrial polyester is polyethylene terephthalate (PET), well known for its application in e.g. beverage bottles, textile fibres, etc. While the diol component ethylene glycol is already produced from renewable biomass (on small industrial scale),4 the diacid component, i.e. biobased terephthalic acid (TA), is still under development.4–6 Given the enormous production volume of TA (>80 Mton per a),7,8 which is expected to continue to grow in the coming decades, biobased TA, or analogues, should be based on abundantly available biobased feedstocks, which do not compete with primary food production. Glucose, which can be obtained from polysaccharides like starch and cellulose, is therefore the most promising feedstock for the industrial production of biobased TA. Despite continuing efforts, the efficient conversion of highly functionalised sugars to TA remains a challenge.4 An alternative approach is the development of a functional analogue of TA, the biobased furan-2,5-dicarboxylic acid (or 2,5-FDCA).9 This diacid, which was already reported by Fittig in 1876![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 can be obtained from sugars with much higher efficiency than TA. While functionally comparable to TA, 2,5-FDCA shows different chemical and physical behaviour in various applications. When used in polyesters such as the PET analogue PEF (polyethylene-2,5-furanoate) it was for instance found that various properties, such as gas barrier properties, were significantly improved by using 2,5-FDCA as diacid.11–13 Many investigators have already shown that 2,5-FDCA based polyesters are functionally comparable to their TA based analogues, in applications such as films, foils, bottles and fibres.14,15 Other polymer applications of 2,5-FDCA include polyamides and unsaturated polyesters resins.15,16 As the dimethyl ester of terephthalic acid (DMT) is an important building block and hence a high-volume chemical substance, it is of particular interest to compare its toxicity profile with that of its (relatively) new FDCA based counterpart.
10 can be obtained from sugars with much higher efficiency than TA. While functionally comparable to TA, 2,5-FDCA shows different chemical and physical behaviour in various applications. When used in polyesters such as the PET analogue PEF (polyethylene-2,5-furanoate) it was for instance found that various properties, such as gas barrier properties, were significantly improved by using 2,5-FDCA as diacid.11–13 Many investigators have already shown that 2,5-FDCA based polyesters are functionally comparable to their TA based analogues, in applications such as films, foils, bottles and fibres.14,15 Other polymer applications of 2,5-FDCA include polyamides and unsaturated polyesters resins.15,16 As the dimethyl ester of terephthalic acid (DMT) is an important building block and hence a high-volume chemical substance, it is of particular interest to compare its toxicity profile with that of its (relatively) new FDCA based counterpart.
In analogy, also medium to long chain diesters of TA and phthalic acid (PA) are industrially important high volume chemicals, which mainly find use as plasticisers for PVC.17 Especially phthalate esters are subject to continuous scrutiny related to (potential) adverse health effects.18–20 However, complete substitution of disputed phthalates is challenging due to both economic as well as performance issues. Hence the development of cost effective, biobased, plasticisers that offer environmental, health and performance benefits compared to existing phthalates is highly desirable. 2,5-FDCA esters can potentially offer such benefits, as was already shown for their technical performance as plasticiser in PVC.21–23 As a next step, more insight is required into the potential health effects of these substances.
Given these developments, it can be expected that industrial 2,5-FDCA production will take off in the near future, and that the number of applications will grow, especially at first in applications where 2,5-FDCA has a clear performance benefit. This could include a positive ecological and toxicological profile. The replacement of disputed phthalate plasticisers by 2,5-FDCA esters is an interesting opportunity.
Despite all the developments on 2,5-FDCA applications, to the best of our knowledge thus far no toxicological data were available for 2,5-FDCA esters. An EFSA report on 2,5-FDCA, however, is available; the document reports 2,5-FDCA as ‘negative’ in in vitro genotoxicity tests, and a 90-day oral toxicity study in rats resulted in a NOAEL of 300 mg kg−1 day−1. Furthermore, no concern for accumulation in man was reported.24 In order to come to a non-regrettable long-term substitution of e.g. TA by 2,5-FDCA it is of utmost importance to get an early insight into the toxicity of these potentially high-volume chemicals.
This prompted us to study a broad series of FDCA esters (short to long chain) by means of in vitro human reporter gene assays. These in vitro human cell-based CALUX® reporter gene assays have been designed to detect interaction of a substance with a specific nuclear receptor or cell signalling pathway, rather than the overall effect of a substance on a complex biological system. The advantage of this approach is that the results are straightforward to interpret, since complicating factors such as metabolism, tissue distribution or receptor crosstalk do not play a role in these reporter gene assays. The panel generates an activity profile that can be used to provide clues on the possible mode-of-action of a substance, to visualise trends for structural analogues, and as a starting point for further investigation.25–32 The substances were analysed on a reporter gene assay panel covering a broad range of endpoints, including nuclear receptor hormone interaction, DNA damage, oxidative stress and cellular stress pathways. This panel of assays has been shown to be predictive for major human toxicological endpoints, including endocrine disruption, reproductive toxicity, genotoxicity and acute toxicity.25–32
Here we report on the in vitro toxicological effects of geometry and substitution of a range of isomeric benzene- and furan dicarboxylic acids and esters. In vitro analysis of the substances was performed on a panel of effect-based CALUX reporter gene assays.
Results and discussion
Substance selection
A list of 27 relevant substances (phthalates, non-phthalate plasticizers, plastic additives, residual monomers and (potential) degradation products) was selected to establish an in vitro reporter gene assay profile as ‘point of departure’. Additionally, furans (dimethyl- to diisodecyl diesters) were selected as test substances, as well as the related free acids. The three benzene dicarboxylic acid isomers tested in this study are all produced on (large) industrial scale, for application in polyesters and polyamides (IPA, TA), as well as plasticisers (PA) and resins (PA). Exposure to these substances can be the result of occupational exposure during production or processing, by leaching/migration from products, or by (bio)degradation or metabolism of e.g. plasticisers or polyesters. Of the three FDCA isomers tested in this study, only the 2,5-isomer is expected to be commercialised on short to medium term. The other two isomers are included to study the effects of positional isomerism. All substances and their abbreviations are listed in ESI Table 1.† The synthesis methods of the substances (when applicable) are described in the ESI.†Assay selection
The phenolic reference substances as well as the benzene dicarboxylic acids analysed in the current study have been previously described as endocrine active substances in vivo.33–39 In several in vitro studies they have been identified as androgen receptor antagonists and/or estrogen receptor agonists.40–46 Therefore, CALUX assays were selected which cover the nuclear hormone receptor endpoints for estrogen agonism (ERα) and androgen antagonism (anti-AR). The OECD guidance document on standardised test guidelines for evaluating chemicals for endocrine disruption,47 additionally mentions thyroid hormone interference as a possible mode-of-action of endocrine disrupting chemicals. Therefore, a CALUX assay assessing thyroid receptor antagonism (anti-TRβ) was also included. Additionally, two more assays were added that are often targeted by endocrine active substances: progesterone antagonism (anti-PR) and glucocorticoid antagonism (anti-GR). These assays have been well validated, and several have been, or are currently in the process of being included in OECD test guidelines.48–52 Additionally, for the benzene dicarboxylic acids, effects on peroxisome proliferation and lipid homeostasis have been described.53–55 To be able to detect such effects, two peroxisome proliferator assays, PPARα and PPARγ CALUX,56,57 involved in lipid homeostasis, were included in the assay selection. Additionally, a cytotoxicity assay was included in the panel.32 This assay detects cell death, but can also be used to identify nonspecific effects of the substances, for example on cell proliferation or luciferase stability.In addition to these key assays directed specifically to the detection of endocrine active substances, several more general assays to assess toxicity of substances were included. The aryl hydrocarbon receptor (AhR) CALUX,58 for example, assesses toxicity of dioxin-like substances and PAHs. AP-1 CALUX detects substances which interfere with cell cycle control, while ESRE CALUX measures an early stage of unfolded protein response as a result of cellular stress. Activity on the Nrf2 CALUX is indicative for oxidative stress; several phenolic substances have been known to undergo redox cycling, which would trigger this particular assay.59 Finally, activation of p53 GENTOX CALUX is indicative for DNA damage.29,32
This resulted in a panel of thirteen CALUX assays (Table 1). To be confident that this selection would enable the detection all effects elicited by the benzene dicarboxylic acids without missing other important endpoints, these substances were also analysed on the twelve non-selected CALUX assays available in our research facility (anti-ERα, AR, GR, PR, TRβ, RAR, LXR, PAH, Hif1α, TCF, NFκB, p21). ESI Table 2† shows that none of these assays were activated by any of the test substances, which confirms that the current selection of assays was adequate for the purpose of this study.
