 Open Access Article
Open Access ArticleSimulating human digestion: developing our knowledge to create healthier and more sustainable foods
Alan
Mackie
 *a,
Ana-Isabel
Mulet-Cabero
b and
Amelia
Torcello-Gómez
*a,
Ana-Isabel
Mulet-Cabero
b and
Amelia
Torcello-Gómez
 a
a
aThe School of Food Science and Nutrition, University of Leeds, Leeds, LS2 9JT, UK. E-mail: a.r.mackie@leeds.ac.uk
bQuadram Institute Bioscience, Norwich Research Park, Norwich, Norfolk NR4 7UQ, UK
First published on 22nd October 2020
Abstract
The gold standard for nutrition studies is clinical trials but they are expensive and variable, and do not always provide the mechanistic information required, hence the increased use of in vitro and increasingly in silico simulations of digestion. In this review, we give examples of the main simulations being used to model upper gastrointestinal tract digestion. This review ranges from the selection of enzymes to the interpretation of results from static models to fully dynamic models. We describe the modifications made to accommodate different demographic groups (infants, the elderly, etc.). We list examples of the application of the different models as well as giving the advantages and disadvantages. A model is only useful if it predicts or aids the understanding of physiological behaviour. Thus, the final section of the review makes a comparison of results obtained from experiments undertaken using in vitro simulations with those obtained in vivo. This comparison will help the reader understand the appropriateness of each model for the type of measurement to be undertaken. In particular, human studies tend to measure bioactive concentrations in blood and not in the gastrointestinal tract whereas in vitro studies often only produce data on release of nutrients into the gut lumen. This is the difficulty of comparing bioaccessibility as generated in vitro with bioavailability as generated in vivo. It is apparent that the models being used are increasingly being validated with in vivo data and this bodes well for the future.
1. Introduction: why use in vitro rather than in vivo?
1.1 The rationale
There has been an increasing emphasis on the link between food and health. As a result, there is a desire for better understanding of the mechanisms involved and this has led to the use of a wide range of models to simulate human digestion.1 These include a variety of in vitro simulations of the gastrointestinal (GI) tract capable of predicting bioaccessibility (i.e. fraction of a compound that is released from the matrix and is available for absorption).2 In reality, it is generally difficult to validate such models because bioaccessibility is hard to measure in vivo in humans.3 The ability to predict the bioaccessibility of specific compounds is important if we are to optimise the health benefits of particular foods in the diet. This optimisation is only possible if the various factors controlling the bioavailability of bioactives are well understood. Bioavailability, i.e. the proportion of a bioactive reaching the site of action, is a balance between rates of clearance or metabolism and rates of absorption and transport. Thus, increasing bioaccessibility is a key step in increasing bioactivity and the predictive power of even simple models becomes important.4 It is essential to understand that bioaccessibility is a prerequisite step for bioavailability5 and in vitro experiments that are representative of physiological conditions can provide useful indications of likely bioavailability in a wide range of food systems that would not be practical to test in human feeding studies. In this review, we discuss the key issues associated with undertaking physiologically relevant simulations of human digestion and how results compare to data from human studies.1.2 Summary of the digestion process
Digestion consists of a number of distinct processes that convert the food we eat into absorbable nutrients that sustain our bodies. The intake of food and the subsequent flow of digesta from one compartment to the next is tightly controlled by a number of different sensing and feedback mechanisms that have evolved to optimally extract the nutrients from food.6The physical process of digestion starts in the mouth where food is chewed and mixed with saliva.7 Solid and semi-solid foods are broken down into small particles and combined with saliva into a bolus. The properties of the bolus can have a significant influence on subsequent digestion.8 The bolus is held together and lubricated by saliva. The saliva also contains amylase that starts the hydrolysis of starch. Although oral processing serves many other functions associated with the sensory perception of food, these are not directly related to digestion. From the mouth, food passes down the oesophagus to the stomach.
The stomach is essentially a storage vessel that controls the release of nutrients into the small intestine, which is the main site of hydrolysis and absorption. The environment in the stomach varies both spatially and temporally. Immediately after a meal has entered the stomach, the pH will be relatively high (just slightly lower than the food itself, depending on buffering capacity) and the enzyme concentration relatively low. However, this soon changes as HCl is secreted and the pH drops, and both pepsinogen, the zymogen of pepsin, and gastric lipase are also secreted. The different enzymes present in the chyme, including salivary amylase, are active over different pH ranges and thus the hydrolysis of macronutrients occurs sequentially. The salivary amylase present in the bolus will be active until the pH drops below about 3.8.9 Next, the gastric lipase will become active as it has an optimum of pH 5–5.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and then finally the pepsin will start to hydrolyse any protein present as the pH drops to its optimum of ∼pH 2.11 In reality, there is significant overlap in the conditions under which these different enzymes can function as they all have some activity outside their optimum pH. After the physical processing of the oral stage, the gastric phase is relatively inactive in relation to physical processing. Only in the antrum and through the pylorus there is significant shearing of the gastric content. In other parts of the stomach there is relatively little mixing and stirring. Thus, phase separation can occur as lipids rise to the top and liquids are emptied in preference to solids. During emptying, particles larger than ∼2 mm will tend to be retained while smaller particles are emptied. The rate at which chyme passes from the stomach to the duodenum is a function of the caloric density of the chyme and its rheological properties12 with nutrient dense and more solid chyme being emptied more slowly than nutrient poor liquids.
10 and then finally the pepsin will start to hydrolyse any protein present as the pH drops to its optimum of ∼pH 2.11 In reality, there is significant overlap in the conditions under which these different enzymes can function as they all have some activity outside their optimum pH. After the physical processing of the oral stage, the gastric phase is relatively inactive in relation to physical processing. Only in the antrum and through the pylorus there is significant shearing of the gastric content. In other parts of the stomach there is relatively little mixing and stirring. Thus, phase separation can occur as lipids rise to the top and liquids are emptied in preference to solids. During emptying, particles larger than ∼2 mm will tend to be retained while smaller particles are emptied. The rate at which chyme passes from the stomach to the duodenum is a function of the caloric density of the chyme and its rheological properties12 with nutrient dense and more solid chyme being emptied more slowly than nutrient poor liquids.
From the stomach, chyme passes into the small intestine that comprises the duodenum, where gastric contents mix with bile and pancreatic enzymes and the pH is neutralised, the jejunum and the ileum. The pancreatic enzymes secreted into the duodenum include a range of lipases and proteases as well as amylase and other enzymes. The contents of the small intestine are well mixed and the relatively high enzyme activity quickly leads to the production of hydrolysis products. These products diffuse through the protective mucus layer to the underlying epithelium for further hydrolysis by brush border enzymes and absorption. Absorption by enterocytes is usually transporter mediated rather than “passively” absorbed. Bile, secreted into the duodenum from the gall bladder is largely reabsorbed in the distal ileum with circa 5% passing into the colon.
Any digesta that has not been hydrolysed and absorbed in the small intestine passes into the colon through the ileocecal valve. The high microbial load in the colon drives the fermentation of dietary fibre, especially soluble fibre, and the metabolism of a wide range of bioactive compounds such as phenolics.
1.3 Advantages of in vitro digestion
The use of in vitro simulations of human digestion has become widespread because they are less labour intensive, less expensive, more rapid and do not have the ethical restriction that in vivo studies have. They are suitable for mechanistic studies and hypothesis building due to their controlled conditions, reproducibility and ease of sampling at the site of interest.13 However, as mentioned in the introduction, such models are only useful if they are predictive of functionality in vivo and can provide some mechanistic insight. The Infogest network was set up in order to provide some guidance in using simple but physiologically relevant simulations of upper GI tract digestion.14 In order to be able to compare studies there is also a need for some standardisation. Indeed, one of the advantages of such simulations is that they avoid inter-individual variations that are often seen in human studies. Despite the widespread recognition that both physiological relevance and standard protocols are important, we still see significant numbers of publications using specific protocols with a modification that means it is no longer standardised.In addition to the advantages listed above, models can be chosen to address specific questions. For example, static models of digestion can be used to determine end-points of digestion or kinetics of very specific steps of digestion such as small intestinal hydrolysis. Thus, food digestibility can be assessed relatively simply and more complex models can be chosen if the question is more about the rate of appearance of specific nutrients or bioactives in the blood stream.15
1.4 Disadvantages of in vitro digestion
As indicated in the Introduction, the health implications of dietary interventions are related to local concentrations of the bioactive under investigation at the site of action. It is often not clear how this relates to bioaccessibility and further it is not easy to validate bioaccessibility as it normally involves either animal experiments or intubation of study participants.16 In the case of validation in animals, there is always the question as to whether the same outcome would be seen in humans. These limitations to validation mean that the reliability of in vitro digestion is questionable. Another potential disadvantage is their ease of use. This initially looks like an advantage but can mean that digestion models are used inappropriately, for example to digest ingredients in isolation.2. Models and ingredients (horses for courses). Tailoring the model to the question
2.1 Ingredients
The activity and specificity of enzymes vary depending on their source and concentration and thus specifying activity is critical when simulating human GI digestion. Of course, the enzymes should be dispersed in physiologically relevant media with appropriate pH and ionic strength, as will be described in later sections for the different models of in vitro digestion. In addition, the presence of biological surfactants will ultimately influence the performance of enzymes on the different substrates, such as lipids or proteins.17–19 Finally, suitable food substrates must be taken into consideration since the effect of the food matrix also plays an important role in the hydrolysis of food components.4 Having this in mind, each of these components is briefly reviewed in this section for suitable use within the context of biological relevance and standardisation of static or semi-dynamic in vitro digestion models to enable comparisons across different research laboratories.13,20,21Regarding the source of the digestive reagents, when human source is not commercially available, enzymes of porcine and bovine origin are usually recommended because of the biological similarity to those of human species. Starting in the oral cavity, the use of human salivary α-amylase is recommended over porcine pancreatic α-amylase13 but this is only relevant if starch is present in the food substrate. Furthermore, amylase will play a more significant role in a semi-dynamic model of digestion, as will be further explained below, since the initial pH in the simulated stomach is more elevated and amylase could still be active. Continuing in the stomach, the use of gastric lipase is now accessible. A rabbit gastric extract is commercially available from Lipolytech®.13 This extract also contains pepsin, which must be accounted for in the total pepsin activity.22 Rabbit gastric lipase shares 85% of sequence homology with human gastric lipase23 and has been shown to mimic well the digestive properties of human gastric lipase24 due to similar regio- and stereo-preference and pH-sensitivity.10 Additional porcine pepsin might be needed to meet the required activity in the simulated stomach. The use of phospholipids in the gastric phase is only recommended if proteolysis is the focus of the study and they are not present in the food substrate. This is because phospholipids have shown to affect the rate of protein digestion in the gastric and intestinal phase,18,25 but this step is optional. The detailed protocol to prepare phospholipids vesicles can be found elsewhere.20 In the intestinal phase, pancreatin from porcine pancreas is used as a cocktail of pancreatic enzymes (proteases, lipases and carbohydrases) and the amount is intended for general purposes in the digestion of food and is based on trypsin activity. For substrates where the focus of study is lipid hydrolysis, the addition of porcine pancreatic lipase is also recommended to meet the required lipase activity. In cases where only starch, protein or lipid comprises the substrate, then individual porcine pancreatic amylase, proteases trypsin and chymotrypsin (from porcine and bovine origin, respectively) or porcine pancreatic lipase and colipase, respectively, can be used. The use of individual enzymes in this specific situation is also an advantage in reducing the interference of additional components in the analysis of the digesta. Digestion in the small intestine occurs in the presence of biosurfactants such as bile salts and phospholipids. For a more physiologically relevant scenario, and when testing complex food matrices comprising proteins and lipids, the use of commercial bile extracts is suggested. These bile extracts contain a biological mixture of bile acids, phospholipids and cholesterol.26 In particular, the bile composition from bovine bile extract has been reported to be closer to human bile composition than porcine source.24 The activity of the enzymes and the content of bile in the extracts should be carefully measured and the appropriate assays are provided in the supplementary material of Brodkorb et al.13
The use of GI juices from human aspirates for in vitro digestion of proteins and lipids has been reported previously.27–29 Despite the closer approach to the in vivo secretions, it may represent more limitations than advantages for the following reasons. The restricted accessibility, the low storage stability of the aspirates in terms of enzyme activity and its complex characterisation, and inter-individual variability30 may lead to poor reproducibility and difficult comparison across studies. The inter-individual variability can be reduced if batches of pooled gastric and intestinal juices from a large cohort are used. Asledottir et al. used the standard protocol of static in vitro digestion20 by replacing the simulated GI fluids with pooled human gastric and intestinal aspirates and measured the enzyme activities with the recommended assays mentioned above.29
2.2 Static in vitro digestion models
The simplest in vitro digestion models are static, which are relatively easy and cheap to use on a daily basis. These models may consist of a mono-compartmental test, simulating for instance only the gastric phase, or a multi-compartment test where oral, gastric and small intestinal phases are simulated in sequence. The parameters of static models are fixed. This means that the meal to secretions ratio, pH, and enzyme/biosurfactant concentrations are set from the beginning of each phase and are constant throughout the respective phase. These fixed values should represent a physiologically relevant average of the GI scenario. For example, a simulation of the gastric phase in the fed state is proposed to be at the gastric emptying half-time.20 Nevertheless, these representative values vary according to human conditions of age, fed/fasted state and healthiness, among others. In addition, the stirring/shaking conditions with constant speed throughout each phase enable homogeneous mixing, which may allow a more representative sampling, but is certainly not characteristic of the very mild mixing found in certain sites, like the fundus in the stomach. Static models oversimplify digestive physiology, failing to mimic the dynamic aspects of the digestive process especially the mechanical forces and fluid dynamics, as well as pH gradients, continuous secretions or gastric emptying, in a series of rigid vessels under continuous stirring. Thus, the static models are mainly used for mechanistic studies and hypothesis building with specific applications for screening purposes.31Various static models used in the past have been developed for very specific purposes. A mono-compartmental protocol has been used for evaluating protein digestion in the context of protein allergenicity, called pepsin resistance test.32,33 This protocol represents the gastric conditions close to the end of gastric emptying or fasted state, which are highly acidic and include a high concentration of pepsin (Table 1). This test has been recommended by the European Food Safety Authority for screening purposes and the comparison with other physiologically relevant scenarios has been encouraged,34 such as simulation of gastric fed state conditions and addition of a subsequent intestinal phase. In this context, a standardised static protocol of in vitro digestion suitable for food was successfully developed by the Infogest network,20 which was based on in vivo data and validated with a ring trial.14 This model is standardised for comparison across laboratories since enzymatic parameters are expressed in standard units of enzyme activity, and experimental assays are explicitly included for accurate enzyme activity and bile acids characterisation. The protocol comprises a multi-compartment model simulating an oral, gastric and small intestinal phase in sequence with GI conditions representative of a healthy fed state human adult. The average gastric pH is higher than in the fasted stated and the average pepsin concentration is lower (Table 1). A recent publication has dealt with some new updates to this protocol,13 such as the inclusion of an oral phase regardless of the nature of the food matrix, and more detail about the use of gastric lipase.
| Infanta | Adult (fed state)b | Adult (fasted state)c | Elderlyd | ||
|---|---|---|---|---|---|
| a Data from Menard et al. (2018).35 b Data from Minekus et al. (2014) and Brodkorb et al. (2019).13,20 c Data from Thomas et al. (2004).33 d Data from Levi et al. (2014) and (2017).36,37 The gastric pH corresponds to that at the emptying half-time. | |||||
| Oral | pH | — | 7 | — | 6.8 |
Meal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SSF (w/v) SSF (w/v) |
— | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
— | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
|
| α-Amylase (U mL−1) | — | 75 | — | 150 | |
| Time (min) | — | 2 | — | 3 s | |
| Gastric | pH | 5.3 | 3 | 1.2 | 4 |
Oral bolus![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SGF (v/v) SGF (v/v) |
63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 initial 60 initial |
|
| Pepsin (U mL−1) | 268 | 2000 | 2500 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U mg−1 test protein) 000 U mg−1 test protein) |
450 initial | |
| Gastric lipase (U mL−1) | 19 | 60 | — | 153 initial | |
| Time (min) | 60 | 120 | 60 | 180 | |
| Intestinal | pH | 6.6 | 7 | — | 6.5 |
Gastric chyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SIF (v/v) SIF (v/v) |
62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
— | — | |
| Trypsin (U mL−1) | 16 | 100 | — | 46 | |
| Pancreatic lipase (U mL−1) | 90 | 2000 | — | — | |
| Bile acids (mM) | 3.1 | 10 | — | 5.34 | |
| Time (min) | 60 | 120 | — | 180 | |
| Suitability | Liquid food | Liquid/solid food | Protein solutions | Liquid food | |
There is also a need for standardising static models targeting different age populations, such as infant and elderly. The immature gut in healthy infants and the deteriorated GI functions in the elderly represent different conditions to those in healthy adults, which may lead to different outcomes of nutrient bioaccessibility.
