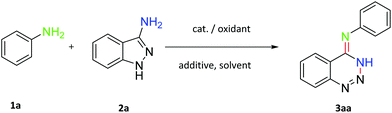Iodine-catalysed N-centered [1,2]-rearrangement of 3-aminoindazoles with anilines: efficient access to 1,2,3-benzotriazines†
Jie
Ren
a,
Xinxin
Yan
a,
Xiaofan
Cui
b,
Chao
Pi
 *a,
Yangjie
Wu
*a,
Yangjie
Wu
 a and
Xiuling
Cui
a and
Xiuling
Cui
 *a
*a
aCollege of Chemistry, Henan Key Laboratory of Chemical Biology and Organic Chemistry, Key Laboratory of Applied Chemistry of Henan Universities, Zhengzhou University, Zhengzhou 450052, P. R. China. E-mail: pichao@zzu.edu.cn; cuixl@zzu.edu.cn
bDepartment of Biological and Chemical Engineering, Nanyang Institute of Technology, Nanyang 473004, P. R. China
First published on 29th November 2019
Abstract
A straightforward and synthetically valuable approach for the synthesis of 1,2,3-benzotriazines has been developed via iodine-catalysed N-centered [1,2]-rearrangement of 3-aminoindazoles with anilines. This reaction tolerated a wide scope of substrates under simple reaction conditions employing I2 as a cheap catalyst.
Introduction
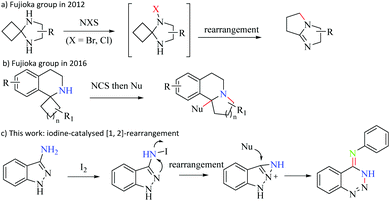
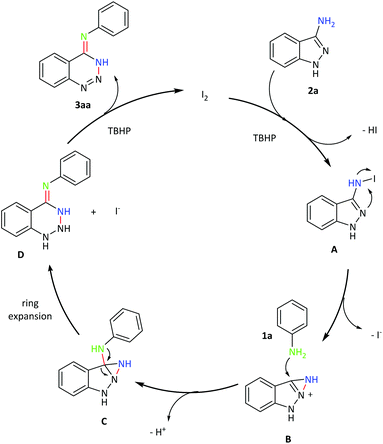
1,2,3-Benzotriazine and its derivatives, as an important class of nitrogen-containing heterocycles, have been widely used in organic synthesis because of their unique chemical properties1 and biological activities.2 Particularly, 4-substituted-1,2,3-benzotriazines are fundamental structural motifs in biologically active molecules that possess significant antitumor, and anti-inflammatory activities and are inhibitors of vascular endothelial growth factor.3 The traditional synthesis approaches to 4-substituted-1,2,3-benzotriazines are roughly divided into three categories: (1) coupling reaction of diazonium salts with alkylamines;4 (2) intramolecular cyclization of o-bromobenzyl azides in the presence of BuLi in THF at −78 °C;5 and (3) diazotization of 2-aminobenzonitrile by treating with a strong acid and NaNO2 at 0 °C.6 However, tedious steps, unstable reagents and harsh reaction conditions are required in these procedures. Consequently, the development of a simple and efficient method to construct such a motif is of great significance.Rearrangement reactions are extremely useful in organic synthesis since they provide complicated molecules from simple and readily available reactants.7N-Haloamine, as an easily available and highly reactive electrophilic nitrogen source, was ingeniously utilized to synthesize nitrogen-containing molecules.8 Generally, an effective pathway to construct substituted amines has become available by the use of N-haloamines together with nucleophilic reagents.9 However, the use of in situ generated N-haloamines directly as electrophilic nitrogen sources in a one-pot manner to carry out rearrangement reactions avoiding prefunctionalization of amines prior to the reaction was still a challenge.10 In 2012, the Fujioka group reported an oxidative rearrangement of spiro cyclobutane cyclic aminals to form bicyclic amidines in one pot and N-halosuccinimides as halogenated reagents that involved initial N-halogenation (Scheme 1a).11 Subsequently, they prepared fused tetrahydroisoquinolines by generating N-chloroamine in situ to participate in oxidative rearrangement reactions in 2016 (Scheme 1b).12 These reactions were limited to the starting materials containing cyclobutane moieties, where releasing a ring strain is an important driving force and an excessive halogenated reagent is essential. On the other hand, iodine-catalysed organic reactions have attracted much attention due to their low cost, high efficiency and green nature of progress.13 Hence, as part of our ongoing efforts to develop clean procedures to heterocycles,14 we reported an iodine-catalysed oxidative N-centered [1,2]-rearrangement reaction of 3-aminoindazoles with anilines for the synthesis of N-phenyl-1,2,3-benzotriazin-4(3H)-imines under simple reaction conditions (Scheme 1c). This transformation involved the formation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and N–N bonds simultaneously, and employed I2 as a cheap catalyst to form N-haloamine in situ.