| Entry number | CAS | Substance | Cytotox | ERα | AR-anti | PR-anti | GR-anti | TRβ-anti | PPARα | PPARγ | AhR | AP-1 | ESRE | Nrf2 | p53 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Reference substance | −6.6 | −12.2 | −7.7 | −11.0 | −9.5 | −6.9 | −9.7 | −7.7 | −12.3 | −9.5 | −7.5 | −5.4 | −9.0 | |
| 2 | 620-92-8 | Bisphenol F | — | −6.6 | −5.4 | −4.8 | — | NA | — | — | — | — | −4.7 | — | −3.3 |
| 3 | 80-05-7 | Bisphenol A | −4.0 | −7.7 | −6.8 | −5.5 | −4.5 | −4.2 | — | — | — | — | −4.3 | — | NA |
| 4 | 140-66-9 | 4-tert-Octylphenol | −5.5 | −7.2 | −6.0 | −6.1 | — | — | — | — | -— | — | — | −4.5 | — |
| 5 | 104-40-5 | 4-Nonylphenol | −4.9 | −5.1 | −5.8 | −5.5 | — | — | — | — | — | — | −4.6 | — | NA |
| 6 | 4376-18-5 | Monomethyl phthalate | — | — | — | — | — | NA | — | — | — | — | — | — | NA |
| 7 | 131-70-4 | Monobutyl phthalate | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 8 | 2528-16-7 | Monobenzyl phthalate | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 9 | 4376-20-9 | Mono(2-ethylhexyl) phtalate | −3.5 | — | — | — | — | — | −5.5 | −4.7 | — | — | — | — | −3.2 |
| 10 | 131-11-3 | Dimethyl phthalate | — | — | −4.7 | −3.6 | — | — | — | — | — | — | — | — | — |
| 11 | 1459-93-4 | Dimethyl isophthalate | — | −3.3 | −3.1 | — | — | — | — | — | — | — | — | — | — |
| 12 | 120-61-6 | Dimethyl terephthalate | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 13 | 84-66-2 | Diethyl phthalate | −3.5 | −4.0 | −5.0 | −4.3 | — | — | — | — | — | — | — | — | — |
| 14 | 636-53-3 | Diethyl isophthalate | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 15 | 636-09-9 | Diethyl terephthalate | — | — | −4.0 | — | — | — | — | — | — | — | — | — | — |
| 16 | 84-74-2 | Dibutyl phthalate | — | −5.2 | −4.7 | −4.5 | — | — | — | — | — | — | — | — | — |
| 17 | 84-69-5 | Diisobutyl phthalate | −4.5 | −5.3 | −5.0 | −5.0 | — | — | — | — | — | — | — | — | — |
| 18 | 84-75-3 | Di(n-hexyl) phthalate | −3.5 | −5.0 | −5.0 | −5.5 | −4.5 | NA | — | — | −4.0 | −4.2 | — | — | NA |
| 19 | 84-61-7 | Dicyclohexyl phthalate | −4.5 | −5.3 | — | −5.4 | −5.1 | NA | — | — | NA | NA | — | — | — |
| 20 | 85-68-7 | Butylbenzyl phthalate | −3.9 | −6.3 | −5.6 | −5.5 | — | — | — | −4.5 | −3.7 | — | — | — | — |
| 21 | 117-81-7 | Di(2-ethylhexyl) phthalate (synthesized) | — | −3.9 | — | — | — | — | — | — | — | — | — | — | — |
| 22 | 117-81-7 | Di(2-ethylhexyl) phthalate (commercial) | — | −4.0 | — | — | — | — | — | — | — | — | — | — | — |
| 23 | 137-89-3 | Di(2-ethylhexyl) isophthalate | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 24 | 6422-86-2 | Di(2-ethylhexyl) terephthalate | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 25 | 117-84-0 | Dioctyl phthalate | — | — | — | — | — | NA | — | — | — | — | — | — | — |
| 26 | 28553-12-0 | Diisononyl phthalate | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 27 | 26761-40-0 | Diisodecyl phthalate | — | — | — | — | — | — | — | — | — | — | — | — | — |
CALUX activity profiles of phenolic substances
Table 1 shows the results for the 13 selected CALUX assays. The values in the table represent lowest observed effect concentrations (LOECs) in LogM. In the absence of internationally established threshold values, substances were arbitrarily considered ‘positive’ if LOEC < 1E−5 M as reported in a previous study on reproductive toxicity using the same CALUX assays.28 Since the aim of the current study was to compare the activity profile of phthalate- vs.furan-based substances rather than to classify substances as ‘positive’ or ‘negative’, this threshold was not applied in the current study, but rather a comparative approach was taken.For the phenolic substances in the list, most of the activity observed was, as expected, on the nuclear hormone receptor assays. The bisphenols (entries 2–3) and alkyl phenols (entries 4–5) were mainly active as estrogen receptor agonists, and androgen- and progesterone receptor antagonists, with a potency in the (sub-) micromolar range. This corresponds with their known activity as endocrine active substances.44,46 4-tert-Octylphenol additionally activated the Nrf2 CALUX, a pathway indicative of oxidative stress. Indeed, oxidative stress has been reported for this substance.59
CALUX activity profiles of benzene dicarboxylic acids
A wide range of benzene dicarboxylic acid derivatives was tested in this study. In Table 1 substances were ranked by degree of substitution (entries 6–9: mono; entries 10–27: di), and by increasing chain length. Most of the reporter gene activity is observed on the nuclear hormone receptor related assays, for the di-substituted phthalates of medium chain length (C4–C6).It is known that in vivo, di-substituted phthalates are readily hydrolysed to their mono-esters.60 In rodent in vivo experiments, exposure to the monoesters results in similar adverse effects as exposure to the diesters.36,61 As a result, the mono-substituted phthalates are thought to be the endocrine active metabolites of the corresponding diesters, displaying endocrine disrupting activity.34,36,41,42 Therefore, in the current study, we determined the in vitro activity profile of four monoesters of (ortho)phthalic acid (entries 6–9) as well as 17 diesters (entries 10–27). Of the four monoesters tested only mono(2-ethylhexyl) phthalate (MEHP) was active (entry 9), yet not on the endocrine assays but on the peroxisome proliferator-assays PPARα and PPARγ, which is in agreement with previous reports.53–55,62 It has been observed before that the phthalate monoesters appear to be able to exert effects in vivo such as reproductive tract anomalies and decreased testosterone synthesis, which are indicative of an anti-androgenic mode of action,35,36,61 but that the phthalates fail to show anti-androgenic activity in in vitro androgen receptor interaction assays,38,42,63,64 suggesting an indirect, non-receptor mediated mode of action.