GI conditions of the infant population have generally been neglected for in vitro studies until the last decade. The static infant models reviewed here are only those with physiologically relevant gastric pH and reasonable levels of gastric and intestinal enzymes and bile acids. One of the first studies using static infant (younger than 6 months) in vitro digestion used a gastric and an intestinal phase in sequence with relevant stomach pH (4), low pepsin and pancreatin activities and bile acid concentration, followed by continuous-flow dialysis.48 Their results showed a good agreement on the bioaccessibility of minerals from human milk, cow's milk-based and soy-based formulas with previous data in term healthy infants. Another study on in vitro digestion of human milk used human neonatal gastric juice adjusted to different pH values (6.5–2) to simulate the stomach conditions in infants.49 The authors found that milk proteins were only digested at relatively low pH values (<4), which are not representative of physiological conditions in infants. Nevertheless, in vitro results at pH 4–6.5 were in good agreement with the in vivo results from human aspirates in newborn infants fed with human milk. This highlights the importance of setting a relevant pH for these models. A static infant model was used by Dupont and co-workers to compare the in vitro digestion of dietary proteins with an adult model.50 This model from Dupont and co-workers has also been applied in combination with the Infogest protocol to study the digestion of processed dairy proteins (glycated or cross-linked) as infant formula models under infant GI conditions.51 The average gastric pH used in these studies was relatively low, pH = 3, and it was increased to pH 4 in an updated protocol to study the digestion of proteins in infant formulas.52 Nevertheless, lipolytic enzymes were not included in this protocol despite the presence of lipids in the food matrix. A gastric model was developed to compare the digestion of milk protein solutions under static pH (2.5) and dynamic pH for adult and infant conditions.53 Since the pepsin levels used for adult and infant conditions were very similar (240 and 210 U mg−1 protein, respectively), proteolysis results were only different under dynamic pH conditions. In a following study, the same research group mimicked the gastric phase with dynamic pH and added a subsequent static intestinal phase to study proteolysis of Maillard products under infant and adult conditions.54 The level of gastric and intestinal enzymes and bile acids were those used previously by Dupont and co-workers.50 Dall'Asta and co-workers modified the adult in vitro model from Versantvoort et al.55 to simulate infant GI conditions to digest human milk.56 Although the model was suitable for real food matrices, and used a relevant gastric pH of 4.5 and pancreatic enzymes concentration, the levels of pepsin included were 10 times lower than those expected for infants. Another infant static digestion protocol was used by Klitgaard and co-workers, based on in vivo data reviewed in the literature,44 to assess drug solubility and bioaccessibility in vitro under different conditions, e.g. fed versus fasted.57 The model was well-designed in terms of relevant physicochemical parameters, however the gastric lipase used was from microbial origin (Rhizopus oryzae). Although this lipase has been widely used for in vitro gastric lipolysis, it is not recommended if rabbit gastric lipase is already commercially available. Rhizopus oryzae lipase has been shown to lead to more extensive in vitro gastric lipolysis of infant formulas (46–49%) as compared to human recombinant gastric lipase and rabbit gastric lipase (10–21%), which replicate both the extent and functionality found in vivo (4.6–14.4%).42 This different behaviour between microbial and mammal source of gastric lipase is in part due to its different fatty acid chain length specificity.42 A static infant protocol considering GI digestion of infant formula has recently been developed based on in vivo data at gastric emptying half-time from literature as a first step towards consensus.35 The parameters of the GI conditions of this infant model are summarised in Table 1 for comparison with adult standard models. This model is intended to simulate 28 days old full-term newborn and includes gastric lipase from rabbit gastric extract. In addition, the presence of reduced pancreatic amylase (in pancreatin) will compensate for the absence of salivary amylase as explained above. According to the authors, the duration of each phase may need to be extended or checked for alternative protein sources, e.g. plant. In addition, the model could be extrapolated to older infants by considering the level of maturity of the enzymes. This model was recently applied to study the micelle formation of carotenoids in human milk at different lactation period (colostrum versus mature milk).58 In general, the infant static protocols have shown limited proteolysis in protein solutions or infant formulas35,50,54 and lower extent of intestinal lipolysis in infant formula as compared to adult models,35 as observed in vivo. This is related to the reduction in enzyme activity (8-fold for pepsin and 6 to 10-fold for pancreatic enzymes: trypsin, chymotrypsin and lipase) and bile acids levels (4 to 10-fold).35,50,54 More sophisticated dynamic models of infant digestion will be discussed in following sections.
What is clear is that there is a need to develop harmonised static infant protocols targeted for different stages, e.g. 0–3, 3–6 months, since the infant conditions also vary largely with postnatal age and pre-term newborns should be differentiated from full-term newborns.38 This may have caused discrepancies in the GI parameters of infant models reviewed in the literature.39 These models would be useful in screening of structural/compositional parameters of new infant formulas (e.g. ultrastructure of emulsion, effect of processing, etc.) that could have important implications for digestion and absorption of nutrients in infants and in the performance of oral drug delivery.35,38
Given the static nature of these models, the inherent limitations are the inability to replicate the dynamics of biochemical secretions, gastrointestinal emptying and motility, digesta absorption, hormonal responses, etc. Thus, the dynamics parameters obtained from these in vitro digestion models, such as the rate of digestion, are not physiologically relevant. It must be considered that the pH gradients within the GI tract, the buffering capacity of the food substrate, the gradual release of enzymes, the gastric sieving and emptying, and the removal of digestion products in the small intestine are likely affecting the kinetics of in vitro digestion and will be explained in more detail in following sections. However, the end-point results in the gastric and intestinal phase simulated with the Infogest protocol have shown good agreement with in vivo data for milk proteins.15
2.3 Semi-dynamic in vitro digestion models
Some digestion models classified as semi-dynamic can simulate at least one of the dynamic features of the GI tract. It is an intermediate system between the complex dynamic models and the simpler static models. Table 2 shows a list of studies in which a semi-dynamic model was used, illustrating the key parameters and applications.| Study | Simulated dynamics | Control unit | Main body | Mixing mechanism | Temperature control | Digestion fluids | Food substrate tested | Application |
|---|---|---|---|---|---|---|---|---|
| SGF: simulated gastric fluid, NS: not specified. | ||||||||
| Bourlieu et al. (2015)47 | Gastric pH changes (from pH 6 to 4 for 180 min) | pH-Stat device | Vessel | NS | 37 °C, water | - SGF electrolytes: 110 mM NaCl, 5 mM CaCl2, 150 mM HCl | Milk emulsion containing native milk fat globules and two processed model infant formulas (homogenized or homogenized/pasteurized) | Proteolysis and lipolysis kinetics, and structural changes in infant digestion |
| - Rabbit gastric lipase (26 U mL−1) | ||||||||
| - Porcine pepsin (261.45 U mL−1) | ||||||||
| Shani-Levi et al. (2013)53 | Gastric pH changes (adult: from pH 4.5 to 1.5 for 120 min, infant: from pH 6.5 to 3.5 for 240 min) | pH-Stat device | Vessel (cylindrical, 50–150 mL) | Rotational stirring (250 rpm) | 37 °C, water | - SGF electrolytes: 2 g L−1 NaCl adjusted to pH 1.2 | β-Lactoglobulin and lactoferrin solutions, and their emulsions using olive oil | Proteolysis kinetics in infant and adult digestion |
| Joubran et al. (2015)54 | - Porcine pepsin (240 or 210 U mg−1 protein for adult or infant, respectively) | Maillard conjugates (α-lactalbumin with fructose or fructo-oligosacharides) | Proteolysis kinetics to study antioxidant capacity | |||||
| Cohen et al. (2017)76 | Vitamin D mixed with sodium caseinate | Protein digestion kinetics and gastric behaviour to study bioavailability and uptake of vitamin D | ||||||
| Dekkers et al. (2016)65 | Gastric pH changes (profile A: from pH 6 to ∼2, profile B: from pH 5 to 4, for 90 min) | - Syringe pump | NS | NS | NS | - SGF electrolytes:2 mg mL−1 NaCl adjusted to pH 1.9 | β-Lactoglobulin-xanthan biopolymers gels | Kinetics/extent of protein digestion |
| - pH module | - Porcine pepsin (4.5 g mL−1) | |||||||
| Liu et al. (2019)66 | - Gastric pH changes (from pH 6.5 to 2 for 30 min) | - pH-Stat device | Vessel | Rotational stirring (95 rpm) | 37 °C, water | - SGF electrolytes: prepared as described by Minekus et al. (2014)20 but not pH adjusted | α-Lactalbumin solution | Protein digestion kinetics and structural changes |
| - Gradual enzyme secretion | - Syringe pump | - Porcine pepsin (2000 U mL−1) | ||||||
| Fahoum et al. (2017)77 | Gastric pH changes (from pH 4.5 to 1.5 for 1 hour) | pH-Stat device | Cylindrical vessel (50–150 mL) | Rotational stirring (250 rpm) | 37 °C, water | - SGF electrolytes based on Minekus et al. (2014)20 adjusted to pH 4.5 | κ-, L-, λ-carrageenan mixed with milk, soy or egg protein isolates | Protein hydrolysis kinetics |
| Stübler et al. (2019)78 | - Porcine pepsin (2000 U mL−1) | Kale juice and strawberry puree and a blend of them | Protein hydrolysis to study protein-polyphenol stability | |||||
| Aarak et al. (2013)28 | Gastric pH changes (from pH 6.5 to 2.5 for 30 min) | pH-Stat device | Vessel | NS | 37 °C, water | - SGF electrolytes: 0.15 M NaCl, pH: 6.5 | Cooked salmon, broccoli and barley (simulating a main dinner course) | Lipid and protein digestion, and digesta viscosity kinetics. |
| - Phosphatidylcholine, 0.17 mM | ||||||||
| - Porcine pepsin (170 U mg−1 protein substrate) | ||||||||
| van Aken et al. (2011)79 | - Gastric pH changes (0.2 mL min−1 of 0.03 M HCl for 60 min) | NS | Vessel (cylindrical, 150 mL) | Rotational stirring, 260 rpm | 37 °C, water | - Porcine pepsin (902 U mL−1) | Oil-in-water emulsion (stabilised by whey protein and Tween 80) and homogenized full fat milk | Gastric behaviour, and protein and lipid distribution after gastric digestion |
| - Gradual enzyme secretion | - Fungal lipase (12 U mL−1) | |||||||
| Ferreira-Lazarte et al. (2017)80 | - Gastric pH changes (to reach pH 2 for 2 hours) | - pH-Stat device | Vessel (v-shape, 70 mL) | Orbital stirring | 37 °C, water | - SGF electrolytes based on Minekus et al. (2014)20 adjusted to pH 7 | Skim milk powder with prebiotic carbohydrates | Carbohydrate and protein hydrolysis |
| - Gradual enzyme and simulated fluids secretion | - Syringe pump | - Porcine pepsin (2000 U mL−1) | ||||||
| Mulet-Cabero et al. (2017)74 | - Gastric pH changes (to reach pH 2, time depending on caloric density of food) | - pH-Stat device | Vessel (v-shape, 70 mL) | Orbital stirring | 37 °C, water | - SGF electrolytes based on Minekus et al. (2014)20 adjusted to pH 7 | Semi-solid meal (mixture of cheese and yogurt) and liquid meal (oil-in-water emulsion stabilised by milk proteins) | Gastric behaviour and, protein and lipid hydrolysis kinetics |
| Mulet-Cabero et al. (2019)69 | - Gradual enzyme and simulated fluids secretion | - Syringe pumps | - Porcine pepsin (2000 U mL−1) | Bovine whole milk with different industrial processes (pasteurisation, ultra-high temperature sterilisation and homogenisation) | Gastric behaviour, lipid released and protein hydrolysis kinetics | |||
| Mulet-Cabero et al. (2020)67 | - Gastric emptying | - Gastric lipase, 40 U mL−1 (from rabbit gastric extract), added in Mulet-cabero et al. 201774) | Protein mixtures solutions with different casein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) whey protein ratios, and inclusions of rapeseed oil whey protein ratios, and inclusions of rapeseed oil |
Gastric behaviour, lipid released and protein hydrolysis kinetics | ||||
| van den Braak et al. (2013)70 | - Gastric pH changes (from pH 6.6 to 2 for 100 min) | Computer-controlled pumps, pH sensors | 8 bioreactors (cylindrical) | Rotational stirring (400 rpm) | 37 °C, water | - SGF electrolytes: 50 mM NaCl, 15 mM KCl, 1 mM CaCl2·H2O, 15 mM NaHCO3 | Soy, pea, whey protein isolates and calcium caseinate, and a blend of them. | Gastric behaviour |
| Luiking et al. (2016)81 | - Gradual secretion of enzymes and simulated fluids | - Porcine pepsin 0.014% (w/v) | Whey protein isolate (leucine-enriched) and calcium caseinate with lipids and carbohydrates | Protein digestion kinetics | ||||
| - Gastric lipase (Rhizopus oryzae) 0.019% (w/v) | ||||||||
| - Artificial saliva (0.1 M NaCl, 30 mM KCl, 2 mM CaCl2, 15 mM NaHCO3, 0.065% (w/v) α-amylase) | ||||||||
| Bicompartmental | ||||||||
| Levi et al. (2014)36 | - Gastric pH changes | - Software programmes (“my-control” and “BioXper”) | 2 vessels (cylindrical, 250 mL) | Rotational stirring, pulsating (gastric) and constant (duodenal) | 37 °C | - Simulated gastric, duodenal and bile fluids containing different concentration of the following salts: KCl, KH2PO4, NaHCO3, NaCl, MgCl2(H2O)6, NH4Cl, NaH2PO4(H2O)2, urea and CaCl2(H2O)2. | β-Lactoglobulin, α-lactalbumin and lactoferrin solutions | Protein digestion kinetics in the elderly and adult digestion conditions |
| Levi et al. (2017)37 | - Gastric emptying | - Peristaltic pump | - Porcine pepsin 1000 (adults) or 750 U mL−1 (the elderly) | β-Lactoglobulin, α-lactalbumin and lactoferrin solutions and, commercial liquid formula (proteins, lipids, carbohydrates and vitamins) | Protein digestion kinetics and generation of bioactive peptides in the elderly and adult digestion conditions | |||
| - Duodenal pH | - pH sensors | - Gastric lipase (from rabbit gastric extract) 1500 (adults) or 255 U mL−1 (the elderly). | ||||||
| - Bile secretion gradient | - Porcine trypsin 100 (adults) or 46 U mL−1 (the elderly), chymotrypsin 50 (adults) or 23 U mL−1 (the elderly). | |||||||
| - Bile salts: sodium glycodeoxycholate and taurocholic acid sodium salt hydrate, 4 (adults) or 2.67 mM (the elderly) | ||||||||
| - Porcine pancreatin lipase 2000 (adults)or 1400 U mL−1 (the elderly) | ||||||||
| - Porcine α-amylase 150 (adults)or 300 U mL−1 (the elderly) | ||||||||
| - Mucin from porcine stomach 0.05 g L−1 | ||||||||
The semi-dynamic models have been mainly used in reference to the gastric phase, simulating the chyme pH and/or the controlled enzymatic release. There are studies that have combined gastric digestion with the simulation of oral and/or intestinal phases but in a static manner. The semi-dynamic models are usually performed in a single simple vessel in contrast to the complex computer-controlled compartments of digestion used in the dynamic models. Most of the studies found in the literature that used some type of semi-dynamic model have focussed on the simulation of the dynamic changes in gastric pH. In vivo studies have shown that meal consumption causes a rapid increase in gastric pH from the fasted pH of around 2 to a pH of 4.5–6.7 within 15 min, following of a steady pH decrease due to gradual HCl secretion.63,64 The main unit for pH control used in these studies is a pH-stat device, which can usually control both pH and fluid secretion. The pH-stat methodology is simple to use and available in most of the laboratories, and allows a computer-controlled auto-titration to simulate the gradient gastric pH profile observed in vivo.63,64
The gastric pH can have a considerable impact on the kinetics of nutrient digestion, especially of proteins. The influence of gastric pH profile on digestion was studied in mixtures of β-lactoglobulin and xanthan gum gels by Dekkers and co-workers.65 Two dynamic gastric pH profiles (initial pH of 6.0 vs. 5.2 and HCl secretion rates of 60 vs. 36 mmol h−1) were compared with a fixed gastric pH (1.9). It is important to mention that the pH changes were based on data from previous studies in vivo. The study by Dekkers and co-workers showed significantly more protein hydrolysis during the first 30 min in the static pH digestion compared to the dynamic pH profile digestions. However, all three pH profiles provided similar extent of digestion after 90 min. Therefore, the static digestion induced the overestimation of pepsin activity during gastric phase. Protein hydrolysis in the stomach is performed by pepsin, whose activity is pH dependent and has a maximum at pH 1.5–2.5.43 Similarly, Liu and co-workers showed slower protein digestion of α-lactalbumin when using semi-dynamic and dynamic models, compared to a static model (pH 3).66 However, the lag phase in the dynamic system was a bit longer than that in the semi-dynamic model. This was suggested to be due to the high force of stirring of the semi-dynamic system whereas the mixing in the dynamic system led to a more heterogeneous pH environment in the body of the simulated stomach.