N and N–N bonds simultaneously, and employed I2 as a cheap catalyst to form N-haloamine in situ.
Results and discussion
We started our investigation with aniline (1a) and 3-aminoindazole (2a) as model substrates to optimize various reaction parameters (Table 1). To our delight, the desired N-phenyl-1,2,3-benzotriazin-4(3H)-imine 3aa was obtained in 20% yield in the presence of I2 and H2O2 in MeOH (methanol) at 80 °C under air for 12 h (Table 1, entry 1). No product could be detected when other iodine sources including NIS (N-iodosuccinimide) and KI, served as a catalyst (entries 2 and 3). Subsequently, a variety of oxidants were investigated (entries 4–7) and TBHP (tert-butyl hydroperoxide) gave the best result. After introduction of TBAI (tetrabutylammonium iodide) as an additive, the yield of 3aa was provided up to 47% (entry 8). Inspired by this interesting result, other additives, including KF, NH4Cl and Na2HPO4 were tested, but resulted in lower yields (entry 9–11). Evaluation of solvents disclosed that 1-propanol was the best choice (entries 12–15). Gratifyingly, when TBHP was increased to 2 equiv., the desired product could be afforded in 78% yield (entry 16). Meanwhile, the higher yield was obtained by increasing the amount of TBAI to 1.5 equiv. (entry 17). When the ratio of 1a and 2a was 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the yield of the desired product 3aa was obtained in an excellent yield of 86% (entry 18). Finally, the optimal reaction conditions were identified as follows: aniline (0.12 mmol), 3-aminoindazole (0.1 mmol) in the presence of I2 (10 mol%), TBHP (2 equiv.) and TBAI (1.5 equiv.) in 1-propanol (2 mL) at 80 °C under an air atmosphere for 12 h.
1, the yield of the desired product 3aa was obtained in an excellent yield of 86% (entry 18). Finally, the optimal reaction conditions were identified as follows: aniline (0.12 mmol), 3-aminoindazole (0.1 mmol) in the presence of I2 (10 mol%), TBHP (2 equiv.) and TBAI (1.5 equiv.) in 1-propanol (2 mL) at 80 °C under an air atmosphere for 12 h.