While the short-chain diesters dimethyl- and diethyl phthalate showed no or low estrogenic- and anti-androgenic activity, the medium chain diesters (dibutyl (entry 16), diisobutyl (entry 17), di(n-hexyl) (entry 18), dicyclohexyl (entry 19) and butylbenzyl (entry 20)) showed estrogenic, anti-androgenic and anti-progestagenic activity with LOECs in the micromolar range. Butylbenzyl phthalate displayed the highest potency for all three endocrine assays. Further increasing the chain length to di(2-ethylhexyl) phthalate (entry 21/22) resulted in a marked decrease in estrogenic potency (10–100 fold), and no detectable anti-androgenic or anti-progestagenic activity. The long chain phthalates dioctyl, diisononyl and diisodecyl phthalate (entries 25–27) showed no activity at all on any of the assays.
These results are in agreement with what is known about these substances;34,41,42,65–68 high molecular weight phthalates such as diisononyl- and diisodecyl phthalate are included in REACH as ‘not toxic for human health’.69,70 The lower molecular weight phthalates dibutyl-, diisobutyl-, butylbenzyl- and di(2-ethylhexyl) phthalate on the other hand, are classified in REACH as ‘very dangerous’, in Category 1B “substances regarded as toxic to reproduction”. The two shortest phthalates in this test, the methyl and ethyl diesters, do show some adverse effects in vivo, but not the reproductive tract developmental abnormalities that have been described for the C4 to C6 chain diesters.66,68 However, in concordance with our results, estrogen receptor agonism and androgen receptor antagonism has been observed in vitro for diethyl phthalate.40,42,66,68
The most prominent representative of the C2–C6 diesters is butylbenzyl phthalate (BBP); in the current study this substance is able to act as an agonist on the estrogen receptor and as an antagonist on the androgen- and progesterone receptor with higher potency than any of the other phthalates tested. This correlates well with other studies; its endocrine disrupting activity has resulted in an industrial phase out of BBP.71,72
Although di(2-ethylhexyl) phthalate (DEHP) is highly scrutinised for its endocrine disrupting properties, two preparations of this substance (synthesized in-house (entry 21) versus commercially available (entry 22)) showed very little activity, apart from estrogen receptor agonism at relatively high LOEC (1 mM). Also the corresponding monoester (entry 9) and presumed active metabolite in vivo, did not show in vitro estrogen- or androgen receptor activation or antagonism. Although in vivo studies in rat, as well as epidemiological studies in men, suggest that DEHP/MEHP exposure results in reproductive tract anomalies and reduced sperm motility indicative for an anti-androgenic mode-of-action,38,61 other in vitro studies have also shown a lack of estrogen- and androgen receptor interaction.41,42,63,68 It has been suggested that these substances exert their effects via other mechanisms, for example by influencing steroidogenesis through CYP19 inhibition, rather than by direct interaction with the androgen receptor.61,68 This is supported by results of two in vitro assays, the H295R steroidogenesis assay and human testis explants, where both MEHP and DEHP were shown to significantly inhibit testosterone synthesis.61,73 For the isobutyl-, butyl- and butylbenzyl diesters, in vitro estrogen receptor transactivation has been reported previously, as well as in vivo endocrine effects,34,36,41,42,66,68 which is in line with our findings. Nonetheless, in the ECHA support document for BBP,74 its adverse effects are considered to be primarily related to effects on steroidogenesis, rather than through direct interaction with steroid hormone receptors.
Trends in positional isomerism and ester chain length
For the methyl-, ethyl- and 2-ethylhexyldiesters, all three phthalic, isophthalic and terephthalic isomers were tested (all commercially available materials). For the dimethyl esters moderate effects were observed for DMP and DMIP, while DMT did not give any response (Table 1, entries 10–12). Increasing the alcohol chain length to C2 results in a dramatic increase in activity of the ortho-phthalate (DEP) on several endocrine assays, while both other isomers are virtually inactive (Table 1, entries 13–15). For the 2-ethylhexyl derivatives, only the ortho-phthalate is active, while again no activity is seen in case of the other isomers (Table 1, entries 21–24). The variation in positional isomerism conclusively shows that ortho-phthalates have the most pronounced endocrine activity. Whether this can be attributed to steric or electronic effects is not clear, and while highly interesting falls beyond the scope of the present study.The data in Table 1 clearly show a dependency of the endocrine activity of the ortho-phthalates on the ester chain length, which appears to have an optimum at C4-C6. Further increasing the chain length to C8 (DEHP entries 21/22 and DOP entry 25) results in a complete lack of activity. This could be due to the dramatic decrease in aqueous solubility of the longer chain phthalates (10−9 M for n-octyl versus 10−5M for n-butyl, see also ESI Table 4†), resulting in a significantly reduced bioavailability.75–78 Furthermore, Thomsen et al.79 reported an inverse relationship between phthalate solubility and temperature; e.g. for DBP solubility drops from 14.6 mg L−1 at 25 °C to 5.5 mg L−1 at 35 °C. Since our cell assays are performed at an incubation temperature of 37 °C, it is reasonable to assume that the actual solubilities for the phthalate esters are even lower than those reported for the 20–25 °C range. Hence, for the long chain phthalates (≥C8) (lack of) bioavailability could simply be the cause for observed lack of activity. A similar lack of bioactivity for long chain phthalates (≥C8) was reported by Ejlertsson et al. in the degradation of phthalic acid esters under methanogenic conditions.80
Another observation that can be made from the results in Table 1 is that there is no significant effect of ester chain branching on endocrine activity (compare DBP (entry 16) and DIBP (entry 17), or DHP (entry 18) and DCHP (entry 19), respectively). Note that in general chain branching leads to a slightly higher aqueous solubility; e.g. approximately two times higher for DIBP compared to DBP (see also ESI Table 4†).78
Overall it can be concluded that solubility, and hence bioavailability is probably the most important factor determining the bioactivity of the phthalate esters.