The changes in gastric pH can also affect protein conformation and structure, which will influence proteolytic susceptibility, the kinetics of protein digestion and gastric emptying.67 Mulet-Cabero and co-workers investigated the behaviour of the main milk proteins, i.e. caseins and whey proteins, during gastric digestion performed by a semi-dynamic model simulating the dynamic pH profile, gradual secretion of enzyme and gastric fluids, and gastric emptying.67 The authors showed that micellar caseins coagulated in the gastric conditions forming hard clots persisting during gastric digestion whereas whey proteins formed soft flocs that were not visible at the end of the gastric digestion. This study confirmed that the gastric behaviour of the milk proteins was the limiting factor of their protein digestion and absorption in the small intestine. This finding relates to the concept of ‘slow’ and ‘fast’ proteins corresponding to caseins and whey proteins, respectively, as observed in the rate of amino acids (AAs) appearance in plasma of an in vivo study.68
The digestion of other macronutrients is also affected by the gastric pH changes. Shani-Levi et al. showed that oil-in-water emulsions stabilised by β-lactoglobulin and lactoferrin underwent several colloidal behaviours (destabilization, flocculation and coalescence) during gastric digestion using a dynamic pH profile, compared to the static profile.53 Similarly, Mulet-Cabero and co-workers observed different colloidal behaviours during the gastric phase of bovine whole milk with different industrial processing, i.e. homogenisation, pasteurisation and ultra-high temperature (UHT) sterilisation.69 The homogenised samples presented the formation of an upper lipid layer, which delayed the subsequent emptying of lipids. However, neither of these two studies used gastric lipase, which could influence the gastric digestion and behaviour of lipids. The availability of relevant gastric lipases, such as rabbit gastric extract, has been recently improved. This will allow the investigation of the effect of dynamic gastric pH on lipid digestion, which involves gastric lipase having a different pH optimum compared to pepsin.10 For instance, using rabbit gastric extract, Bourlieu and co-workers showed that the structural parameters of the infant milk formulas tested were a limiting factor to control gastric lipolysis and the patterns of fatty acids released, when using a dynamic gastric pH based on infant digestion conditions.47
The conditions of digestion in the GI tract vary significantly between age populations i.e. infants, adults and the elderly.39 This includes pH gradient, enzymatic levels and gastric emptying rates. These differences can lead to changes in the hydrolysis of food nutrients. Some of the reported semi-dynamic models have been adapted to simulate the digestion of other age groups, apart from adults. For example, in vivo data has been used to adjust the pH gradient to simulate the digestion of infants47,53 and the elderly digestion.36 One of the advantages of the semi-dynamic model is that it can be easily adapted to other parameters of digestion, such as pH, since they do not usually involve complex software. However, there are also more advanced semi-dynamic models. Shani-Levi and co-workers used a bi-compartmental system, two reactors connected, simulating the gastric and duodenal phases.36 In this model, a computer controls the temperature, the different secretions and the gastric emptying. This bi-compartmental model was used to simulate the digestion of both adult and the elderly using in vivo parameters specific to the relevant age. The digestive fate of protein was determined in some milk protein solutions (β-lactoglobulin, α-lactalbumin and lactoferrin) to assess the efficacy of the system and compare the two sets of digestion conditions. As seen in Table 2, the model uses complex digestion fluids, individual enzymes and bile salts. Using the same semi-dynamic bi-compartment system, the use of gastric lipase from rabbit gastric extract in a dynamic gastric pH profile led to differences in the colloidal gastric phase.39 The state of coalescence was observed under adult conditions while flocculation was dominant under elderly conditions. This behaviour could have affected the subsequent protein digestion in the duodenum as seen by the generation of different bioactive peptides. Another sophisticated semi-dynamic model consists of eight parallel computer-controlled bioreactors, offering high throughput capabilities.70 Each unit is equipped with a pH electrode and four pumps with dosing-lines (1 M HCl and 1 M NaHCO3 and 3 M NaOH, for pH adjustment, simulated gastric juice and simulated saliva). During the digestion, both simulated saliva and gastric fluids were added gradually. This model was applied to some plant proteins (pea and soy) to assess their gastric behaviour compared to whey proteins and sodium caseinate.
The use of a semi-dynamic simulation for studying gastric behaviour needs to consider the mixing mechanism carefully. Most of the semi-dynamic models discussed used rotational stirring using, usually, a magnetic stirrer. This probably disrupts the formation of any possible structural changes within the simulated stomach. The aim of the mixing in this model should be to disperse the acid solution that is secreted over time. This does not mean that the pH needs to be homogenous within the compartment. Indeed, the human intragastric pH is heterogeneous and the chyme is not distributed homogenously within the stomach.71 In addition, the shape of the simulated stomach should be considered since it can influence the shear forces. The stomach is a J-shaped organ and the construction of this kind of shape can be difficult. The cylindrical shape vessel is the most common shape used in the present semi-dynamic models. However, a V-shape vessel has been used,67 which simulates the inverted cone shape observed in some dynamic models such as the dynamic gastric model72 and the human gastric simulator.73
The semi-dynamic model has been seen of relevance to gain more insight into the digestion of food that is susceptible to pH change and obtain kinetics of protein digestion. However, as seen in Table 2, the parameters in which digestion is performed vary from one study to another and even in the same research group. In addition, some of the parameters such as enzyme source and activities, and mixing have not been described or clearly specified. In order to allow comparison, a standardised protocol for a semi-dynamic model has been recently developed.21 The authors provide a detailed description of how to perform this semi-dynamic model, which includes crucial kinetic aspects associated with the gastric phase of digestion, including gradual acidification, fluid and enzyme secretion and emptying. Static small intestinal digestion of the aliquots that are collected over time simulating the gastric emptying can be used to evaluate the kinetics of nutrient bioaccessibility. Adaptations of this model have already been used to provide kinetic data on nutrient digestion and structural changes during the gastric phase, which influenced nutrient absorption.67,69,74 Moreover, it provides a simple tool that can be used in a wide range of laboratories.
In conclusion, the semi-dynamic model provides a simple approach to perform a more physiologically relevant simulation of the gastric phase, by which digestion kinetics can be obtained. It could help to provide new and more physiologically relevant insights into protein and emulsion gastric behaviour, which is crucial in the understanding of the kinetics of nutrient bioavailability and absorption. For instance, by the simulation of the gastric emptying, the degree of digestion of the initial fractions leaving the stomach may have a great influence in the neuro-hormonal regulation of the subsequent gastric emptying.75 It requires simple and widely accessible devices, which allows its use in many laboratories. It is much less expensive than dynamic models since it is based on small volumes, therefore, the amount of enzymes needed is less. However, running this model is more time-consuming and a lower throughput system, compared to the static model. Furthermore, the gastric emptying may be difficult with some foods, especially solids.
2.4 Dynamic in vitro digestion models
Dynamic model systems are designed to mimic a series of variable factors of the GI tract, such as the secretion of digestion fluids, variable enzyme concentration, pH changes, the transit of chyme and the appropriate mixing at each stage due to peristalsis. Because of these characteristics, they can provide a meaningful mechanistic understanding of food digestion by assessing the nutrient bioaccessibility rate and extent. A few dynamic in vitro models have been developed in comparison to static models. Dynamic models can be mono-compartmental, bicompartmental or multicompartmental, which simulates one, two and several compartments of the digestive tract, respectively. The use of the different models will depend on the purpose of study. In this section, the specific mouth simulators and colon models will not be discussed but have been reviewed elsewhere.82,83| System | Photo | Simulated dynamics | Digestion fluids | Application | Food substrate tested | Ref. |
|---|---|---|---|---|---|---|
| Dynamic gastric model (DGM) |
|
- Gastric pH changes | - Electrolytes (0.2 M HCl, 0.08 M NaCl, 0.03 mM CaCl2, 0.9 mM NaH2PO4) | Bioaccessibility kinetics of bioactives (polyphenols, carotenoids) | Raw, toasted pistachios, and muffins with raw pistachios | Mandalari et al. (2013)94 |
| - Gradual enzyme secretions | - Shelled lecithin liposomes (0.127 mM) | Biscuits, crisp-bread and full-fat milk with almond skins | Mandalari et al. (2016)105 | |||
| - Peristaltic contractions | - Pepsin (9000 U mL−1, porcine) | Edible agar film with green tea extract | Lopez de Lacey et al. (2012)106 | |||
| - Gastric emptying and sieving | - Gastric lipase (60 U mL−1, fungal, Rhizopus oryzae) (conditions from Curto et al.201195) | - Extent of lipid hydrolysis | Raw and roasted almonds | Mandalari et al. (2014)90 | ||
| - Structural changes | Muffins with small or large almond particles | Grassby et al. (2017)91 | ||||
| Hydrolysis kinetics of protein | Chocolate mouse and Victoria sponge cake made with almond flour | Mandalari et al. (2014)92 | ||||
| - Hydrolysis kinetics of starch | White bread, cornflakes, micronized barley and extruded barley flakes, oat flour and extruded oat flakes | Ballance et al. (2013)93 | ||||
| - Structural changes | Extruded snack with blends of wheat flour and (native/hydrolysed) pea protein | López-Barón et al. (2018)107 | ||||
| Hydrolysis kinetics of starch and protein | Porridge meal with flour (different particle size) and semolina (different particle size) | Mandalari et al. (2018)108 | ||||
| Human gastric simulator (HGS) |
|
- Gastric pH changes | - Electrolytes: NaCl (8.775 g L−1), adjusted to pH 1.3 | Physical disintegration kinetics | Emulsion (whey protein and soybean oil) gels. Hard vs. soft | Guo et al. (2015)101 |
| - Gradual gastric secretions | - Pepsin (1 g L−1, porcine) | Lipid stability, protein hydrolysis and digesta microstructure | Guo et al. (2014)100 | |||
| - Gradual gastric emptying in a set time and sieving | - Gastric mucin (1.5 g L−1) (conditions from Kong et al. 2010![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73) 73) |
Physical disintegration kinetics and rheology properties of gastric digesta | Cooked white and brown rice | Kong et al. (2011)98 | ||
| - Continuous peristaltic contractions and antrum forces | Gastric structural changes and protein hydrolysis kinetics | Skim milk (unheated vs. heated, 90 °C for 20 min) | Ye et al. (2016)102 | |||
| Gastric structural changes, protein/lipid release kinetics, protein hydrolysis kinetics and digesta microstructure | Whole milk (untreated/homogenised/homogenised + heated) | Ye et al. (2016)103 | ||||
| Ye et al. (2017)104 | ||||||
| Gastric structural changes, protein hydrolysis kinetics and digesta microstructure | Milk protein ingredients (skim milk powder, milk protein concentrate, calcium-depleted milk protein concentrate, sodium caseinate, whey protein isolate (unheated, heated at 90 °C for 20 min) | Wang et al. (2018)109 | ||||
| Gastric structural changes, protein hydrolysis kinetics, lipid release kinetics and digesta microstructure | Oil-in-water emulsions stabilised by milk protein ingredients (milk protein concentrate, calcium-depleted milk protein concentrate, sodium caseinate) | Wang et al. (2019)110 | ||||
| Oil-in-water emulsions stabilised by whey protein isolate. Unheated vs. heated (90 °C, 20 min) | Ye et al. (2020)111 |
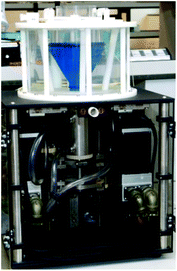
The dynamic gastric model (DGM). The DGM was developed at the former Institute of Food Research (Norwich, UK).86 It is a computer-controlled single compartment with an inverted cone shape, made of latex and capacity of ∼800 mL. Within the fundus/main body of the DGM, the acid and enzyme fluids are secreted depending on the food composition. The rate of acid secretion is controlled by the pH response of the food sample. The mechanical forces are applied by water (kept at 37 °C) pressure with a piston and barrel, and differ in the main body and the antrum. In the main body of the DGM, the bolus is subjected to gentle peristaltic contractions (three contractions per minute). Portions of gastric contents are moved into the antral part and subjected to high shear stress and mixing. There is also the addition of an initial gastric basal volume. The gastric residence time is calculated by the software associated with the DGM and depends on the meal size, composition and caloric content. The emptying is set at pre-defined intervals and after emptying, samples may be subjected to further digestion depending on the purpose of the study. The efficiency of the antral mechanical forces were calibrated by studying the breakdown of agar beads of various fracture strengths in high- and low-viscosity meals.87 The results obtained from the DGM were comparable to those reported in a human study using similar meals.88
The DGM has been extensively used for food-based research. The system allows the use of complex and realistic food matrices, as used in in vivo studies. It has widely been used to assess the hydrolysis rate and extent of macronutrients and bioactives in the GI upper tract through the rate of nutrient released from the gastric digestion. The effect of food matrix and processing has been mainly investigated. In addition, the study of the structural changes during the digestion has been performed using microscopy to gain more insight into the mechanisms of food structure on nutrient delivery and nutrient interactions. For instance, the in vitro study of the lipid digestion in almonds is important to understand the mechanisms by which the consumption of almonds can reduce a number of risk factors associated with non-communicable diseases such as obesity.89 The effect of processing (roasting) and mastication on the lipid bioaccessibility of almonds was studied using the DGM followed by static small intestine digestion.90 In addition, the effect of the almond particle size in a muffin matrix was investigated.91 These studies have concluded that the cell wall in almonds governs the lipid digestion and therefore give plausible mechanisms of the physiological responses observed after the consumption of almonds. The effect of the food matrix was also studied in the protein digestion and immunoreactivity of polypeptides from almond flour,92 which provides insight into the interrelationship between food matrix and almond allergy. The DGM was used to assess the GI digestion of starch in different cereals to provide mechanisms associated with glycaemic response.93 The DGM has also been used to study the bioaccessibility kinetics of other bioactives. The release of polyphenols and tocopherols from pistachios, with different processing and in the presence of food matrix (muffin), was assessed during GI digestion.94 This research highlights the relevance of the food matrix in the uptake of beneficial bioactives. The DGM has also been used to study probiotic survival in the GI tract.95
The limitations of the model include the vertical orientation of the gastric compartment, which differs from that in vivo, which might affect the simulation of fluid dynamics and distribution of gastric contents. The compartment is not transparent, which does not allow visual observations during digestion. Moreover, it is open from the top exposed to air, which might lead to pH fluctuations.