| Entry | Catalyst | Oxidant | Additive | Solvent | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), 2a (0.1 mmol), catalyst (10 mol%), oxidant (1 equiv.), additive (1 equiv.) and solvent (2 mL), 80 °C, 12 h, air. b Isolated yields. NR: no reaction. c TBHP (2 equiv.). d TBAI ( 1.5 equiv). e 1a (0.12 mmol). | |||||
| 1 | I2 | H2O2 | — | MeOH | 20% |
| 2 | NIS | H2O2 | — | MeOH | NR |
| 3 | KI | H2O2 | — | MeOH | NR |
| 4 | I2 | K2S2O8 | — | MeOH | 15% |
| 5 | I2 | BPO | — | MeOH | 21% |
| 6 | I2 | TBHP | — | MeOH | 35% |
| 7 | I2 | O2 | — | MeOH | Trace |
| 8 | I2 | TBHP | TBAI | MeOH | 47% |
| 9 | I2 | TBHP | KF | MeOH | 24% |
| 10 | I2 | TBHP | NH4Cl | MeOH | NR |
| 11 | I2 | TBHP | Na2HPO4 | MeOH | 17% |
| 12 | I2 | TBHP | TBAI | DCM | 37% |
| 13 | I2 | TBHP | TBAI | CH3CN | 32% |
| 14 | I2 | TBHP | TBAI | 1-Propanol | 53% |
| 15 | I2 | TBHP | TBAI | THF | NR |
| 16c | I2 | TBHP | TBAI | 1-Propanol | 78% |
| 17d | I2 | TBHP | TBAI | 1-Propanol | 82% |
| 18 | I 2 | TBHP | TBAI | 1-Propanol | 86% |
With the optimized reaction conditions in hand, the applicability of this reaction system was investigated (Scheme 2). We firstly examined the effect of the substituents in anilines. Satisfyingly, it was observed that anilines with both electron-withdrawing and electron-donating groups at the para-position all coupled smoothly with 3-aminoindazole to deliver the corresponding products in 61–90% yields (3aa–3ia). However, only trace amounts of the desired products were obtained for 4-CF3 and 4-NO2 substituted anilines, perhaps due to their strong electron-withdrawing ability (3ja–3ka). The meta- and ortho-substituted (including F, Me and OMe) anilines were all compatible in this transformation, affording the corresponding products in moderate yields (3la–3qa). Meanwhile, the polysubstituted anilines were also coupled with 2a to provide products in 70–94% yields (3ra–3ta). This result indicated that the steric hindrance on the benzene had no significant effect on this reaction. In addition, the transformation showed an excellent tolerance toward benzylamine and 4-aminobiphenyl (3ua–3va). The secondary amines were also applicable as in the isolation of products in 63–72% yields (3wa–3xa).
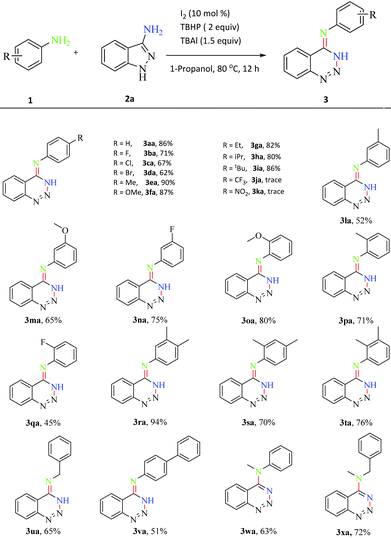 | ||
| Scheme 2 Scope of the anilines. Reaction conditions: 1 (0.12 mmol), 2a (0.1 mmol), I2 (10 mol%), TBHP (2 equiv.), TBAI (1.5 equiv.) and 1-propanol (2 mL) at 80 °C for 12 h under air. | ||
Furthermore, a series of substituted 3-aminoindazoles were employed to probe the scope of the reaction (Scheme 3). When halogen (including Cl and Br), Me and OMe were at the C-4 position of 3-aminoindazoles, this transformation showed satisfactory tolerance in 47–91% yields (3ab–3ae). The C-5 position of 3-aminoindazoles also proceeded smoothly to afford the desired products in moderate yields (3af–3ag). This catalytic system was not applied to 5-nitro-1H-indaol-3-amine, perhaps due to the low electron density (3ah). In addition, 4-methoxy-1H-indaol-3-amine also performed well, affording the product in 82% yield (3ai). These results indicated that steric hindrance of 3-aminoindazoles did not dramatically influence this transformation. However, it was unsuccessful to synthesize product 3aj from N-methyl-1H-indaol-3-amine, which indicated that the N-protected 3-aminoindazole was not applied to the reaction.