Physico-chemical properties of furan- vs. benzene dicarboxylic acids
There are significant physico-chemical differences between terephthalic and phthalic acid on the one hand and 2,5-FDCA on the other. For instance, as can be seen from Chart 1 and Table 2, due to the different geometries of the benzene vs. furan nucleus both the angles and the interatomic distances between carboxylic acid groups differ significantly for the respective analogues. While TA is linear (angle 180°), the angled structure of 2,5-FDCA is closer to that of isophthalic acid (IPA). In fact, the highly unsymmetrical 2,4-FDCA is the most linear furan derivative. The differences between PA and 2,5-FDCA are even larger than for TA and the latter. A more suitable structural comparison would hence be based on PA vs. 3,4-FDCA. Another difference between the analogous benzene and furan dicarboxylic acid derivatives is the higher polarity of the latter due to different symmetries, the presence of the oxygen, and the more pronounced diene character in the furan ring. These differences are also related to the significantly higher acidity of the FDCA isomers, as is clear from their pKa values. Interestingly, while 2,5-FDCA more closely resembles IPA with respect to geometry, the aqueous solubility is closer to (or actually surpasses) that of PA.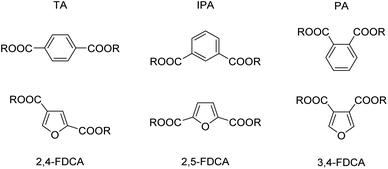 | ||
| Chart 1 Structures of benzene-, and related furan dicarboxylic acid (FDCA) isomers used in this study; TA = terephthalic acid, IPA = isophthalic acid, PA = phthalic acid. | ||
| Substance | Anglea (°) | Db (Å) | pKa1c | pKa2c | Solubilityd |
|---|---|---|---|---|---|
| a Angle between carboxylic acid groups.82,85 b Distance between carboxylic acid groups.82 c Data from ref. 83. d Solubility under ambient conditions in mg ml−1 (data from https://pubchem.ncbi.nlm.nih.gov/). e NR: Not reported. | |||||
| TA | 180 | 5.73 | 3.51 | 4.82 | 0.015 |
| 2,4-FDCA | 150 | 5.08 | 2.63 | 3.77 | NRe |
| IPA | 120 | 4.40 | 3.46 | 4.46 | 0.12 |
| 2,5-FDCA | 129 | 4.83 | 2.60 | 3.55 | 1 |
| PA | 60 | 2.67 | 2.98 | 5.28 | 0.7 |
| 3,4-FDCA | 83 | 3.37 | 2.55 | 7.23 | NRe |
Hence, FDCA's are in general more polar than their benzene counterparts. Based on these differences, we expect different effects in the interactions with human nuclear receptors and cell signalling pathways.
CALUX activity profiles of furan- vs. benzene dicarboxylic acids
After having generated an activity profile for benzene dicarboxylic acid esters in the CALUX assays, we switched our focus to the analogous furan dicarboxylic acid esters, which are potential biobased alternatives to the former.Most of the diesters that are the focus of this study are prepared from the corresponding diacids. Furthermore, under environmental and in vivo conditions it can be expected that all diesters will (eventually) undergo hydrolysis to the monoesters first, and subsequently to the diacids.78,81,82 Hence, in order to exclude any toxicological effects of the parent diacids themselves, they were analysed on the CALUX assay panel.
Interestingly, none of the free acids used in this study showed any effect on the assays used (data not shown, see ESI Table 3†). This could imply that the free acids are not active in the cells. However, lack of activity could also be due to inability to enter the cells. Under physiological conditions, i.e. pH 7.2, all of the diacids used will be deprotonated given their pKa values (see Table 2), which could inhibit uptake in the cells, explaining the lack of observed effect.83,84
As discussed previously, while the dimethyl esters of phthalic acid (DMP) and isophthalic acid (DMIP) showed activity, no effects were found for the terephthalic acid (DMT) isomer. In contrast, none of the furan derivatives tested were active on any of the assays (Table 3, entries 28–33). The 2,5-FDCA and 3,4-FDCA dimethyl esters were commercial samples that were subsequently purified, while the 2,4-FDCA derivative was prepared and purified in our labs according to a previously published procedure (see ESI†).86
| Entry number | CAS | Substance | Abbreviation | Cytotox | ERα | AR-anti | PR-anti | GR-anti | TRβ-anti | PPARα | PPARγ | AhR | AP-1 | ESRE | Nrf2 | p53 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 28 | 131-11-3 | Dimethyl phthtalate | DMP | — | — | −4.7 | −3.6 | — | — | — | — | — | — | — | — | — |
| 29 | 1459-93-4 | Dimethyl isophthalate | DMIP | — | −3.3 | −3.1 | — | — | — | — | — | — | — | — | — | — |
| 30 | 120-61-6 | Dimethyl terephthalate | DMT | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 31 | 4282-33-1 | Dimethyl-3,4-furandicarboxylate | DM-3,4-FDCA | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 32 | 4282-32-0 | Dimethyl-2,5-furandicarboxylate | DM-2,5-FDCA | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 33 | 1710-13-0 | Dimethyl-2,4-furandicarboxylate | DM-2,4-FDCA | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 34 | 84-66-2 | Diethyl phthalate | DEP | −3.5 | −4.0 | −5.0 | −4.3 | — | — | — | — | — | — | — | — | — |
| 35 | 636-53-3 | Diethyl isophthalate | DEIP | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 36 | 636-09-9 | Diethyl terephthalate | DET | — | — | -4.0 | — | — | — | — | — | — | — | — | — | — |
| 37 | 53662-83-2 | Diethyl-2,5-furandicarboxylate | DEF | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 38 | 84-69-5 | Diisobutyl phthalate | DIBP | −4.5 | −5.3 | −5.0 | −5.0 | — | — | — | — | — | — | — | — | — |
| 39 | N/A | Diisobutyl-2,5-furandicarboxylate | DIBF | — | −4.3 | — | — | — | — | — | — | — | — | — | — | — |
| 40 | 117-81-7 | Di(2-ethylhexyl) phthalate | DEHP (synthesized) | − | −3.9 | — | — | — | — | — | — | — | — | — | — | — |
| 41 | 117-81-7 | Di(2-ethylhexyl) phthalate | DEHP (commercial) | — | -4.0 | — | — | — | — | — | — | — | — | — | — | — |
| 42 | 137-89-3 | Di(2-ethylhexyl) isophthalate | DEHIP | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 43 | 6422-86-2 | Di(2-ethylhexyl) terephthalate | DEHT | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 44 | 158099-01-5 | Di(2-ethylhexyl)-2,5-furandicarboxylate | DEHF (method 1) | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 45 | 158099-01-5 | Di(2-ethylhexyl)-2,5-furandicarboxylate | DEHF (method 2) | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 46 | 26761-40-0 | Diisodecyl phthalate | DIDP | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 47 | N/A | Diisodecylfuran-2,5-dicarboxylate | DIDF | — | — | — | — | — | — | — | — | — | — | — | — | — |
For the furan dicarboxylic acid esters no effect of isomerism, and hence of symmetry or dipole moment, is observed under the conditions tested in our assays.
A possible reason why the FDCA methyl esters show no effect while their benzene analogues do, could lie in a different susceptibility to (enzymatic) hydrolysis of these esters. Oae et al. reported significant differences between the rate of hydrolysis of dimethyl 2,5-FDCA and 3,4-FDCA versus DMT (17 and 1.3 times higher respectively), which could result in a relatively fast hydrolysis of the furan derivatives, successively to the respective mono esters and diacids.87 The latter were found to be completely inactive (vide supra).
In order to investigate the effect of ester chain length, next the diethyl esters of the phthalate family were compared with diethyl 2,5-FDCA (purified commercial material). As discussed in the previous chapter, diethyl phthalate and diethyl terephthalate showed moderate to low activity on the endocrine assays, while the isophthalic acid analogue was inactive. Once again, no activity was found for the analogous 2,5-FDCA derivative (Table 3, entries 34–37).