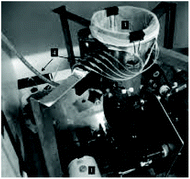
The human gastric simulator (HGS). The HGS was created at the University of California (USA) and also reproduced at the Riddet Institute (New Zealand).73 The stomach compartment is simulated by a cylindrical flexible chamber made of latex in an inverted conical shape and capacity of about 5.7 L. The delivery of gastric secretion is controlled by a peristaltic pump and usually set at the rate of 2.5 mL min−1. Gastric emptying is performed by a peristaltic pump connected to the bottom of the gastric compartment with a mesh bag (pore size ∼1.5 mm) placed inside the chamber to mimic the human gastric sieving. The rate of gastric emptying is usually set at 3 mL min−1 and the time of gastric digestion varies from 3 to 5 hours. The system is operated at 37 °C using a heater and fan. It has a series of rollers supported by belts controlled by a variable speed motor. The rollers compress the simulated stomach wall with an increasing amplitude (set usually at three contractions per minute). The compression forces are higher on the bottom of the compartment as the rollers come closer, which increases the amplitude and mimic the antral contractions. The HGS was designed aiming to closely mimic the peristaltic activity of the antrum contractions waves and, thus, provide a physiologically relevant range of amplitude and frequency of mechanical forces as those presented in vivo. The performance of the HGS was evaluated by digesting cooked rice and apple slices in the simulated stomach. The maximum stresses recorded within the HGS were 6738 N m−2 and 8922 N m−2, for 50% and 70% of compression, respectively. This is, according to the authors, in good agreement with in vivo data that reported mechanical stresses varying from 5134 to 67
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 292 N m−2 (ref. 88 and 96) and the comparison of the contraction force profile.97
292 N m−2 (ref. 88 and 96) and the comparison of the contraction force profile.97
The HGS has been widely used in the research of food materials. The main applications have been to investigate the effect of physical breakdown and gastric stability during digestion on nutrient release kinetics. This research is of high relevance to understand the mechanisms of the physiological responses of foods. For instance, the physical digestion of brown and white rice was studied using the HGS.98 It was shown that the bran layer on brown rice limited the physical disintegration of the rice, delaying the gastric emptying and, as a result, the possible starch digestion. This research has offered a plausible mechanism by which some human studies showed that brown rice lowered glycaemic responses when compared to white rice.99 The disintegration kinetics in the stomach are of crucial relevance to assess the nutrient bioaccessibility in the small intestine. The consistency of the matrix in which the nutrients are confined governs the released of proteins and lipids, and their gastric emptying. This was investigated using emulsion gels with different hardness digested in the HGS.100,101 The dynamics of the HGS provided the structural changes during gastric digestion of milk, forming a structured clot. This restructuring impacted protein hydrolysis102 and lipid released103 during gastric digestion. Furthermore, this gastric behaviour was affected by the different milk processing treatments of homogenisation and heat.104
The limitations of the HGS include the fact that the rates of gastric fluid secretion and emptying, as well as the time of residence, are not based on the properties of the food materials, which is not physiologically relevant. The HGS, similar to the DGM, does not provide a physiological shape of the stomach and the gastric contents depend on gravity. Additionally, the progression of the digestion cannot be observed in situ since the material of the compartment is not transparent.
| System | Photo | Simulated dynamics | Digestion fluids | Application | Food substrate tested | Ref. |
|---|---|---|---|---|---|---|
| TNO gastro-intestinal model (TIM-1) |
|
Gastric compartment: | Gastric compartment: | Protein and starch digestion rate | Short-dough biscuits (enriched in proteins and/or fibre) | Villemejane et al. (2016)116 |
| - Gastric pH changes | - Electrolytes: 1.1 g L−1 KCl, 3.1 g L−1 NaCl, 0.15 g L−1 CaCl2, 0.6 g L−1 NaHCO3 | Viscosity kinetics of chyme | Villemejane et al. (2015)115 | |||
| - Gradual enzyme secretion | - Pepsin (porcine, 520 U min−1) | - Starch hydrolysis rate | Fermented barley and oat Tempe | Alminger et al. (2012)133 | ||
| - Mixing | - Lipase (amano lipase, 7 U min−1) | - Digesta microstructure | ||||
| - Gastric emptying and sieving | From pH 6.2 to 2.3, for 240 min using 0.6 M HCl | Glucose bioaccessibility rate | Rye products (sourdough-fermented bread, fermented and unfermented crispbread, extrusion-cooked rye, and porridge) | Johansson et al. (2018)134 | ||
| Small intestinal compartment: | Small intestinal compartment: | - Viscosity kinetics of chyme | Glucose and maltodextrin solutions | Karthikeyan et al. (2019)135 | ||
| - Controlled pH at pre-set values for each compartment | - Electrolytes: 5 g L−1 NaCl, 0.6 g L−1 KCl, 0.3 g L−1 CaCl2·2H2O | - Glucose bioaccessibility rate | Milk protein concentrate added to instant oats and steel cut oats | AlHasawi et al. (2018)136 | ||
| - Gradual secretion of bile, intestinal electrolytes and pancreatin | - Bile salts (porcine, 20 mg min−1 for 30 min, then 10 mg min−1) | Instant oats, steel cut oats and oat bran | AlHasawi et al. (2017)117 | |||
| - Ileal emptying rate | - Pancreatin (porcine, 89 mg min−1) with 1% w/w pancreatin lipase | Bioaccessibility rate of fatty acids | Oil-in-water emulsions with tricaprylin and nonionic surfactants (Tween-20, Tween-40, Tween-60, Span-20, Span-60, or Span-80) and anionic surfactants (sodium lauryl sulfate, sodium stearoyl lactylate, and sodium stearyl fumarate) | Speranza et al. (2013)137 | ||
| - Gradual removal of digestion products and water | Duodenum pH 6, jejunum pH 6.9 and ileum pH 7.2 using 1 M NaHCO3 (conditions from Villemejane et al. 2015115) | Kinetics of nitrogen bioaccessibility | Salmon/whey protein hydrolysate | Framroze et al. (2014)138 | ||
| Antioxidant and anti-inflammatory properties of bioaccessible compounds over digestion time | Wheat fractions: aleurone, bran, flour or starch | Anson et al. (2010)139 | ||||
| Bioaccessibility rate of iron and phosphorus | Rapeseed, sunflower seed, whole-wheat and white wheat flour | Larsson et al. (1997)140 | ||||
| Bioaccessibility rate of phytochemicals | β-Carotene from vegetable or supplemented β-carotene | Van Loo-Bouwman et al. (2014)141 | ||||
| Blueberry and maqui berry extract (with and without a standardised high fat meal) | Lila et al. (2012)142 | |||||
| Red or yellow tomato puree in a standard meal (western diet) | Blanquet-Diot et al. (2009)119 | |||||
| Boiled, fried and scrambled eggs | Nimalaratne et al. (2015)118 | |||||
| Breads from different wheat fractions (aleurone, bran and flour) | Anson et al. (2009)5 | |||||
| Bioaccessibility rate of vitamins | UHT processed and pasteurised milk fortified with folic acid or 5-methyltetrahydrofolate | Verwei et al. (2003)143 | ||||
| DIDGI® |
|
Gastric compartment: | Gastric compartment: | Protein hydrolysis kinetics | Bovine skim milk powder | Egger et al. (2019)15 |
| - Gastric pH changes | - Electrolytes: Na+ 100 mmol L−1, Ca2+ 1 mmol L−1, pH 6.5 | Heated and unheated bovine skim milk powder | Sánchez-Rivera et al. (2015)126 | |||
| - Gradual enzyme secretion | - Pepsin (porcine, 2000 U mL−1, 1 mL min−1 for 5 min then 0.5 mL min−1) | Liquid yoghurts (enriched in protein/fibre) with different viscosity | Ménard et al. (2018)144 | |||
| - Gastric emptying and sieving | Small intestinal compartment: | Lipid digestion kinetics | Fat-free yogurt enriched with whey protein isolate emulsified safflower oil (in free emulsion or encapsulated with calcium-alginate beads) | Corstens et al. (2018)128 | ||
| Small intestinal compartment: | - Intestinal electrolytes: Na+ 100 mmol L−1, Ca2+ 100 mmol L−1, pH 6.6 | Starch hydrolysis kinetics | White bread, wheat-based and gluten-free pasta | Freitas et al. (2019)129 | ||
| - Controlled pH | - Pancreatin (porcine, 7%, 0.25 mL min−1)) | White bread with water, tea or lemon juice | Freitas et al. (2019)145 | |||
| - Gradual secretion of bile, intestinal electrolytes and pancreatin | - Bile salts (bovine, 4% for 30 min then 2%) (conditions from Menard et al. 2018144) | In an infant digestion model | Human milk (pasteurised and raw) | de Oliveira et al. (2016)125 | ||
| - Kinetics of lipolysis and proteolysis | Deglaire et al. (2016)146 | |||||
| - Structural changes | Infant milk formula | Ménard et al. (2014)124 |
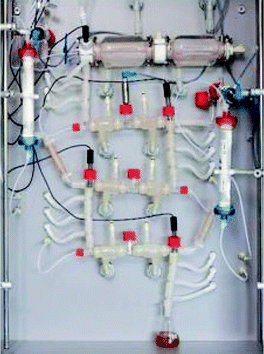
TNO's gastrointestinal model (TIM). The TIM was developed at TNO Nutrition and Food Research (The Netherlands).114 It consists of tubular compartments made of glass jackets (capacity of ∼300 mL) with flexible inner wall that can be controlled independently. The conditions of digestion in each compartment, such as pH and secretion rates, are controlled by software based on in vivo studies in animals and human volunteers. Peristaltic pumps enable the transit of chyme between compartments. The programmable gastric emptying curve depends on type and amount of food, and follows Elashoff's equation, as well as the intestinal delivery. To imitate peristalsis, the tubes are squeezed periodically by a pump action on the surrounding water, which also maintains a physiological temperature (37 °C). It is usually applied 3 and 9 contractions per minute in the gastric and intestine compartment, respectively. Furthermore, the TIM allows the constant removal of digestive products and water by filtration and membrane dialysis to simulate the nutrient uptake in the small intestine.
Some TIM systems have been developed focussing on the upper GI tract; TIM-1, with a stomach compartment and three compartments for the small intestine, i.e. the duodenum, jejunum and ileum, and the tiny-TIM that consists of a stomach compartment and one single compartment for the small intestine. The accuracy and reproducibility of the model for gastric delivery, intestinal transit and ileal delivery of chyme were assessed in simulating a slow and fast transit time.114 For that, the data from human volunteers who consumed yoghurt and milk was applied, respectively, and a solution of blue dextran was used as a test meal. The efficacy of the model was assessed by testing the glucose levels in the dialyzed aliquots from the simulated ileum. The in vitro data from the digesta transit simulated closely the in vivo data.