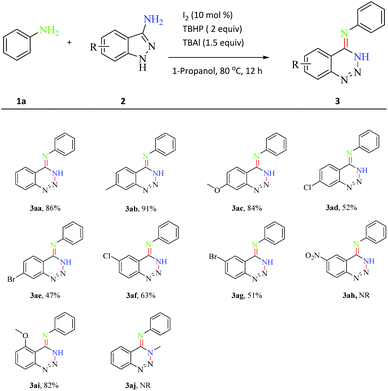 | ||
| Scheme 3 Scope of the 3-aminoindazoles. Reaction conditions: 1a (0.12 mmol), 2 (0.1 mmol), I2 (10 mol%), TBHP (2 equiv.), TBAI (1.5 equiv.) and 1-propanol (2 mL) at 80 °C for 12 h under air. | ||
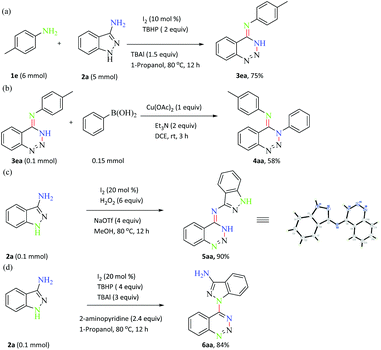
To evaluate the utility of this method in organic synthesis, a gram-scale synthesis of N-phenyl-1,2,3-benzotriazin-4(3H)-imine 3aa was performed under the standard reaction conditions in 75% yield (Scheme 4a). In addition, a series of chemical transformations of 3aa and 1a were carried out. The aryl boronic acid was coupled with 3aa to give the corresponding N-arylated product 4aa, which can be exploited as a precursory platform for the synthesis of other nitrogen-containing heterocycles (Scheme 4b). Homo-coupling of 3-aminoindazole gave product 5aa in 90% yield (Scheme 4c). The structure of compound 5aa was unambiguously confirmed by X-ray crystallographic analysis (see the ESI†). In the presence of I2 (20 mol%), TBHP (4 equiv.), 2-aminopyridine (2.4 equiv.) and TBAI (3.0 equiv.) in 1-propanol (2 mL) at 80 °C for 12 h, the secondary amine of 3-aminoindazole took part in the reaction to get product 6aa in 84% yield (Scheme 4d). We deduced that 2-aminopyridine might act as the base in this transformation. These results confirmed the practicability of this current developed protocol to afford N-phenyl-1,2,3-benzotriazin-4(3H)-imines.
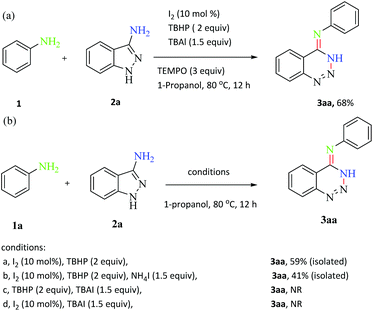
In order to understand the reaction mechanism, a series of control experiments were carried out. N-Phenyl-1,2,3-benzotriazin-4(3H)-imine 3aa could be obtained in 68% yield when TEMPO (3 equiv., TEMPO = 2,2,6,6-tetramethylpiperidinyl) as a radical trapper was added to the reaction of 1a and 2a (Scheme 5a). This result indicated that a radical process might not be involved in the reaction. In addition, the reaction proceeded smoothly to afford the desired product in 59% yield in the absence of TBAI (condition a). Meanwhile, when NH4I (1.5 equiv.) instead of TBAI was added to the reaction of 1a and 2a, 3aa was obtained in 41% yield (condition b), which implied that the iodine ion of TBAI was not the initial step of the reaction and TBAI might act as the phase transfer catalyst in this transformation. Moreover, no product was observed when the reaction was performed in the absence of I2 or TBHP (conditions c and d), which suggested that I2 and oxidant were crucial for the initial step of the reaction.