In the current study, we observed a significant increase in both the potency and the number of active assays for the C4 phthalate derivatives (Table 1, entries 16–17). To investigate if 2,5-FDCA diesters with similar chain length likewise show an increased activity compared to their diethyl- and dimethyl counterparts, the CALUX profile of diisobutyl phthalate was compared to the profile of diisobutyl-2,5-FDCA (Chart 2). While diisobutyl phthalate (DIBP) was the second most active phthalate derivative tested, showing micromolar-range activity as an estrogen, anti-androgen and anti-progestin, in contrast diisobutyl-2,5-FDCA (DIBF) acted as a weak estrogen only (Table 3, entries 38–39). While DIBF is the only active furan derivative tested thus far, the observed effects are not as pronounced as those observed with DIBP.
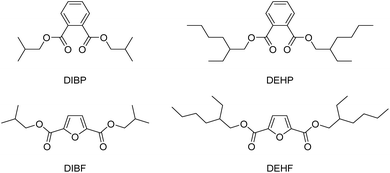 | ||
| Chart 2 Chemical structures of isobutyl and 2-ethylhexyl diester of PA (DIBP and DEHP) and 2,5-FDCA (DIBF and DEHF). | ||
Further increasing the alcohol chain length from isobutyl to 2-ethylhexyl (2EH), i.e. an extension of the isobutyl motif (Chart 2), results in a significant reduction of effect in our assays, as is apparent from the weak estrogenic activity only in the case of DEHP, with very similar LOEC values for the synthesized and the commercial preparation (Table 3, entries 40 and 41). The absence of any response of the iso- and terephthalate isomers in our assay (as opposed to the ortho-phthalate) shows that positional isomerism in the phthalate family has a significant impact (Table 3, entries 40–43). The furan-based analogue of DEHP, di(2-ethylhexyl)-2,5-FDCA, prepared for this study using two different methods, did not activate any of the CALUX assays (entries 44 and 45).
Further increasing the chain length of the ester group to isodecyl results in complete absence of effects, both for the phthalate (DIDP) and the FDCA diester (DIDF) (Table 3, entries 46 and 47). This observation is in line with the industrial move from DEHP as general purpose plasticiser to the longer chain analogues DINP and DIDP.70,88
Experimental
Origin of chemicals
4-Nonylphenol, 4-tert-octylphenol, bisphenol A, bisphenol F, dibutyl phthalate, dicyclohexyl phthalate, diethyl phthalate, diisobutyl phthalate, dimethyl isophthalate, di(2-ethylhexyl) terephthalate, dimethyl phthalate, dimethyl-3,4-furan dicarboxylic acid ester, phthalic acid and terephthalic acid were obtained from Sigma-Aldrich. Monoethylhexyl phthalate was obtained from Wako. Butylbenzyl phthalate, di(2-ethylhexyl) phthalate, dimethyl terephthalate and isophthalic acid were obtained from Fluka. Di(n-hexyl) phthalate was obtained from Dr Ehrenstorfer. Di(2-ethylhexyl) isophthalate was obtained from TCI. Dimethyl-2,5-furandicarboxylic acid ester was obtained from Bepharm (China). 2,5-Furandicarboxylic acid was obtained from V&V Pharma Industries (India) and Bepharm (China), and by independent synthesis from galactaric acid according to ref. 89.Cell lines
CALUX® assays have been constructed and are licensed world-wide by BioDetection Systems BV, Amsterdam, The Netherlands. The CALUX® battery of stable reporter gene assays29 comprised assays and test conditions that were selected from a larger panel of CALUX cells because of their possible relevance. The selected panel consisted of: the DR CALUX, consisting of rat H4IIE liver cells expressing the aryl hydrocarbon receptor endogenously.58 In addition a range of highly selective human U2-OS cell based lines was used including doubly (i.e. reporter gene and receptor) transfected estrogen receptor subtype alpha (ERα)-, progesterone receptor (PR)-, glucocorticoid receptor (GR)- and androgen receptor (AR)-CALUX cell lines,90 and an extension of the panel with peroxisome proliferator activated receptor PPARα- and PPARγ CALUX assays56,57 and thyroid receptor subtype beta (TRβ)-CALUX.29 This screening panel was completed with a range of U2-OS-based singly transfected lines expressing a reporter gene only, which are designed to selectively measure the activity of main intracellular signalling pathways. This included the assays to assess transcriptional activation by the p53 protein (p53 CALUX), the oxidative stress responsive nrf-2 pathway (Nrf2 CALUX),32 the endoplasmic reticulum stress pathway (ESRE CALUX), and the activator protein 1 pathway (AP1 CALUX).29 The Cytotox CALUX was used to determine cytotoxicity of the substances; it consists of human U2-OS cells stably transfected with an expression construct constitutively expressing the luciferase gene.32CALUX assay procedure
The CALUX cells were cultured essentially as described before.90 U2-OS cells were routinely subcultured every 3–4 days in growth medium consisting of DMEM (Gibco) supplemented with 7.5% fetal calf serum, 1× nonessential amino acids (Gibco) and 10 U ml−1 penicillin and 10 μg ml−1 streptomycin. H4IIE-CALUX cells were routinely subcultured every 3–4 days in growth medium consisting of αMEM (Gibco) supplemented with 10% fetal calf serum. All cell types were maintained at 37 °C and 5% CO2 at all times.All CALUX assays were performed, as described in detail in the publicly available DB ALM protocol 197, in assay medium, consisting of DMEM without phenol-red indicator (Gibco) supplemented with 5% DCC-stripped fetal calf serum, 1× non-essential amino acids (Gibco) and 10 U ml−1 penicillin and 10 μg ml−1 streptomycin. For seeding, a cell suspension in assay medium was made of 1 × 105 cells per ml (U2-OS) or 4 × 105 cells per ml (H4IIE), and the white 384-wells plates were seeded with 30 μl per well cell suspension using a MicroFlo Select dispenser (BioTek). After 24 h, exposure medium was prepared by adding 0.2% of test substance dilution series in DMSO to a 96-wells plate with assay medium. Of this exposure mixture, 30 μl was added to the assay plates containing the CALUX cells, resulting in final DMSO concentrations of 0.1%. The final concentrations of the substances in the wells were 1E−4–3E−10 M in 0.5 log unit increments. Additionally, DMSO blanks and a full dose response curve of the relevant reference substance were included on each plate. All samples were tested in triplicate. The preparation of the substance dilution series as well as the exposure of the cells were performed on a Hamilton Starlet liquid handling robot coupled to a Cytomat incubator. In order to be able to detect receptor antagonism, the assays were also performed in antagonistic mode. The assay procedure was as described above, with the only exception that the EC50 concentration of the reference agonist was present during the exposure.