The unique feature of the incorporated dialysis or filtration membrane systems allows the assessment of the rate at which the absorbable digestion products are generated. The TIM has been used for food-related studies to examine the bioaccessibility of nutrients and the stability of phytochemicals, providing mechanistic insights into some physiological responses of foods. For instance, dietary fibre has been shown to exert some specific physiological responses (e.g. reduced postprandial glycaemic response) and one of the mechanisms proposed is the increase of digesta consistency in the GI tract. The effect of the enrichment of biscuits in viscous soluble fibres, combined or not with protein, on the viscosity of the chyme was investigated throughout the different upper digestive compartments of the TIM-1 system.115 The results showed a significant effect of the soluble fibres on the chyme viscosity during GI digestion. Using the same biscuits, the effects of enrichment in fibre and proteins on starch and protein hydrolysis was determined during the digestive process.116 These studies highlight the relevance of the effect of dietary fibre in a dynamic system, as well as the mechanisms driving the physiological responses and the limiting factors. Viscosity is one of the major physical properties of food having a major role in influencing the flow behaviour and gastric emptying rate, which can affect the rates of digestion, nutrient release and intestinal absorption. Real-time luminal viscosity in the simulated GI tract of TIM-1 was assessed using optical chromophores that exhibit molecular rotor behaviour.117 The physicochemical properties of three commercially available oat-based products (instant oats, steel cut oats and oat bran) were assessed on starch digestion. The authors showed that steel cut oats presented the slowest rate of starch digestion, which was linked to the gastric luminal viscosity.
The TIM-1 system has been widely used in the assessment of the bioaccessibility of different phytochemicals during digestion. This system has provided relevant information about the digestive stability of bioactives, especially at the level where degradation occurs throughout digestion, and about their sensitivity to the different factors of the GI digestion, in particular, the pH changes. The main effects tested are food matrix and processing. Some examples are the investigation of the effect of processing of eggs in the bioaccessibility of lutein and zeaxanthin,118 and the study of the bioaccessibility of zeaxanthin, lutein, β-carotene and lycopene in a standard meal with the addition of red or yellow tomato puree.119 The compartment of dialysis and membrane filtration for removal of low molecular compounds from the digestion is not able to simulate the absorption processes occurring in vivo such as active transport, efflux and intestinal wall metabolism. For that, TIM-1 samples can be combined with intestinal absorption systems to predict nutrient bioavailability. For instance, TIM-1 coupled with Caco-2 cells was used to assess the absorption of lycopene and α-tocopherol from a whole western-type meal containing puree of red tomatoes.120 TIM-1 has been used as a relevant tool to assess the efficacy of drug delivery systems in the GI tract, providing also the effect of meal consumption on the drug uptake.121
Some limitations of the TIM are that many of the food matrices tested in the TIM-1 model do not present the complexity of those used in other dynamic models, such as the DGM. This could be due to the performance and shape of the gastric compartment. Therefore, the model could be limited in the study of the structure stability in the gastric phase. The intragastric behaviour and extent of lipolysis of various emulsions stabilised by different stabilisers was studied.122,123 For that, the authors used the tiny-TIM with a specifically designed gastric compartment. It consisted of a main body and lower antral part with an L-shape, in contrast to the tubular shape of the TIM-1. The authors showed that the extent of cream layer formation was the limiting factor for the delay in the entry of lipid into the small intestinal compartment and, hence, a delay in absorption. This adaptation has been taken further resulting in the developed of the TIM advanced gastric compartment (TIMagc).
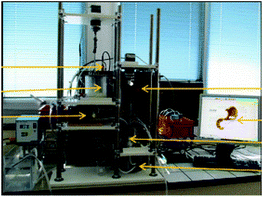
DIDGI®. The DIDGI® system was developed at INRAE, the French National Research Institute for Agriculture, Food and Environment.124 It consists of two consecutive compartments simulating the stomach and small intestine (capacity of 200 mL). It is a computer-controlled system that simulates the pH changes and sequential addition of digestive secretions, applied by peristaltic pumps. Each compartment is surrounded by a glass jacket filled with water at 37 °C. Gastric emptying is performed using a peristaltic pump together with a 2 mm membrane. The digestion parameters such as the digestion time, pH curve, secretion and emptying rates are based on in vivo data from human volunteers and animals and supported by the StoRM computer software. The gastric and intestinal emptying are controlled based on the Elashoff's equation. The DIDGI® was validated by comparing the in vitro digestion of an infant milk formula with the in vivo data collected in 18 piglets.124 The parameters of digestion such as pH curves and transit time were obtained using in vivo data related to infant physiological conditions. The chyme transit presented no significant differences, as observed in the remaining volumes in the gastric compartment, confirming the physiological relevance of the DIDGI® system. The proteolysis kinetics of the main milk proteins (caseins and β-lactoglobulin) in both gastric and small intestinal compartments were, in general, comparable between the in vitro dynamic and in vivo digestion.
The DIDGI® has mainly been used to study the digestion of dairy products/ingredients. Since it was primarily designed considering the infant digestion, some studies have focussed on the study of the protein and lipid digestion of human milk and infant milk formula. The effect of Holder pasteurisation (62.5 °C, 30 min) of human milk on the proteolysis and lipolysis kinetics, was compared to raw human milk.125 The authors found differences in the susceptibility of proteolysis and intestinal release of some amino acids in the heated human milk. Pasteurisation also limited lipolysis but not the evolution of lipid classes. These differences could be linked to the structural changes observed during gastric digestion using microscopy. This study showed that the use of the DIDGI® could provide some understanding of the digestive kinetics of the human milk. The impact of heat treatment on the hydrolysis kinetics of milk proteins and peptide release has also been studied.126 Bovine skim milk powder solution was heated at 90 °C for 10 min and gastric digestion was performed using the DIDGI® system. The half-time of gastric emptying used was set at 191 and 283 min for unheated and heated milk, respectively, as estimated in mini-pigs fed with the same milks.127 The authors showed that the heat treatment applied induced an increase of the resistance of caseins and higher susceptibility of β-lactoglobulin upon digestion. Furthermore, there were differences in the identity and time of peptides release. The knowledge of the kinetics of peptide formation could have important implications in their possible bioactivity and allergenic response.
The control of lipid digestion is crucial in the development of products for weight management strategies. The delay of lipid digestion might allow the presence of undigested lipid in the distal intestine (ileum), which may increase the feelings of satiety (i.e. ileal brake). The behaviour of the lipid kinetics during digestion was investigated in an encapsulated oil-in-water emulsion in alginate beads when incorporated in a yoghurt matrix.128 This was performed using the DIDGI® system but adding a third compartment to mimic the second part of the small intestine (jejunum + ileum). The encapsulation of the emulsion delayed lipolysis by 3 hours when compared to the free emulsion. This showed that the DIDGI® system could be used for assessing differences in lipid digestion kinetics and was adapted to be representative of the different sections of the small intestine, so far applied to liquid foods. The DIDGI® was also used to gain more information about the glycaemic response by studying the digestion kinetics of starch and the contribution of the salivary and pancreatic α-amylases.129 This was assessed in wheat-based and gluten-free pasta, and white bread. The importance of the use of salivary α-amylase was highlighted in the study since some studies have skipped oral digestion due to its shortness. Furthermore, salivary α-amylase remains active in the stomach until it is inactivated at pH between 3.0 and 3.8, highlighting the importance of using more physiological digestion models to investigate carbohydrate digestion.
The main limitations of the DIDGI® are that the vertical orientation of the main body and antrum does not mimic the gastric anatomy. The mixing in the compartments consists only in a basic stirring using a propeller, which is not physiologically relevant, and the gastric physical contractions are not simulated.
The established examples given above were selected since they are the most representative and are widely cited in the literature. However, other more recent but less validated dynamic models have been published in recent years that are working towards the development of more realistic GI digestion simulation. For instance, the in vitro mechanical gastric system (IMGS),130 the new dynamic in vitro human stomach system (DIVHS),131 the gastric simulation model (GSM)132 and the artificial gastric digestive system (AGDS).66 Their general features are described in Table 5 for comparison. All these models incorporate the J-shaped gastric morphology and anatomical structures with the aid of 3-D printing technology although the biochemical aspects of the model may be rather more primitive. The AGDS exhibited a similar digestion behaviour of the α-lactalbumin protein solution to the in vivo data.66 The DIVHS was able to generate consistent gastric emptying profiles of both solid and liquid fractions in the mixed meal of beef stew and orange juice with that reported in vivo. Furthermore, this model showed qualitative correlations regarding gastric pH, particle size distribution and emptying profiles of cooked rice with the in vivo data from the literature.
| System | Photo | Gastric dynamics | Main body | Control units | T control | Mixing mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| In vitro mechanical gastric system (IMGS) |

|
- pH control ✓ | J-shape; latex; 900 mL | pH stat automatic titration unit | 37 °C; water | Adjusting acrylic pistons acting in pairs, each piston is driven by a motor with different torques | Barros et al. (2016)130 |
| - Digestive fluids secretion X | |||||||
| - Gastric emptying X | |||||||
| - Mixing and shear stresses ✓ | |||||||
| Gastric simulation model (GSM) |
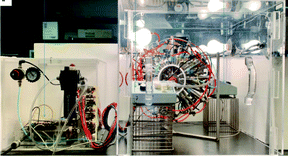
|
- pH control X | J-shape; latex; 600 mL | Peristaltic pumps | 37 °C; light bulbs and fan | A series of syringes that work independently with gas | Li et al. (2019)132 |
| - Digestive fluids secretion ✓ | |||||||
| - Gastric emptying ✓ | |||||||
| - Distribution, amplitude and frequency of contractions ✓ | |||||||
| Dynamic in vitro human stomach system (DIVHS) |
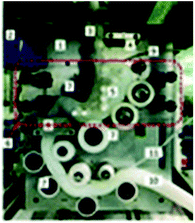
|
- pH control X | J-shape with folds/wrinkles inside, silicone, 400 mL | Peristaltic pumps | 37 °C, lamp | Electromechanical instrument composed of a series of motors, rollers and eccentric wheels | Wang et al. (2019)131 |
| - Digestive fluids secretion ✓ | |||||||
| - Gastric emptying ✓ | |||||||
| - Peristaltic contractions ✓ | |||||||
| Artificial gastric digestive system (AGDS) |
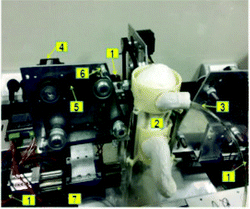
|
- pH control ✓ | J-shape; silicone; 1700 mL | pH stat automatic titration unit; peristaltic pumps | 37 °C, lamp | Two sets of symmetrical rollers and one set of contrary movement of the roller | Liu et al. (2019)66 |
| - Digestive fluids secretion ✓ | |||||||
| - Gastric emptying ✓ | |||||||
| - Peristaltic contractions ✓ |
As general conclusion, the dynamic models can be useful to gain insights into food digestion and establish mechanistic relationships with physiological responses related to specific food consumption. The dynamic models have been used to study nutrient breakdown, structural changes and release of food components showing the kinetics of digestion. However, they have mostly provided a partial view of the fate of food digestion focused on the aim of the study. The system can be very useful for the simulation of specific populations such as the infant or the elderly. However, the specific conditions of digestion need to be standardised for systematic use. In general, these dynamic models need further validation with in vivo parameters in light of the new advanced techniques for studying in vivo digestion. These systems vary in the biochemical composition of the digestive solutions used in each phase, for example the type and enzyme concentrations, the electrolytes and buffers, between the dynamic models as well as in the studies using the same system. There is also no consensus agreement on how these models reproduce the relevant motility of the stomach. In addition, the shape of stomach in most of the dynamic models is not representative of the actual morphological and anatomical features.
The use of dynamic systems is technically very complex and costly, compared to the static model. It is time-consuming due to the long preliminary preparations enabling the performance of, usually, one digestion per day. Furthermore, the data provided by these models are usually more difficult to interpret. They do not include any feature related to feedback hormonal mechanisms, resident microbiota or immune system. In addition, products of digestion are not progressively removed from the compartments, except in the case of TIM, and they do not provide the simulation of further hydrolysis by the brush border enzymes. Therefore, there are still opportunities for further improvements in dynamic in vitro models.
2.5 Digestion models relevant to absorption
After luminal GI digestion, there is a last step of further degradation of hydrolysis products from dietary lipids, proteins and carbohydrates by brush border membrane (BBM) enzymes at the level of small intestinal epithelium before absorption. The term brush border is often used to refer to the microvilli and glycocalyx, on the apical side of the enterocytes lining the small intestinal surface. More than twenty different BBM-associated enzymes have been identified,147,148 among which peptidases (exopeptidases, endopeptidases, enteropeptidases and dipeptidases) and di(oligo)saccharidases are most abundant. Thus, this section aims to focus on the action of BBM peptidases and to a lesser extent BBM carbohydrases.Exopeptidases progressively erode amino acids generated by gastric and pancreatic proteases, whereas endopeptidases can hydrolyse larger polypeptides. However, the hydrolysis of high molecular weight polypeptides is very slow compared to the hydrolysis of short peptides with less than 20 residues.149 A significant amount of BBM enzymes are also present in the intestinal lumen, because of the rapid turnover and shedding of epithelial cells.150 The distribution of BBM enzymes is not homogeneous along the small intestine. In general, the activity increases longitudinally from the proximal duodenum to the distal ileum, as the bulk of the chyme also decreases. Thus, the optimum BBM digestion/absorption of gastro-pancreatic generated peptides takes place in the distal duodenum and proximal jejunum.151 To date, there are no standard protocols to approach this final step of digestion.151 The lack of consensus regarding incubation conditions (pH, media composition, times, enzyme to substrate ratio, etc.), along with the limited availability of BBM enzymes are the main reasons why this step is omitted in most of in vitro digestion models. In turn, the standardisation requires data about the activity of BBM enzymes, which are still incomplete, and adequate validation of the use of BBM enzymes. The characterisation of BBM material is complex due to the presence of active intracellular components and variability in enzyme activity because of the different expression among the different segments of the small intestine as well as the type of diet, host genetics, age, etc. In addition, it is not yet clear whether the final degradation by BBM enzymes is sufficiently significant to be physiologically relevant, and/or the dependence on the substrate. One of the simplified methods is the use of isolated BBM vesicles, although it may lack sufficient physiologically relevance, while more complex procedures using tissue or model organ are not widely applicable and not sufficiently established.150 Regarding the use of BBM vesicles, studies have mostly used BBM enzymes isolated from the jejunum of pig, rat and cow, when access to human source is restricted.152,153 This is because major peptidases have been found structurally and functionally homologous in these animal species and human.150 Nevertheless, the porcine source is a better substitute for human BBM enzymes due to the relatively high stability and yield, and well-correlated physiology of the small intestine, in both anatomical and dietary terms.154,155 One of the most broadly used procedures to isolate BBM vesicles is from Shirazi-Beechey et al.156 For protein digestion, the use of a last step of in vitro intestinal digestion with BBM peptidases supported the discovery of wheat prolamin derived peptides that elicit celiac disease in vivo.152 Picariello and co-workers showed that BBM enzymes significantly increased the degree of hydrolysis of milk proteins from 55–60% after GI digestion up to 70–77% after BBM hydrolysis.153 If properly validated in vivo, these results might question the role of putative bioactive peptides supplied by an average daily intake of milk, which would be present at too low concentrations for exerting any local or systemic activity. A recent study showed that this step only affected short peptides (<20 residues) and was not relevant for the study of allergenicity of native and aggregated egg ovalbumin since shorter peptides may have lost already the antigenic properties by pancreatic proteases.157 In contrast, Di Stasio and co-workers found that BBM digestion after oral-gastro-duodenal digestion reduced the allergenicity of roasted peanuts compared to raw peanuts, or compared to raw/roasted peanuts after only oral-gastro-duodenal digestion.158 This suggests that BBM peptidases contributed to further hydrolyse the shorter peptides generated from digestion of roasted peanuts destroying specific domains of peanut allergens. Similarly, Gianfrani and co-workers found that in vitro gastro-pancreatic digesta of gliadin from einkorn (a diploid ancestor of wheat) have comparable immunogenic potential to that of common wheat when assayed against specific intestinal T-cell lines from celiac disease patients, but this immunotoxicity of einkorn is considerably reduced after BBM digestion when compared to wheat.159 They found that BBM digestion dramatically affected the stability of einkorn resistant peptides, retaining a lower number of minor T-cell stimulatory epitopes. These results support in vivo evidence of the lower-toxicity of certain wheat species for the celiac disease patients and the correspondence of the digestome. Therefore, the use of BBM enzymes has to be reviewed case by case. In these studies, the incubation conditions of BBM peptidase activity to substrate ratio was 100 mU/100 mg peptides, the incubation times were 4–6 hours, and the incubation pH was in the range of 7–8, based on previous procedures demonstrating certain in vitro–in vivo consistency.152,160 Nevertheless, these conditions were rather empirical. The BBM enzymes activity is critical and even if accurately evaluated individually or in total, the challenge remains in calculating the amount of chyme encountering the intestinal mucosa.150 Therefore, the parameter of BBM enzymes to substrate ratio has yet to be fairly extrapolated for in vitro purposes.