On the basis of the above discussion and previous reports,12,13d a plausible mechanism of the [1,2]-rearrangement reaction is proposed in Scheme 6. First, the iodination of 2a with TBHP would lead to the formation of N-iodoaminoindazole A and HI.12,13d Then, intermediate A underwent an intramolecular nucleophile to generate intermediate B and the electron-rich nucleophile of 1a attacked B to afford intermediate C. Subsequently, intermediate C underwent intramolecular C–N bond cleavage to deliver D. Finally, with the aid of the oxidant, D was oxidized to the desired product 3aa. In addition, the molecular iodine as the catalyst can be regenerated by the oxidation of hydrogen iodide and TBHP.13d
Conclusions
In conclusion, we have successfully developed a novel and straightforward iodine-catalysed N-centered [1,2]-rearrangement reaction of 3-aminoindazoles with anilines. A broad range of 1,2,3-benzotriazines were conveniently obtained in moderate to excellent yields with good functional group tolerance under simple reaction conditions. Significantly, the formation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and N–N bonds was simultaneously involved and I2 was used as a cheap catalyst to form N-haloamine in situ. Further studies on the application of this strategy are in progress in our laboratory.
N and N–N bonds was simultaneously involved and I2 was used as a cheap catalyst to form N-haloamine in situ. Further studies on the application of this strategy are in progress in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We greatly acknowledge partial financial support from the Ministry of Science and Technology of China (2016YFE0123600).Notes and references
- (a) T. Miura, M. Yamauchi, A. Kosaka and M. Murakami, Angew. Chem., Int. Ed., 2010, 49, 4955 CrossRef CAS PubMed; (b) M. Yamauchi, M. Morimoto, T. Miura and M. Murakami, J. Am. Chem. Soc., 2010, 132, 54 CrossRef CAS; (c) Z.-J. Fang, S.-C. Zheng, Z. Guo, J.-Y. Guo, B. Tan and X.-Y. Liu, Angew. Chem., Int. Ed., 2015, 54, 9528 CrossRef CAS; (d) N. Wang, S.-C. Zheng, L.-L. Zhang, Z. Guo and X.-Y. Liu, ACS Catal., 2016, 6, 3496 CrossRef CAS; (e) V. H. Thorat, N. S. Upadhyay, M. Murakami and C.-H. Cheng, Adv. Synth. Catal., 2018, 360, 284 CrossRef CAS; (f) M. H. Balakrishnan, K. Sathriyan and S. Mannathan, Org. Lett., 2018, 20, 3815 CrossRef PubMed.
- (a) F. Hu, X. Cheng, Z. Niu, X. Yang, D. Li, L. Wan, J. Li, B. Liu, X. Zou, H. Yang and B. Li, J. Heterocycl. Chem., 2015, 52, 793 CrossRef CAS; (b) K. S. Kumar, R. Adepu, S. Sandra, D. Rambabu, G. R. Krishna, C. M. Reddy, P. Misra and M. Pal, Bioorg. Med. Chem. Lett., 2012, 22, 1146 CrossRef PubMed; (c) G. Wang, X. Chen, Y. Deng, Z. Li and X. Xu, J. Agric. Food Chem., 2015, 63, 6883 CrossRef CAS; (d) Y. Chang, J. Zhang, X. Chen, Z. Li and X. Xu, Bioorg. Med. Chem. Lett., 2017, 27, 2641 CrossRef CAS.