After 24 h exposure the exposure medium was removed and 10 μl per well Triton-lysis buffer was added by the MicroFlo Select. Subsequently, the luciferase signal was measured in a luminometer (Infinite Pro reader coupled to a Connect stacker, both TECAN), essentially as described before.90
Data analysis
The luminometer data was analysed as follows; the average of the triplicate wells was determined, and the average blank (DMSO) value was subtracted. The maximum response elicited by the reference substance was set to 100% (full receptor activation), and the other values were scaled accordingly. The lowest observed effect concentrations (LOECs) were determined; for agonist assays, the LOEC was defined as the PC10 value, while for antagonist assays a PC20 value was used. The PC10 concentration was defined as the concentration where the response elicited by the test substance equals 10% of the maximum response of the reference substance. For antagonist experiments, PC20 values were determined instead, which was defined as the concentration where the test substance causes 20% inhibition of the basal signal elicited by the receptor agonist. In this case, the maximum inhibition achieved by the reference antagonist was set to 100%.Conclusions
FDCA esters are highly relevant biobased alternatives for currently used benzene dicarboxylic acid esters, and can in the case of (ortho) phthalate based plasticisers offer a sustainable alternative. However, in contrast to the ubiquitous phthalate family, thus far no toxicological data were available for 2,5-FDCA esters, although a recent paper mentions that DEHF shows no cytotoxicity in mouse 3T3-L1 cells up to 500 μM for 72 h.91In the present study, an in vitro reporter gene assay approach was used to compare the activity profile of commonly used phthalates to that of their furan-based counterparts on a broad series of toxicological endpoints. The assay selection was aimed at the detection of endocrine activity, since several phthalates are heavily scrutinised for their endocrine disrupting properties. However, to avoid missing other relevant toxicological endpoints, several assays able to detect various forms of cellular stress were also included in the panel.
The results showed that the benzene dicarboxylic acid esters were predominantly active on the endocrine assays ERα, anti-AR and anti-PR, while effects on other endpoints such as peroxisome proliferation (PPARs) or genotoxicity (p53) were seen only sporadically. The results obtained for the benzene dicarboxylic acid esters correspond well with literature, showing that endocrine activity (mainly estrogenic and anti-androgenic) increases with ester chain length, reaching a maximum at C4-C6, while longer chains result in a rapid decrease in activity.
Furthermore, it was shown that positional isomerism in the phthalate family has significant effects, with ortho-phthalates being by far the most active substances.
In comparison, six of the seven furan dicarboxylic acid based diesters tested here showed no activity in any of the 13 assays used. Only the isobutyl derivative DIBF showed moderate estrogenic activity on one assay, compared to much more pronounced activities on four assays for the ortho-phthalate analogue.
As a follow-up of the current study it would be relevant to also assess whether the 2,5-FDCA based diesters are able to interfere with steroidogenesis, since it has been suggested for phthalates that they exert their adverse activity not only via direct interaction with nuclear steroid hormone receptors, but also by affecting steroidogenesis.61,68,73 This could be assessed using a steroidogenesis assay (H295R, OECD TG456), or with enzyme inhibition assays for the main enzymes involved in steroidogenesis, CYP17 and CYP19. Additionally, since plastics often end up in the environment, it would be advisable to investigate the biodegradation- and ecotoxicological properties of 2,5-FDCA based diesters and derived products. For example, Jia et al. have shown that PEDF-30 + co-polyesters are enzymatically degradable.92
Overall, the results presented in this paper are a strong indication that 2,5-FDCA based diesters in general are not only technically viable alternatives to phthalates, but also offer significant toxicological benefits, which supports a non-regrettable substitution.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the BE Basic consortium. Special thanks to Dr A. E. Frissen (WFBR) for NMR analyses, and Dr S. Thiyagarajan (WFBR) for substance purification.Notes and references
- J. H. Clark, T. J. Farmer, L. Herrero-Davila and J. Sherwood, Green Chem., 2016, 18, 3914–3934 RSC.
- M. Hong and E. Y. X. Chen, Green Chem., 2017, 19, 3692–3706 RSC.
- I. A. Ignatyev, W. Thielemans and B. Vander Beke, ChemSusChem, 2014, 7, 1579–1593 CrossRef CAS.
- J. Pang, M. Zheng, R. Sun, A. Wang, X. Wang and T. Zhang, Green Chem., 2016, 18, 342–359 RSC.
- M. Volanti, D. Cespi, F. Passarini, E. Neri, F. Cavani, P. Mizsey and D. Fozer, Green Chem., 2019, 21, 885–896 RSC.
- D. I. Collias, A. M. Harris, V. Nagpal, I. W. Cottrell and M. Schultheis, Ind. Biotechnol., 2014, 10, 91–105 CrossRef CAS.
- P. Insight, Purified Terephthalic Acid (PTA) Properties, Production, Price, and Market, https://www.plasticsinsight.com/resin-intelligence/resin-prices/purified-terephthalic-acid-pta/.
- G. V. Research, Purified Terephthalic Acid Market Size, Share & Trends Analysis Report By Application, https://www.grandviewresearch.com/industry-analysis/purified-terephthalic-acid-market.
- E. de Jong, M. A. Dam, L. Sipos and G. J. M. Gruter, in Biobased Monomers, Polymers, and Materials, American Chemical Society, 2012, vol. 1105, ch. 1, pp. 1–13.
- R. Fittig, Ber. Dtsch. Chem. Ges., 1876, 9, 1189–1199 CrossRef.
- S. K. Burgess, O. Karvan, J. R. Johnson, R. M. Kriegel and W. J. Koros, Polymer, 2014, 55, 4748–4756 CrossRef CAS.
- S. K. Burgess, R. M. Kriegel and W. J. Koros, Macromolecules, 2015, 48, 2184–2193 CrossRef CAS.
- S. K. Burgess, J. E. Leisen, B. E. Kraftschik, C. R. Mubarak, R. M. Kriegel and W. J. Koros, Macromolecules, 2014, 47, 1383–1391 CrossRef CAS.
- R. J. I. Knoop, W. Vogelzang, J. van Haveren and D. S. van Es, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 4191–4199 CrossRef CAS.
- A. F. Sousa, A. C. Fonseca, A. C. Serra, C. S. R. Freire, A. J. D. Silvestre and J. F. J. Coelho, Polym. Chem., 2016, 7, 1049–1058 RSC.
- T. Cousin, J. Galy, A. Rousseau and J. Dupuy, J. Appl. Polym. Sci., 2018, 135, 45901 CrossRef.
- G. Wypych, in Handbook of Plasticizers, ed. G. Wypych, ChemTec Publishing, 3rd edn, 2017, pp. 333–483, DOI:10.1016/B978-1-895198-97-3.50013-5.
- J. L. Lyche, A. C. Gutleb, Å. Bergman, G. S. Eriksen, A. J. Murk, E. Ropstad, M. Saunders and J. U. Skaare, J. Toxicol. Environ. Health, Part B, 2009, 12, 225–249 CAS.
- A. J. Martino-Andrade and I. Chahoud, Mol. Nutr. Food Res., 2010, 54, 148–157 CrossRef CAS.
- M. Matsumoto, M. Hirata-Koizumi and M. Ema, Regul. Toxicol. Pharmacol., 2008, 50, 37–49 CrossRef CAS.
- B. I. Chaudhary, B.-D. Nguyen and A. Zamanskiy, J. Appl. Polym. Sci., 2015, 132, 42382 CrossRef.
- R. D. Sanderson, D. F. Schneider and I. Schreuder, J. Appl. Polym. Sci., 1994, 53, 1785–1793 CrossRef CAS.
- Z. Yu, J. Zhou, J. Zhang, K. Huang, F. Cao and P. Wei, J. Appl. Polym. Sci., 2014, 131, 40938 Search PubMed.
- E. F. S. A. (EFSA), EFSA J., 2014, 12, 3866 Search PubMed.