For carbohydrate digestion, Ferreira-Lazarte and co-workers studied the GI digestion of reconstituted skim milk powder with incorporated prebiotic oligosaccharides and reported some differences between the in vitro protocols applied, gastro-intestinal Infogest versus gastric Infogest complemented with intestinal phase using rat small intestinal extract.80 The authors attributed the different results to the presence of lactase (a BBM enzyme) in the small intestinal extract from rat, which would represent a more physiologically relevant scenario for carbohydrate digestion. Recent advances on the in vitro digestion of carbohydrates are addressing the use of mammal or recombinant mucosal carbohydrases and have been reviewed elsewhere.161,162
BBM digestion can be coupled with absorption studies since the uptake is the ultimate barrier between an active compound and the systemic circulation. Regarding in vitro models of absorption, these span from simple membrane systems to cell model systems, and more complex ex vivo models include intestinal tissues, although the viability time ex vivo is a limitation if shorter than the typical intestinal transit time. In all these techniques, sampling and composition analysis of the collected aliquots are required to measure the release/transport of the bioactive compound of interest. At least eight of the main BBM peptidases have been identified at the cell surface of Caco-2 cells.163 However, the digestion patterns generated by the BBM vesicles are appreciably different from those obtained by Caco-2 cell monolayer.164 This may be related to the heterogeneous and dissimilar distribution of Caco-2 peptidases from the enterocyte peptidases.165 Investigations are still needed to define physiologically relevant operative parameters.
The simplest in vitro membrane systems to study the transport of nutrients or bioactive compounds across the intestinal epithelium are dialysis bags or tubing containing the digesta and suspended in simulated intestinal fluids for a more accurate approach166 or dialysis membrane implemented in more sophisticated in vitro dynamic digestion models, such TIM-1.167 Nevertheless, this approach does not mimic the epithelial cell behaviour and bioaccessibility is rather the measuring parameter than bioavailability. A more realistic model of the human intestinal epithelium is achieved with Caco-2 monolayer cell cultures168 or co-cultures of Caco-2. These can be grown either in a single cell culture plate well for uptake studies or in a membrane insert in a Transwell® system for transport studies.169 Caco-2 monolayer only reproduces enterocytes and therefore exhibit microvilli and the expression of enzymes and transporters.170 Co-cultures of Caco-2 and other cell lines are alternatively used to better reproduce aspects of the multicellular intestinal epithelium by including M cells (Caco-2 and RajiB or lymphocyte co-culture) and mucus secreting Goblet cells (Caco-2 and HT29-MTX co-culture). Good descriptions of the methods for these types of cell cultures are given elsewhere.171 The integrity of the cell monolayer is usually evaluated by measuring the transepithelial electrical resistance (TEER). Ideally, coupling in vitro digestion models with human cell cultures would provide a more realistic scenario of absorption than using cell culture on undigested/pure systems. These tandem studies can use simple static in vitro digestion, including the Infogest harmonized protocol, or dynamic models (e.g. TIM) prior to cellular uptake and account for the effect of the food matrix and digestive media.120,172,173 For instance, Deat et al. combined the TIM dynamic model of GI digestion of whole food with cultures of Caco-2 cells to determine the bioavailability of phytochemicals.120 Their in vitro results, namely lower bioavailability of lycopene compared to α-tocopherol, were consistent with in vivo data from literature. Vors and co-workers coupled static in vitro GI lipolysis of emulsions with Caco-2 cells to study lipid absorption and metabolism.172 The authors showed that the emulsion composition influenced the activation of lipid metabolism and triglyceride secretion by Caco-2 cells. Felice et al. coupled the Infogest digestion protocol with Caco-2 cell model to study the bioavailability of magnesium from marine-derived multimineral supplement and compared with other sources of magnesium.173 The marine-derived product had comparable magnesium in vitro bioavailability to the source with reported higher in vivo bioavailability. To maintain the barrier integrity of the Caco-2 cell monolayers in these experiments, digested samples may require previous ultracentrifugation, filtration and dilution before adding to the cell cultures or, alternatively, filtering the enzyme and buffer solutions before in vitro GI digestion and diluting digested samples afterwards, in order to minimise bacterial contamination and avoid toxicity.
A step further on cell model systems has been achieved with the biomimetic microfluidic device gut-on-a-chip. This consists of two hollow microchannels separated by a porous flexible membrane coated with extracellular matrix and lined by human intestinal cells, e.g. Caco-2.174 Fluid flow and mechanical stress are applied via vacuum microchambers alongside the microchannels, which mimic the intestinal peristalsis and increase the paracellular transport without compromising the integrity of the cell monolayer. In addition, human gut isolated microflora can be cultured on the luminal surface of the Caco-2 monolayer to account for the presence of living microorganisms.
Ex vivo absorption in segments of animal or human gut offers a better representation of the morphological and physiological features of the intestinal wall, such as the presence of all the relevant cell types and architecture, and the presence of a mucus layer allowing the simulation of the possible further hydrolysis by BBM enzymes.175 These tissue segments can be used to follow the transport of nutrients or bioactive compounds across the intestinal epithelium, permeability, absorption or interactions with the mucus layer.176 The main limitation is that the tissue is only viable for 1 to 3 hours depending on whether the muscle layers are present or removed. The simpler techniques are intestinal rings or segments, which are immersed into highly oxygenated buffer containing the compound of interest. The main disadvantage in this procedure is that there is no isolation of the luminal (apical) side from the serosal (basolateral) side, thus mainly the accumulation of nutrients or bioactive compounds into the enterocytes is determined rather than transport.177 The separation of apical and basolateral compartments can be achieved with the everted sac model, whereby a segment of intestine can be sutured at one end, the digesta or compound under study introduced and the open end also sutured and immersed in physiological solution, such as Ringer.178 Another alternative is opening the intestinal segment by cutting longitudinally so that the tissue can be mounted in an Ussing chamber. Here, the tissue is pinned to a frame dividing two semi-chambers, one facing the apical side, where the system of interest is loaded, and the other facing the basolateral side, where the appearance of the loaded system is monitored.179,180 The electrophysiological properties: TEER of the tissue, potential difference between the two chambers and short-circuit current of the tissue are monitored throughout the experiment as indicators of tissue integrity and viability. Relatively high TEER values reflect the activity of the ion pumps on the epithelial membrane, which is characteristic of viable tissue. The permeability of hydrophilic markers is often monitored as well to follow tissue integrity, where a low permeability is associated with intact tissue.181 Most of these intestinal tissue models make use of an animal source, rats, rabbits and pigs being the more common, due to the limited availability of healthy human intestinal tissue.182 Although pigs share more physiological and immunological similarities to human than rodents, the extrapolation of data to humans is complicated due to interspecies differences.183 Even the large inter-individual variability in humans make the interpretation of the results difficult in small studies. As for cell models, tandem in vitro digestion with Ussing chambers studies have been reported in the literature. In these studies, the digested samples only require dilution before being in contact with intestinal tissues. García et al. used the digesta of commercial fish products (obtained with the static Infogest harmonized protocol) in the apical side of Ussing chambers to determine the ex vivo bioavailability of antioxidants.184 They found faster rates of transport for the products counterpart enriched with protein hydrolysates from sea cumcumber. Mackie and co-workers coupled the static in vitro digestion (Infogest) of emulsions stabilised by cellulose nanocrystals (CNC) with Ussing chambers technique and compared the GI stability and lipid transport across murine intestinal epithelium with that of conventional protein-stabilised emulsions.185 It was shown that CNC clusters post-digestion were entrapped in the mucosa layer probably limiting the bile and lipid transport, in particular of saturated free fatty acids, in comparison with protein emulsion. These findings have important implications in the modulation of lipid absorption and bile recycling in the context of cholesterol-lowering potential ascribed to dietary fibre. In a more recent study, Mulet-Cabero and co-workers coupled an in vitro semi-dynamic gastric digestion with in situ static intestinal digestion in the apical side of the Ussing chamber to study absorption of amino acids from dairy formulations.67 This approach is even more realistic since intestinal digestion and absorption are simultaneous processes. More importantly, a positive correlation was found between gastric emptying, GI bioaccessibility and absorption within the context of “fast” and “slow” proteins.
The limitations of using freshly excised intestinal tissues for transport studies are on one hand associated with the removal of the serosa and muscularis layers. The lack of circulation and lymphatic drainage leads to water accumulation and tissue swelling. This is overcome to some extent by reducing the osmotic gradient between the apical and basolateral side (when individualised as in Ussing chambers). To this end, glucose is normally substituted with mannitol in the apical compartment.181 Still the lack of circulation can hinder the passage of especially larger molecules through the subepithelial layer and it is thus possible that the subepithelium presents more of a barrier in ex vivo experiments than it does in vivo. Furthermore, toxicological responses dependent on the systemic immune system cannot be studied and the lack of innervation and the subepithelial muscle layer means that responses dependent on nerve signalling and peristaltic movements, respectively, will not be captured ex vivo.182 Additionally, the reduced area of the intestinal tissue may result in saturation in the tissue hampering the transport. Overall, the technical challenge of these systems, limits the throughput, nonetheless good in vitro–in vivo correlation has been shown for small molecules, such as drug and peptides.186–188 Promising ex vivo–in vivo correlation in nanoparticle uptake in human intestinal tissue has also been reviewed.182
2.6 In silico models of digestion
In general, the complex nature of the problem and the limited amount of reliable data to base models on has meant that only a limited amount of in silico models have been used. Although in many ways one might consider the oral cavity a difficult compartment to model, Harrison et al. were able to model the fracture of agar gels.189 They were able to show that gel fragment size results were mildly sensitive on the initial position of the agar samples relative to the position of the jaw and highly sensitive to the measure of fracture toughness. In other words, foods break according to the properties of the food not their location in the mouth.Most of the research into in silico models of digestion has come from the pharmaceutical arena.190,191 For example, in their review, Fois et al. discussed a number of computational fluid dynamics (CFD) approaches that have been used to understand mass transfer in the GI tract. Although there is no absorption of nutrients during the gastric phase, the extent of mixing and shearing has a significant impact of breakdown and emptying of solid and semi-solid meals. Additionally, the change in pH and the presence of food in relation to drug delivery is now coming under more scrutiny.191 The difficulty of assessing these physical processing parameters in vivo has led to a heavy reliance on data from modelling in understanding gastric shear fields.192 These simulations highlight the very limited mixing in the gastric compartment under postprandial conditions relating to the consumption of a meal where the chyme will have significant rheological properties. More recently, Harrison et al. used a coupled biomechanical smoothed particle hydrodynamics model to show that the rate of gastric emptying increased with a high frequency of contractile waves and had a nonlinear relationship with content viscosity.193 Increased resistance to flow into the duodenum was also shown to reduce the rate of emptying. The degree of gastric mixing was found to be insensitive to changes in the frequency of contractile waves for fluid with a viscosity of water but to be substantially affected by the viscosity of the gastric content and this confirms the findings of Drechsler and co-workers.192
In addition to gastric processing, mixing in the small intestine has also been simulated by a number of researchers. These include the work by Moxon et al. on luminal mixing194 and Lentle and co-workers on intestinal mixing and flow around villi.195 The CFD simulations suggest that there is ongoing augmentation of luminal mixing at the periphery of the lumen to promote flow around and between adjacent villi. Thus making mixing and absorption of nutrients and bioactives more efficient. The final part of the intestine is the colon and Sinnott et al. have modelled colonic motility with regard to the transport of fluid.196 They were able to show that in the absence of descending inhibition (DI), poor transport properties and high intra-luminal pressures were observed. The importance of DI as a physical mechanism for peristalsis appeared to be that it enabled excellent compact transport with reduced muscular work. It is clear that luminal composition has an important effect on mixing and thus transport and absorption at all stages of digestion.
More recently, improvements in data and modelling tools have led to ideas about their use for improving the bioacessibility of lipophilic nutrients197 and more realistically predicting protein hydrolysis.198 In the latter article a combination of in vivo and in silico methods were used to predict bioactive peptide release from meat. This mixed type approach has also been used to predict appetite responses to foods based on gastric rheology combined with amino acid and glucose absorption.199 The researchers used an artificial neural network approach with a number of training sets to build the predictive capacity and link the in vitro measures to human participant visual analogue scale scores for fullness and hunger. Although this area of modelling is still in its infancy, it is clear that developments in CFD and artificial intelligence approaches have the potential to make in silico models powerful predictive tools with the ability to predict physiological responses to foods.