- (a) T. S. Ibrahim, A. A. Rashad, Z. K. Abdel-Samii, S. A. El-Feky, M. K. Abdel-Hamid and W. Barakat, Med. Chem. Res., 2012, 21, 4369 CrossRef CAS; (b) D. J. Kohlsmith, K. Vaughan and S. J. Luner, Can. J. Physiol. Pharmacol., 1984, 62, 396 CrossRef CAS PubMed; (c) J.-L. Lv, R. Wang, D. Liu, G. Guo, Y.-K. Jing and L.-X. Zhao, Molecules, 2008, 13, 1427 CrossRef CAS; (d) X.-W. Zhao, D. Liu, S.-L. Luan, G.-D. Hu, J.-L. Lv, Y.-K. Jing and L.-X. Zhao, Bioorg. Med. Chem. Lett., 2013, 21, 7807 CrossRef CAS.
- (a) P. L. Faye, K. Vaughan and D. L. Hooper, Can. J. Chem., 1983, 61, 179 CrossRef CAS; (b) K. K. Lal, C. H. Schwalbe, K. Vaughan, R. J. Lafrance and C. D. Whiston, Can. J. Chem., 1985, 63, 581 CrossRef; (c) G. W. Rewcastle, W. A. Denny, A. J. Bridges, H. Zhou, D. R. Cody, A. Mcmichael and D. W. Fry, J. Med. Chem., 1995, 38, 3482 CrossRef CAS PubMed.
- K. Kobayashi and Y. Chikazawa, Helv. Chim. Acta, 2016, 99, 33 CrossRef CAS.
- J.-L. Lv, R. Wang, D. Liu, G. Guo, Y.-K. Jing and L.-X. Zhao, Molecules, 2008, 13, 1427 CrossRef CAS PubMed.
- (a) Y. Wang, L. Liu and L. Zhang, Chem. Sci., 2013, 4, 739 RSC; (b) S. Shang, D. Zhang-Negrerie, Y. Du and K. Zhao, Angew. Chem., Int. Ed., 2014, 53, 6216 CrossRef CAS PubMed; (c) A. A. Tabolin and S. L. Loffe, Chem. Rev., 2014, 114, 5426 CrossRef CAS; (d) R. Costil, H. J. A. Dale, N. Fey, G. Whitcombe, J. V. Matlock and J. Clayden, Angew. Chem., Int. Ed., 2017, 56, 12533 CrossRef CAS; (e) M. Li, J.-H. Wang, W. Li and L.-R. Wen, Org. Lett., 2018, 20, 7694 CrossRef CAS; (f) C. M. Pearson, J. W. B. Fyfe and T. N. Snaddon, Angew. Chem., Int. Ed., 2019, 58, 10521 CrossRef CAS; (g) X. Chang, Q. Zhang and C. Guo, Org. Lett., 2019, 21, 10 CrossRef CAS.
- (a) C. He, C. Chen, J. Cheng, C. Liu, W. Liu, Q. Li and A. Lei, Angew. Chem., 2008, 120, 6514 CrossRef; (b) T. J. Barker and E. R. Jarvo, J. Am. Chem. Soc., 2009, 131, 15598 CrossRef CAS; (c) T. Kawano, K. Hirano, T. Satoh and M. Miura, J. Am. Chem. Soc., 2010, 132, 6900 CrossRef CAS; (d) B. Shen, D. M. Makley and J. N. Johnsron, Nature, 2010, 465, 1027 CrossRef CAS; (e) T. Hatakeyama, Y. Yoshimoto, S. K. Ghorai and M. Nakamura, Org. Lett., 2010, 12, 1516 CrossRef CAS.
- (a) T. G. Barker and E. R. Jarvo, Angew. Chem., Int. Ed., 2011, 50, 8325 CrossRef CAS; (b) T. Miura, M. Morimoto and M. Murakami, Org. Lett., 2012, 14, 5214 CrossRef CAS PubMed; (c) R. Vanjari, T. Guntreddi and K. N. Singh, Green Chem., 2014, 16, 351 RSC; (d) F. Zhang, D. Zheng, L. Lai, J. Cheng, J. Sun and J. Wu, Org. Lett., 2018, 20, 1167 CrossRef CAS.