- E. D. Kroese, S. Bosgra, H. E. Buist, G. Lewin, S. C. van der Linden, H. Y. Man, A. H. Piersma, E. Rorije, S. H. Schulpen, M. Schwarz, F. Uibel, B. M. van Vugt-Lussenburg, A. P. Wolterbeek and B. van der Burg, Reprod. Toxicol., 2015, 55, 11–19 CrossRef CAS PubMed.
- A. H. Piersma, S. Bosgra, M. B. van Duursen, S. A. Hermsen, L. R. Jonker, E. D. Kroese, S. C. van der Linden, H. Man, M. J. Roelofs, S. H. Schulpen, M. Schwarz, F. Uibel, B. M. van Vugt-Lussenburg, J. Westerhout, A. P. Wolterbeek and B. van der Burg, Reprod. Toxicol., 2013, 38, 53–64 CrossRef CAS PubMed.
- B. Pieterse, I. J. Rijk, E. Simon, B. M. van Vugt-Lussenburg, B. F. Fokke, M. van der Wijk, H. Besselink, R. Weber and B. van der Burg, Environ. Sci. Pollut. Res., 2015, 22, 14442–14454 CrossRef CAS PubMed.
- B. van der Burg, B. Pieterse, H. Buist, G. Lewin, S. C. van der Linden, H. Y. Man, E. Rorije, A. H. Piersma, I. Mangelsdorf, A. P. Wolterbeek, E. D. Kroese and B. van Vugt-Lussenburg, Reprod. Toxicol., 2015, 55, 95–103 CrossRef CAS PubMed.
- B. van der Burg, S. van der Linden, H.-y. Man, R. Winter, L. Jonker, B. van Vugt-Lussenburg and A. Brouwer, in High-Throughput Screening Methods in Toxicity Testing, John Wiley & Sons, Inc., 2013, pp. 519–532, DOI:10.1002/9781118538203.ch28..
- B. van der Burg, E. B. Wedebye, D. R. Dietrich, J. Jaworska, I. Mangelsdorf, E. Paune, M. Schwarz, A. H. Piersma and E. D. Kroese, Reprod. Toxicol., 2015, 55, 114–123 CrossRef CAS PubMed.
- S. C. Van der Linden, H. Y. Man, E. Sonneveld, L. M. Puijker, A. Brouwer and B. Van der Burg, Environ. Sci. Technol., 2008, 42, 5814–5820 CrossRef CAS PubMed.
- S. C. van der Linden, A. R. von Bergh, B. M. van Vught-Lussenburg, L. R. Jonker, M. Teunis, C. A. Krul and B. van der Burg, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2014, 760, 23–32 CrossRef CAS.
- M. Aydogan Ahbab and N. Barlas, Food Chem. Toxicol., 2013, 51, 123–136 CrossRef CAS PubMed.
- X. Chen, S. Xu, T. Tan, S. T. Lee, S. H. Cheng, F. W. Lee, S. J. Xu and K. C. Ho, Int. J. Environ. Res. Public Health, 2014, 11, 3156–3168 CrossRef PubMed.
- S. M. Duty, A. M. Calafat, M. J. Silva, J. W. Brock, L. Ryan, Z. Chen, J. Overstreet and R. Hauser, J. Androl., 2004, 25, 293–302 CrossRef CAS.
- M. Ema, Congenital Anomalies, 2002, 297–308 CrossRef CAS PubMed.
- L. Liu, H. Bao, F. Liu, J. Zhang and H. Shen, Environ. Int., 2012, 42, 78–83 CrossRef CAS PubMed.
- J. D. Meeker, A. M. Calafat and R. Hauser, J. Androl., 2009, 30, 287–297 CrossRef CAS PubMed.
- N. Pant, A. Pant, M. Shukla, N. Mathur, Y. Gupta and D. Saxena, Hum. Exp. Toxicol., 2011, 30, 507–514 CrossRef CAS.
- V. Christen, P. Crettaz, A. Oberli-Schrammli and K. Fent, Chemosphere, 2010, 81, 1245–1252 CrossRef CAS PubMed.
- M. Ghisari and E. C. Bonefeld-Jorgensen, Toxicol. Lett., 2009, 189, 67–77 CrossRef CAS PubMed.
- C. A. Harris, P. Henttu, M. G. Parker and J. P. Sumpter, Environ. Health Perspect., 1997, 105, 802–811 CrossRef CAS PubMed.
- C. Kirchnawy, J. Mertl, V. Osorio, H. Hausensteiner, M. Washüttl, J. Bergmair, M. Pyerin and M. Tacker, Packag. Technol. Sci., 2014, 27, 467–478 CrossRef CAS.
- R. T. Pedersen and E. M. Hill, Chem.-Biol. Interact., 2000, 128, 189–209 CrossRef CAS PubMed.
- B. Pinto and D. Reali, Int. J. Hyg. Environ. Health, 2009, 212, 228–232 CrossRef CAS PubMed.
- M. G. ter Veld, B. Schouten, J. Louisse, D. S. van Es, P. T. van der Saag, I. M. Rietjens and A. J. Murk, J. Agric. Food Chem., 2006, 54, 4407–4416 CrossRef CAS PubMed.
- OECD, Guidance document on standardised test guidelines for evaluating chemicals for endocrine disruption, Report 150, 2012 Search PubMed.
- B. van der Burg, R. Winter, M. Weimer, P. Berckmans, G. Suzuki, L. Gijsbers, A. Jonas, S. van der Linden, H. Witters, J. Aarts, J. Legler, A. Kopp-Schneider and S. Bremer, Reprod. Toxicol., 2010, 30, 73–80 CrossRef CAS.
- B. van der Burg, R. Winter, H.-Y. Man, C. Vangenechten, P. Berckmans, M. Weimer, H. Witters and S. van der Linden, Reprod. Toxicol., 2010, 30, 18–24 CrossRef CAS.
- B. Collet, E. Simon, S. van der Linden, N. El Abdellaoui, M. Naderman, H. Y. Man, I. Middelhof, B. van der Burg, H. Besselink and A. Brouwer, Reprod. Toxicol., 2019 DOI:10.1016/j.reprotox.2019.05.011.
- OECD, Test No. 455: Performance-Based Test Guideline for Stably Transfected Transactivation In Vitro Assays to Detect Estrogen Receptor Agonists and Antagonists, 2016 Search PubMed.
- V. Zuang, A. Dura, D. Asturiol Bofill, J. Barroso, S. Batista Leite, S. Belz, E. Berggren, C. Bernasconi, S. Bopp, M. Bouhifd, G. Bowe, I. Campia, S. Casati, S. Coecke, R. Corvi, L. Gribaldo, E. Grignard, M. Halder, M. Holloway, A. Kienzler, B. Landesmann, F. Madia, A. Milcamps, S. Morath, S. Munn, A. Paini, F. Pistollato, A. Price, P. Prieto Peraita, A. Richarz, J. Sala Benito, I. Wilk-Zasadna, C. Wittwehr, A. Worth and M. Whelan, EURL ECVAM Status Report on the Development, Validation and Regulatory Acceptance of Alternative Methods and Approaches, EURL ECVAM, Luxembourg, 2018 Search PubMed.
- M. T. Bility, J. T. Thompson, R. H. McKee, R. M. David, J. H. Butala, J. P. Vanden Heuvel and J. M. Peters, Toxicol. Sci., 2004, 82, 170–182 CrossRef CAS PubMed.