3 In vivo–in vitro comparisons
In vitro models aim to mimic the situation in vivo although some models simulate the physiological conditions more closely than others do. These models aim to provide data that can predict human digestion but there is the need to understand their limits and accuracy. This should be performed by direct in vivo–in vitro comparison using the same food samples including raw material and processing. These studies are limited due to constrictions of in vivo studies and the challenge of how to compare in vitro bioaccessibility data with in vivo bioavailability data. Table 6 shows examples of this in vivo–in vitro correlation studies illustrating the relevance and some of them are discussed in this section.| In vivo model | In vitro model | Food substrate tested | Parameters assessed | Physiological relevance | Ref. |
|---|---|---|---|---|---|
| AA: amino acid, FFA: free fatty acid, GI: gastrointestinal, AUC: area under the curve. | |||||
| Pig (n = 8) | Static | Skim milk powder | Protein hydrolysis (qualitatively) and, peptide and AA analysis during GI digestion | - The two in vitro endpoints (gastric and intestinal phase) reflected the in vivo situation of protein bioaccessibility | Egger et al. (2019)15 |
| In vivo: sample at 1.5 h of gastric/intestinal and at 3 and 6 h of intestinal digestion | - Peptide correlation of 0.8 and 0.6 of overall proteins in the endpoints of gastric and intestinal, respectively. | ||||
| In vitro: during gastric phase (2 h) and intestinal phase (2 h) | - Different gastric behaviour | ||||
| Piglet (n = 18) | Dynamic model, DIDGI® | Infant milk formula | In vitro and in vivo: kinetics of residual immunoreactivity of β-lactoglobulin and caseins during gastric (210 min) and intestinal (210 min) digestion | The residual concentrations of caseins and β-lactoglobulin in gastric and intestinal digesta were statistically not different (p < 0.05) | Menard et al. (2014)124 |
| Pig (n = 8) | Dynamic model, DIDGI® | Skim milk powder | Protein hydrolysis (qualitatively) and, peptide and AA analysis during GI digestion | - Peptide correlation of 0.85 and ∼0.5 of overall proteins in the endpoints of gastric and intestinal, respectively. | Egger et al. (2019)15 |
| In vivo: sample at 1.5 h of gastric/intestinal and at 3 and 6 h of intestinal digestion | - Formation of gastric solid coagula but different consistency | ||||
| In vitro: during gastric phase (1.5 h) and intestinal phase (3 h) | |||||
| Calf | Semi-dynamic model | Bovine whole milk | Non-protein and protein nitrogen, AA analysis, estimation of caseins/whey proteins during gastric digestion | The in vitro kinetics of protein patterns generally agrees with in vivo data, applying in vitro time/2 | Yvon et al. (1992)203 |
| In vivo: monitoring for 6 h | |||||
| In vitro: monitoring for 3 h | |||||
| Human (n = 8) | Static | Oil-in-water emulsion using phospholipids, sodium stearoyl lactylate liquid oil and liquid oil/solid fat | In vivo: blood triglycerides rate | Partial correlation rates of FFA (in vitro intestinal phase) and triglyceride concentration in plasma | Golding et al. (2011)205 |
| In vitro: FFA release during intestinal digestion after gastric step, microstructure, particle size emulsion | |||||
| Human (n = 8) | Static | Phospholipid stabilised emulsion, sodium caseinate + monoglyceride emulsion, sodium caseinate + monoglyceride emulsion in gelatine gel and sodium caseinate + monoglyceride emulsion in starch dispersion | In vivo: blood triglycerides rate for 6 h | Partial correlation rates of FFA (in vitro intestinal phase) and triglyceride concentration in plasma | Wooster et al. (2014)223 |
| In vitro: FFA release during intestinal digestion (3 h) after static gastric step (2 h), microstructure and particle size emulsion | |||||
| Human (n = 12) | Semi-dynamic model | Whey protein (leucine-enriched) and calcium casein protein at two different concentrations containing carbohydrates and fat | In vivo: AAs analysis, glucose and insulin concentrations in blood samples for 4 h | The samples presented similar variation of essential AAs after intestinal digestion | Luiking et al. (2016)81 |
| In vitro: protein digestion rate and, peptide and AAs analysis during intestinal digestion (90 min) after gastric phase (90 min) | |||||
| Human (n = 10) | Semi-dynamic model | Semi-solid sample (cheese and yoghurt) and liquid sample (oil-in-water emulsion stabilised by milk proteins) | In vivo: gastric structuring, satiety assessment and CCK determination for ∼3 h | - Correlation in gastric behaviour | Mulet-Cabero et al. (2017)74 |
| In vitro: gastric structuring and, protein and lipid digestibility during intestinal digestion (1 h) after gastric digestion (∼3 h) | - The higher levels of proteins and lipids released from semi-solid sample in vitro can correlate with its higher satiety | Mackie et al. (2013)207 | |||
| Human (n = 5–10) | Static | Cooked and sweet potatoes, white and brown rice, white and wholemeal spaghetti, buckwheat, millet, oats, kidney beans, soya beans, lentils and marrowfat pea. | In vivo: glucose concentration in blood samples for 2 h | Positive correlation between the concentration of carbohydrate liberated in vitro and the glycaemic index at 1 h (r = 0.8627) and 5 h (r = 0.8618) | Jenkins et al. (1982)224 |
| In vitro: glucose, maltose and oligosaccharides concentration at 1 and 5 h | |||||
| Human (n = 8) | Static | Corn flakes, white bread, cooked white spaghetti, and cooked pearled barley | In vivo: glucose concentration in blood for 2 h | Correlation between the mean glycaemic response values for all the subjects and the rapid available glucose content of the test meals (20 min) (r = 0.981) | Englyst et al. (1999)211 |
| In vitro: glucose concentration during intestinal digestion at 20 and 120 min | |||||
| Human (n = 30) | Static | Cereals: white bread, spaghetti, rice and biscuits. Legumes: lentils, chick-peas and beans. Vegetables: peas, boiled potatoes and crisp potatoes | In vivo: glucose concentration in blood for 3 h | Correlation between the starch hydrolysis (%) and glycaemic index: r = 0.837 (30 min), r = 0.883 (60 min), r = 0.909 (90 min), r = 0.890 (120 min) and r = 0.888 (180 min) | Goñi et al. (1997)212 |
| In vitro: glucose concentration during intestinal digestion for 3 h | |||||
| Human (n = 18) | Static | Muffins and breads | In vivo: blood glucose concentration for 3 h | In vitro method overestimated in vivo method, the trend in the reduction in glycaemic index seemed to be similar in both methods | Ferrer-Mairal et al. (2012)225 |
| In vitro: reducing sugar concentration during intestinal digestion (3 h) after gastric digestion (1 h) | |||||
| Human (n = 9) | Static | Two wheat porridge meals: coarse (2 mm particles) and smooth (<0.2 mm particles) | In vivo: blood glucose, insulin, C-peptide, lipids, and gut hormones concentrations for 4 h | Same differences in the extent of hydrolysis and bioavalability of glucose between the samples | Edwards et al. (2015)226 |
| In vitro: hydrolysis index (quantification of maltose) for 1.5 h | |||||
| Human (n = 12) | Dynamic model, DGM | Barley flakes (micronized and extruded) | In vivo: glucose concentration in blood for 2 h | Similar range in the glycaemic index | Ballance et al. (2013)93 |
| In vitro: microstructure, gastric viscosity and glucose concentration during intestinal digestion (1 h) after gastric phase (30 min) | |||||
| Human (n = 13) | Dynamic model, TIM | Barley and oat Tempe | In vivo: glucose concentration in blood for 2 h | Similar range in the AUC values | Alminger et al. (2012)133 |
| In vitro: quantification maltose formation, microstructure during intestinal digestion for 3 h | Alminger et al. (2008)215 | ||||
| Human (n = 10) | Static | Meal containing α- and γ-tocopherol, β-carotene, lycopene, and lutein bioaccessibility from their main dietary sources | In vivo: all trans and cis-carotenoids content from stomach (for 3 h) and duodenal digesta (for 3 h) | Bioaccessibility values measured with the in vitro model were in the same range than those measured in vivo (r = 0.90) | Reboul et al. (2006)218 |
| In vitro: carotenoids and vitamin E (micellar phase) in endpoint of digestion (gastric phase for 3 h and intestinal phase for 2 h) | The spinach lutein bioaccessibility was about 5-fold higher in vitro than in vivo | Tyssandier et al. (2003)219 | |||
| Human (n = 72) | Static | A high-polyphenol diet, the main dietary sources were artichokes, fennel, onion, spinach, arugula salad (rucola), orange, dark chocolate bar, decaffeinated coffee and green tea | In vivo: phenolic metabolites (for hydroxylated phenylpropionic and phenylacetic acids, some benzoic acid derivatives and mammalian lignans) in 24 h urine samples | Low correlation (r = 0.280) found for 15 metabolites | Vetrani et al. (2016)220 |
| In vitro: phenolic metabolites formation after 6 h incubation | |||||
| Human (n = 15) | Static | Tortillas fortified with various form of iron (native iron, ferrous fumarate, ferrous bis-glicinate, FeNa EDTA) | In vivo: iron status and bioavailability in blood samples on day 15 and 29 | The iron solubility in vitro and the bioavailability in vivo were highly correlated (r = 0.89) | Walter et al. (2003)227 |
| In vitro: dialyzable iron after GI digestion | |||||
| Human (n = 69) | Dynamic model, TIM-1 | Folic acid fortified milk (ultra-high temperature sterilised and pasteurised) | In vivo: folate concentration in blood for 4 weeks | In vitro/in silico approach can predict in vivo folate concentrations in blood | de Jong et al. (2005)228 |
| In vitro: folate concentration after GI digestion and absorption | Verwei et al. (2006)221 | ||||
3.1 In vivo–in vitro correlation in protein research
In vivo studies have been usually applied to assess the nutritional quality of food proteins. This is most frequently evaluated by the protein digestibility-corrected amino acid score (PDCAAS) or digestible indispensable amino acid score (DIAAS).200 DIAAS is based on the true ileal digestibility of each indispensable AA. The true ileal digestibility should preferably be determined in vivo by, for instance, sampling via a naso-ileal tube after intake of 15N-labeled AAs. These scores are based on the overall ability of humans to digest proteins, which does not include the rate of digestion. However, the postprandial profile by which AAs are present in the blood (i.e. aminoacidemia) is of great relevance and can exert different physiological effects. This is a similar concept of glycemia in relation to starch digestion but it has not been studied in that depth. For instance, a rapid presence of AAs, especially essential AAs such as leucine, was observed to enhance muscle protein synthesis response in the elderly,201 which could help in the problem of sarcopenia.Some studies have aimed to compare the protein digestion between animal and dynamic in vitro models by analysing the digesta after gastric or gastrointestinal digestion. For instance, Egger and co-workers compared the digestion of skim milk powder using the data of protein hydrolysis by gel electrophoresis and peptide patterns obtained by a pig model and the dynamic DIDGI® system.15 The authors observed the formation of a firm coagulum in the pig stomach whereas the coagulation in the in vitro model was much less compact. The kinetics of protein hydrolysis were difficult to compare since there was no time-following during in vivo digestion, just one sample from the stomach and three points in different parts of the intestine were collected. Peptide identifications were performed for the five most abundant milk proteins (β-casein, β-lactoglobulin, αs1-casein, αs2-casein, and κ-casein). The peptide patterns of the gastric and intestinal endpoints were compared to those from the pig in vivo.202 The total correlation calculated over all proteins was 0.85 and ∼0.5 in the endpoints of the gastric and intestinal digestion, respectively. This lower outcome in the intestinal compartment, in particular, in the ileum, could be due to absorption processes are not mimicked in the in vitro model. Similar results were obtained when the same in vivo protocol was compared to a static digestion using standardised Infogest conditions.202 In contrast, Yvon and co-workers obtained a good agreement in protein digestion data of gastric emptying obtained using a calf and semi-dynamic models.203
Luiking and co-workers determined in humans the AA concentration in plasma after the consumption of different concentrations of calcium caseinate and whey proteins solutions.81 In the first 90 min, there was a rapid and higher increase in the whey protein samples compared to the caseinate samples. However, in vitro the initial intestinal protein digestion rate of casein supplements was higher than that of whey protein supplements. Nevertheless, the overall cumulative release of AAs over 90 min was higher for whey protein samples as observed in vivo. The semi-dynamic model used provided the kinetics of pH changes and the gradual secretion of enzymes, which could lead to the formation of some kind of coagulation in the casein samples. However, the time of digestion and the stirring applied could influence the outcomes.
In most cases, the use of an in vivo model to compare in vitro data in the same study is not possible. Some studies have used relevant human clinical data for reproducing in vitro studies. For example, Maathuis and co-workers determined the true ileal protein digestibility kinetics and DIAAS of a goat and cow milk-based infant formula, and human milk.204 The dynamic system tiny-TIM was used applying relevant infant (1–6 months) conditions of digestion. Over time, the digested compounds were dialyzed from the intestinal compartment and the content of nitrogen and AAs determined. The DIAAS for the goat milk formula, cow milk formula and human milk was 83%, 75%, and 77%, respectively, the latter being comparable to the value given by the FAO in humans.200 This suggests that the DIAAS can be determined by an in vitro dynamic model.
In general, the static models can provide a good approximation of overall protein digestion. The (semi)dynamic models have the potential to simulate the kinetics of protein digestion, in particular, when the gastric phase can induce structural changes in proteins. However, it is important to consider the digestion conditions in the (semi)dynamic models since the stirring mechanism has been observed to provide the main cause of difference from the in vivo data.
3.2 In vivo–in vitro correlation in lipid research
In vivo studies have usually assessed lipid breakdown by following the triglyceride appearance in blood, i.e. lipidemia. Although lipidemia is an indirect measure of lipid digestion, there is also an increasing interest in the study of the colloidal properties of lipids using, for example, MRI during gastric digestion to gain insights into gastric structural changes. This research has currently been of great interest since digestion of lipid is not only important in nutrient delivery and absorption but also plays a critical role in satiation and subsequent regulation of energy intake.75A few studies have investigated the in vivo–in vitro correlation. Golding et al. studied the free fatty acid (FFA) release in oil-in-water emulsions (stabilised by phospholipid, polysorbate, whey proteins and sodium stearoyl lactylate) designed to exert specific gastric behaviours (stable, coalesced, partially coalesced and fully broken).205 This study was performed using a 2-state static digestion, a gastric phase (pH 1.9) and intestinal phase using a pH stat device controlled at pH 6.8, which enable the measurement of the extent of lipolysis. The authors showed that the low surface area induced by lipid coalescence or breaking, resulted in low lipolysis when compared to emulsions that were stable under gastric conditions. Three emulsions (phospholipids, sodium stearyl lactylate liquid oil and liquid oil/solid fat) were used in an in vivo study using healthy participants to investigate the impact on lipid absorption/metabolism. The rates of increase in triglyceride concentration in plasma after the consumption of the emulsions were partially correlated to the rates of FFAs released in the in vitro intestinal phase. However, there was a poor correlation with the emulsion that coalesced under simulated gastric conditions (i.e. emulsion with sodium stearyl lactylate liquid oil/solid fat). The in vivo results showed a delayed but fast intestinal lipid uptake in contrast to the slow lipolysis in vitro. The stirring applied in the static model and the use of a static gastric pH (1.9) could have influenced this outcome. The latter provides an overestimation of pepsin hydrolysis and limited action of the fungal gastric lipase at that pH. These conditions differed from the in vivo situation and therefore, could influence the gastric stability of the emulsions and the subsequent triglyceride appearance in blood. Indeed, the incorporation of solid fat into an acid-unstable emulsion induced phase separation observed by MRI, which delayed the emptying of lipid.206 Therefore, when studying the effect of lipid gastric stability on lipid digestion it is crucial to consider the physiologically relevant conditions of the gastric digestion that are better provided by (semi)dynamic models.