- (a) F. M. Schell and A. M. Smith, Tetrahedron Lett., 1983, 24, 1883 CrossRef CAS; (b) K. Murai, M. Shimura, R. Nagao, D. Endo and H. Fujioka, Org. Biomol. Chem., 2013, 11, 2648 RSC; (c) K. Kurai, D. Endo, N. Kawashita, T. Takagi and H. Fujioka, Chem. Pharm. Bull., 2015, 63, 245 CrossRef.
- K. Murai, H. Komatsu, R. Nagao and H. Fujioka, Org. Lett., 2012, 14, 772 CrossRef CAS.
- K. Murai, K. Matsuura, H. Aoyama and H. Fujioka, Org. Lett., 2016, 18, 1314 CrossRef CAS PubMed.
- (a) C. Varszegi, M. Ernst, F. V. Laar, B. F. Sels, E. Schwab and D. E. D. Vos, Angew. Chem., Int. Ed., 2008, 47, 1477 CrossRef CAS PubMed; (b) J. Zhang, D. Zhu, C. Yu, C. Wang and Z. Wang, Org. Lett., 2010, 12, 2841 CrossRef CAS PubMed; (c) W.-J. Hao, S.-Y. Wang and S.-J. Ji, ACS Catal., 2013, 3, 2501 CrossRef CAS; (d) W.-K. Luo, X. Shi, W. Zhou and L. Yang, Org. Lett., 2016, 18, 2036 CrossRef CAS PubMed; (e) Z. Chen, H. Li, W. Dong, M. Miao and H. Ren, Org. Lett., 2016, 18, 1334 CrossRef CAS PubMed; (f) Z. Zhang, C. Pi, H. Tong, X. Cui and Y. Wu, Org. Lett., 2017, 19, 440 CrossRef CAS PubMed; (g) T. Liu, J. Zhu, X. Sun, L. Cheng and L. Wu, Adv. Synth. Catal., 2019, 361, 3532 CrossRef CAS; (h) S. Du, C. Pi, T. Wan, Y. Wu and X. Cui, Adv. Synth. Catal., 2019, 361, 1766 CrossRef CAS; (i) W.-H. Bao, C. Wu, J.-T. Wang, W. Xia, P. Chen, Z. Tang, X. Xu and W.-M. He, Org. Biomol. Chem., 2018, 16, 8403 RSC; (j) W.-H. Bao, M. He, J.-T. Wang, X. Peng, M. Sung, Z. Tang, S. Jiang, Z. Cao and W.-M. He, J. Org. Chem., 2019, 84, 6065 CrossRef CAS; (k) Z. Shi, L. Wang and X. Cui, Chin. J. Org. Chem., 2019, 39, 1596 CrossRef.
- (a) Z. Yang, C. Pi, X. Cui and Y. Wu, Org. Chem. Front., 2019, 6, 2897 RSC; (b) Z. Yang, Z. Song, L. Jie, L. Wang and X. Cui, Chem. Commun., 2019, 55, 6094 RSC; (c) J. Ren, C. Pi, Y. Wu and X. Cui, Org. Lett., 2019, 21, 4067 CrossRef CAS; (d) S. Huang, H. Li, X. Sun, L. Xu, L. Wang and X. Cui, Org. Lett., 2019, 21, 5570 CrossRef CAS PubMed; (e) Y. Yu, Y. Feng, R. Chauvin, S. Ma, L. Wang and X. Cui, Org. Lett., 2018, 20, 4209 CrossRef CAS PubMed; (f) T. Yuan, C. Pi, C. You, X. Cui, S. Du, T. Wan and Y. Wu, Chem. Commun., 2019, 55, 163 RSC; (g) Z. Shen, C. Pi, X. Cui and Y. Wu, Chin. Chem. Lett., 2019, 30, 1374 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1955883. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9gc03567b |
| This journal is © The Royal Society of Chemistry 2020 |