- C. H. Hurst and D. J. Waxman, Toxicol. Sci., 2003, 74, 297–308 CrossRef CAS PubMed.
- P. J. Lapinskas, S. Brown, L. M. Leesnitzer, S. Blanchard, C. Swanson, R. C. Cattley and J. C. Corton, Toxicology, 2005, 207, 149–163 CrossRef CAS PubMed.
- L. Gijsbers, H. Y. Man, S. K. Kloet, L. H. de Haan, J. Keijer, I. M. Rietjens, B. van der Burg and J. M. Aarts, Anal. Biochem., 2011, 414, 77–83 CrossRef CAS.
- L. Gijsbers, H. D. van Eekelen, L. H. de Haan, J. M. Swier, N. L. Heijink, S. K. Kloet, H. Y. Man, A. G. Bovy, J. Keijer, J. M. Aarts, B. van der Burg and I. M. Rietjens, J. Agric. Food. Chem., 2013, 61, 3419–3427 CrossRef CAS.
- P. M. Garrison, K. Tullis, J. M. Aarts, A. Brouwer, J. P. Giesy and M. S. Denison, Fundam. Appl. Toxicol., 1996, 30, 194–203 CrossRef CAS.
- E. Perez-Albaladejo, D. Fernandes, S. Lacorte and C. Porte, Toxicol. In Vitro, 2017, 38, 41–48 CrossRef CAS PubMed.
- J. L. Lyche, A. C. Gutleb, A. Bergman, G. S. Eriksen, A. J. Murk, E. Ropstad, M. Saunders and J. U. Skaare, J. Toxicol. Environ. Health, Part B, 2009, 12, 225–249 CAS.
- C. Desdoits-Lethimonier, O. Albert, B. Le Bizec, E. Perdu, D. Zalko, F. Courant, L. Lesne, F. Guille, N. Dejucq-Rainsford and B. Jegou, Hum. Reprod., 2012, 27, 1451–1459 CrossRef CAS PubMed.
- E. K. Maloney and D. J. Waxman, Toxicol. Appl. Pharmacol., 1999, 161, 209–218 CrossRef CAS PubMed.
- P. M. Foster, E. Mylchreest, K. W. Gaido and M. Sar, Hum. Reprod. Update, 2001, 7, 231–235 CrossRef CAS PubMed.
- J. D. Meeker, S. Sathyanarayana and S. H. Swan, Philos. Trans. R. Soc., B, 2009, 364, 2097–2113 CrossRef CAS PubMed.
- M. Di Lorenzo, M. Forte, S. Valiante, V. Laforgia and M. De Falco, Ecotoxicol. Environ. Saf., 2018, 147, 565–573 CrossRef CAS PubMed.
- L. E. Gray Jr., J. Ostby, M. Furr, D. N. Price, L. Veeramachaneni and L. Parks, Toxicol. Sci., 2000, 58, 350–365 CrossRef CAS.
- B. R. Hannas, C. S. Lambright, J. Furr, K. L. Howdeshell, V. S. Wilson and L. E. Gray Jr., Toxicol. Sci., 2011, 123, 206–216 CrossRef CAS.
- R. Hauser and A. M. Calafat, Occup. Environ. Med., 2005, 62, 806–818 CrossRef CAS PubMed.
- P. Ventrice, D. Ventrice, E. Russo and G. De Sarro, Environ. Toxicol. Pharmacol., 2013, 36, 88–96 CrossRef CAS PubMed.
- ECHA, Proposing harmonised classification and labelling at EU level of DINP, 2018 Search PubMed.
- ECHA, Consultation on draft recommendation to amend DEHP, BBP, DBP and DIBP Annex XIV entries following their identification as SVHC due to additional intrinsic properties, 2018 Search PubMed.
- ECHA, Substance information benzyl butyl phthalate, https://echa.Europa.eu/substance-information/-/substanceinfo/100.001.475.
- N. K. Kambia, I. Severin, A. Farce, E. Moreau, L. Dahbi, C. Duval, T. Dine, V. Sautou and M. C. Chagnon, J. Appl. Toxicol., 2019, 39, 1043–1056 CrossRef CAS PubMed.
- ECHA, Support document to the opinion of the member state committee for identification of BBP as a substance of very high concern, 2014 Search PubMed.
- P. Myrdal, G. H. Ward, R. M. Dannenfelser, D. Mishra and S. H. Yalkowsky, Chemosphere, 1992, 24, 1047–1061 CrossRef CAS.
- J. J. Ellington, J. Chem. Eng. Data, 1999, 44, 1414–1418 CrossRef CAS.
- D. J. Letinski, M. J. Connelly, D. R. Peterson and T. F. Parkerton, Chemosphere, 2002, 48, 257–265 CrossRef CAS.
- C. A. Staples, D. R. Peterson, T. F. Parkerton and W. F. Adams, Chemosphere, 1997, 35, 667–749 CrossRef CAS.
- M. Thomsen, L. Carlsen and S. Hvidt, Environ. Toxicol. Chem., 2001, 20, 127–132 CrossRef CAS.
- J. Ejlertsson, M. Alnervik, S. Jonsson and B. H. Svensson, Environ. Sci. Technol., 1997, 31, 2761–2764 CrossRef CAS.
- D.-W. Gao and Z.-D. Wen, Sci. Total Environ., 2016, 541, 986–1001 CrossRef CAS PubMed.
- D.-W. Liang, T. Zhang, H. H. P. Fang and J. He, Appl. Microbiol. Biotechnol., 2008, 80, 183 CrossRef CAS PubMed.
- F. Kamp and J. A. Hamilton, Biochemistry, 1993, 32, 11074–11086 CrossRef CAS PubMed.
- F. Kamp and J. A. Hamilton, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2006, 75, 149–159 CrossRef CAS PubMed.
- M.-S. Cui, J. Deng, X.-L. Li and Y. Fu, ACS Sustainable Chem. Eng., 2016, 4, 1707–1714 CrossRef CAS.
- S. Thiyagarajan, A. Pukin, J. van Haveren, M. Lutz and D. S. van Es, RSC Adv., 2013, 3, 15678–15686 RSC.
- S. Oae, M. Hamada, Y. Otsuji and N. Furukawa, Ann. Rep. Radiation Centre Osaka Perfect., 1961, 2, 106–110 CAS.
- ECHA, Evaluation of new scientific evidence concerning the restrictions on DINP and DIDP contained in entry 52 of annex XVII to regulation (EC) NO 1907/2006 (REACH), 2012 Search PubMed.
- A. C. Cope and R. T. Keller, J. Org. Chem., 1956, 21, 141–141 CrossRef CAS.
- E. Sonneveld, H. J. Jansen, J. A. Riteco, A. Brouwer and B. van der Burg, Toxicol. Sci., 2005, 83, 136–148 CrossRef CAS PubMed.
- M. Matos, R. A. Cordeiro, H. Faneca, J. F. J. Coelho, A. J. D. Silvestre and A. F. Sousa, Materials, 2019, 12, 2336 CrossRef CAS PubMed.
- Z. Jia, J. Wang, L. Sun, J. Zhu and X. Liu, J. Appl. Polym. Sci., 2018, 135, 46076 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Full substance list, synthesis methods and full bioassay results tables. See DOI: 10.1039/c9gc04348a |
| This journal is © The Royal Society of Chemistry 2020 |