A semi-dynamic model was able to simulate the gastric restructuring that was observed in an in vivo study.74 This model simulated the gradual pH decrease, the sequential addition of digestives enzymes and gastric fluid and the gastric emptying. The latter parameter was simulated according to a pre-set curve based on the in vivo study data207 and each aliquot that was taken simulating the gastric emptying underwent a static small intestinal digestion. The same samples were tested in both studies; semi-solid (a mixture of cheese and yoghurt) and liquid (oil-in-water emulsion stabilised by milk proteins). The in vivo study, using MRI, showed phase-separation and sedimentation in the liquid and semi-solid samples, respectively. The same gastric behaviour was obtained in the in vitro model providing a good in vivo–in vitro correlation. However, the phase separation of the liquid sample was obtained in a later stage, which might be due to the complex peristaltic movements that were not well simulated in the gastric in vitro model used. Furthermore, in the in vivo study, the semi-solid sample induced substantially more fullness than the liquid sample after just 15 min of digestion. This could potentially be explained by the high levels of proteins and lipids released from the semi-solid sample in vitro. This study shows how in vivo and in vitro models can complement one another to gain mechanistic insights into in vivo outcomes, which could be highly complex to obtain using only in vivo data. However, experiments need to be performed using relevant in vitro models based on the research question of interest.
In general, the static models can predict trends of the overall lipid digestion. However, semi-dynamic and dynamic models can be recommended as the most appropriate model to simulate the gastric colloidal stability of lipids that occurs in vivo.
3.3 In vivo–in vitro correlation in carbohydrate research
In clinical studies, the digestion of digestible carbohydrates, mainly starch, is followed by the rate of postprandial glucose appearance in blood, i.e. glycaemia. The most popular metric to classify the in vivo availability of digestible carbohydrate sources is the glycaemic index.208 This is a kinetic parameter that measures the postprandial incremental area under the curve (AUC) for 120 min in a test food when compared to a reference food (white bread or glucose solution) with the equivalent amount of available carbohydrate (ISO, 2010). The study of the postprandial glucose of foods is of high interest since the consumption of food products containing slowly digested starch has been associated with health benefits such as the decreased risk of type II diabetes.209Static in vitro models have been widely applied to study starch digestibility to predict the glycaemic response to foods. The study of Jenkins et al. is one of the first to investigate the correlation between the glycaemic index of foods and their in vitro starch hydrolysis.208 The in vitro static digestion included pooled human saliva and post-prandial jejunal secretions using dialysis bags. The authors found a high positive correlation between the concentration of carbohydrate liberated in vitro and the glycaemic index at 1 hour (r = 0.8627) and 5 hours (r = 0.8618) of digestion in foods with a different starch source. Englyst et al. developed an enzymatic in vitro method to classify food carbohydrates based on their digestibility,210 where rapidly available glucose was defined as the fraction of glucose that was obtained at 20 min of hydrolysis and slowly available glucose was related to the fraction obtained at 120 min of hydrolysis. This in vitro method provided a significant correlation (r = 0.981) between the proportions of rapidly digestive starch when compared to the glycaemic index values obtained in vivo.211 Similarly, Goñi et al. developed a static in vitro procedure to follow the rate of starch digestion of a series of 10 starchy foods.212 The authors showed a possible estimation of glycaemic index based on the time monitoring of starch hydrolysis, considering 90 min of intestinal digestion as the best correlation (r = 0.909) with the glucose responses (AUCs for 120 min).
There is a multitude of other in vitro carbohydrate digestion models that can predict the glycaemic properties of foods.213,214 However, they do not simulate the glycaemic response over time, which needs to take into account the rate of gastric emptying. The rate of digestion and absorption of glucose is controlled by the pancreatic α-amylase accessibility and amount of starch present in the duodenum. The latter is very much dictated by gastric emptying. Furthermore, salivary α-amylase can play a significant role in gastric starch hydrolysis since it is still active at the highest pH values found in the early stage of gastric digestion.129 Therefore, the simplification of a static gastric pH or its absence could lead to underestimation of starch hydrolysis.
Some dynamic in vitro models have been used to calculate glycaemic index based on the kinetics of digestible starch hydrolysis in the small intestine and correlate with in vivo data. For example, Ballance et al. investigated starch digestion in six cereal-based meals (white bread, cornflakes, micronized barley flakes, extruded barley flakes, oat flour and extruded oat) performed by a dynamic gastric digestion using the DGM and static intestinal digestion for 60 min.93 The samples were chewed by a subject and expectorated. There was no significant difference in the glycaemic index between the calculated in vitro values and the corresponding in vivo values from the literature for cornflakes and the two barley products. The two barley flake samples (micronized and extruded) were also assessed in vivo. The micronised barley flake meal had a low digestible starch content, peak rate of starch hydrolysis and a long duration of starch digestion in both in vitro and in vivo. The in vitro calculated glycaemic index was 66, which was similar to that calculated in vivo. However, the peak of the glucose curve in plasma in vivo occurred earlier than the one predicted in vitro. It is important to highlight that the supernatant of the digesta samples was treated with amyloglucosidase to convert the starch oligomers to glucose and, therefore simulating the further hydrolysis that occurs in the brush border. Therefore, the in vitro data represented the potential plasma curves. This study shows the potential of the dynamic in vitro model to simulate the in vivo glycaemic response of a simple starch-rich cereal.
The multi-compartmental digestion system, TIM-1, has been used to examine the impact of fermented barley and oat microstructure on the rate of in vitro starch hydrolysis,133 and compared to the glycaemic index values of the human study performed with identical raw material and tempe fermentations.215 The AUC calculated from the starch hydrolysis curves, measured as maltose generated during digestion, in barley was 40% of that for oat tempe. Similarly, in the parallel human study, the AUC for plasma glucose for barley was 46% of the AUC for oat tempe. The findings showed that both the oat and barley samples showed an increase in starch hydrolysis within the 60 min in contrast to the rise of blood glucose that occurred within the 30 min. However, after that increase the levels reached a plateau and decreased steadily both in vitro and in vivo. Therefore, the close agreement of the in vitro–in vivo data indicates this dynamic model as a potential tool of predicting the rates and extent of glucose in plasma. The TIM model has been validated with clinical data on carbohydrate digestion, against different in vivo plasma glucose response curves after the intake of carbohydrate food products (R = 0.91) and named TIM-Carbo technology.216
The use of in vitro models has been seen to provide a valuable tool to give indications about glycaemic index. However, most of the studies have used individual food products that are commonly consumed as part of a complex meal. For instance, corn flakes are usually consumed with milk. The co-ingestion of other macronutrients will influence the emptying rate of carbohydrate foods and, consequently the glycaemic response.217 Consequently, it is important to bear in mind, that the gastric emptying of foods can change their inherent glycaemic index.
3.4 In vivo–in vitro correlation in micronutrients and phytochemicals research
Bioavailability of micronutrients and phytochemicals in vivo is estimated using animal models or in human studies by measuring long-term plasma responses. The absorption efficiency of food micronutrients and phytochemicals is highly variable and dependent on a range of factors such as colonic changes and transport metabolism. Some of them are highly absorbed in the small intestine (i.e. carotenoids) whereas the colonic fermentation is highly important in others (i.e. polyphenols).Reboul et al. compared the bioaccessibility of the micellar fraction of food sources rich in carotenoids (α- and γ-tocopherol, β-carotene, lycopene, and lutein) between an in vitro static model218 and in vivo.219 The results showed a high correlation (r = 0.90) between the in vitro–in vivo bioaccessibility. However, spinach lutein bioaccessibility was about 5-fold higher in vitro than in vivo. The authors also compared the in vitro bioaccessibility values obtained with the bioavailability values measured in other published human studies using similar test meals, obtaining a significant relationship (r = 0.98). However, the range of bioavailability ratios observed in human studies were very wide.
The digestion of polyphenols is dictated by the extent of transformation into different metabolites. A small amount of ingested polyphenols are absorbed in the small intestine and converted by enzymes in brush border. Many polyphenols reach the colon where several microbial metabolites are formed and can be absorbed. Vetrani et al. compared the in vitro microbial phenolic metabolite profile of a series of high polyphenol content foods with the metabolites that were excreted by subjects after consuming the same foods in an 8-week study.220 There was a moderate correlation (r = 0.280) for the main 15 metabolites between the average calculation of 6-hour colonic metabolites from in vitro colon model and 24-hour urinary.
Verwei et al. developed an approach to predict human serum concentrations after the consumption of folate-fortified milk products.221 The GI digestion was performed using a TIM-1 providing folate bioaccessibility data and Caco-2 cells were used to provide in vitro data about the folate absorption. This data was used as input for a mathematical (in silico) model to predict serum concentrations. The in vitro–in silico approach was compared to the in vivo data using the same fortified milks consumed for 4 weeks. The predicted serum folate concentrations would increase from 9.2 nmol L−1 to 14.6 nmol L−1 and 15.3 nmol L−1 for UHT and pasteurized milk, respectively, whereas the serum concentrations increased to 14.1 and 13.9 nmol L−1in vivo. According to the authors, this in vitro–in silico approach provided a correct prediction of serum folate concentrations in humans.
Overall, the high complexity and variability of the bioavailability observed in micronutrients and phytochemicals leads to the use of specific approaches in each case to try to enhance the in vivo–in vitro correlation. The review by Etcheverry et al. provides more detailed information.222
4 Conclusions
In this article, we have reviewed the wide range of approaches to simulate the human GI tract. It is evident that although progress has been made towards more physiologically relevant and standardised approaches such as those developed by the Infogest Network, there is still a wide range of models being used for different purposes. One of the key recent developments has been the extension of models to enable the simulation of the GI environment in different segments of the population. These have seen particular focus on infants and the elderly but a number of other groups are also being studied. In addition to this approach targeting more specific demographic groups, the use of combinations of in silico, in vitro and in vivo models offers many opportunities for more powerful predictive scenarios.A model is only useful if it is either predictive or offers mechanistic insight. In either case, models need to be validated against in vivo data and this can often be an obstacle due to limitations in the availability of relevant data. In particular, human studies tend to measure bioactive concentrations in blood and not in the GI tract whereas in vitro studies often only produce data on release into the gut lumen. This is the difficulty of comparing bioaccessibility as generated in vitro with bioavailability as generated in vivo. In general, bioaccessibility tends to overestimate bioavailability. Indeed, the luminal hydrolysis by the brush border enzymes and the mucosal absorption might be considered as limiting factors, which can be considered for further improvements to in vitro models. It is crucial to build up a proper correlation between in vitro and in vivo to better elucidate nutrient digestion mechanisms in vitro and, consequently, gain understanding of the linked physiological responses.
It is apparent that the models being used are increasingly being validated with in vivo data and this bodes well for the future. In particular, the combination of artificial intelligent methods and validated in vitro models offers the possibility of replacing animal and human studies in some areas of research.
Abbreviations
| GI | Gastrointestinal |
| UHT | Ultra-high temperature |
| AA | Amino acid |
| DGM | Dynamic gastric model |
| HGS | Human gastric simulator |
| BBM | Brush border membrane |
| CFD | Computational fluid dynamics |
| DIAAS | Digestible indispensable amino acid score |
| MRI | Magnetic resonance imaging |
| FFA | Free fatty acid |
| AUC | Area under the curve |
Conflicts of interest
The authors have no conflicts of interest to declare.References
- R. Lucas-Gonzalez, M. Viuda-Martos, J. A. Perez-Alvarez and J. Fernandez-Lopez, In vitro digestion models suitable for foods: Opportunities for new fields of application and challenges, Food Res. Int., 2018, 107, 423–436 CrossRef CAS.
- D. J. McClements and S.-F. Peng, Current status in our understanding of physicochemical basis of bioaccessibility, Curr. Opin. Food Sci., 2020, 31, 57–62 CrossRef.
- F. Granado-Lorencio, E. Donoso-Navarro, I. Blanco-Navarro and B. Perez-Sacristan, Predictive value of in vitro digestion models: in vivo validation using absorption modifiers, Ann. Nutr. Metab., 2011, 58, 102–102 Search PubMed.
- T. Bohn, F. Carriere, L. Day, A. Deglaire, L. Egger, D. Freitas, M. Golding, S. Le Feunteun, A. Macierzanka, O. Menard, B. Miralles, A. Moscovici, R. Portmann, I. Recio, D. Remond, V. Sante-Lhoutelier, T. J. Wooster, U. Lesmes, A. R. Mackie and D. Dupont, Correlation between in vitro and in vivo data on food digestion. What can we predict with static in vitro digestion models?, Crit. Rev. Food Sci. Nutr., 2018, 58, 2239–2261 CrossRef CAS.
- N. M. Anson, R. van den Berg, R. Havenaar, A. Bast and G. Haenen, Bioavailability of ferulic acid is determined by its bioaccessibility, J. Cereal Sci., 2009, 49, 296–300 CrossRef.
- M. Hajishafiee, V. Bitarafan and C. Feinle-Bisset, Gastrointestinal Sensing of Meal-Related Signals in Humans, and Dysregulations in Eating-Related Disorders, Nutrients, 2019, 11, 1298 CrossRef CAS.
- A. Okada, M. Honma, S. Nomura and Y. Yamada, Oral behavior from food intake until terminal swallow, Physiol. Behav., 2007, 90, 172–179 CrossRef CAS.
- M. Assad-Bustillos, J. Palier, H. Rabesona, Y. Choiset, G. Della Valle and G. Feron, Role of the bolus degree of structure on the protein digestibility during in vitro digestion of a pea protein-fortified sponge cake chewed by elderly, J. Texture Stud., 2020, 51, 134–143 Search PubMed.
- M. Fried, S. Abramson and J. H. Meyer, Passage of salivary amylase through the stomach in humans, Dig. Dis. Sci., 1987, 32, 1097–1103 CrossRef CAS.
- L. Sams, J. Paume, J. Giallo and F. Carriere, Relevant pH and lipase for in vitro models of gastric digestion, Food Funct., 2016, 7, 30–45 RSC.
- D. W. Piper and B. H. Fenton, pH stability and activity curves of pepsin with special reference to their clinical importance, Gut, 1965, 6, 506–508 CrossRef CAS.
- L. Marciani, P. A. Gowland, R. C. Spiller, P. Manoj, R. J. Moore, P. Young and A. J. Fillery-Travis, Effect of meal viscosity and nutrients on satiety, intragastric dilution, and emptying assessed by MRI, Am. J. Physiol.: Gastrointest. Liver Physiol., 2001, 280, G1227–G1233 CrossRef CAS.
- A. Brodkorb, L. Egger, M. Alminger, P. Alvito, R. Assunção, S. Ballance, T. Bohn, C. Bourlieu-Lacanal, R. Boutrou, F. Carrière, A. Clemente, M. Corredig, D. Dupont, C. Dufour, C. Edwards, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. R. Mackie, C. Martins, S. Marze, D. J. McClements, O. Ménard, M. Minekus, R. Portmann, C. N. Santos, I. Souchon, R. P. Singh, G. E. Vegarud, M. S. J. Wickham, W. Weitschies and I. Recio, INFOGEST static in vitro simulation of gastrointestinal food digestion, Nat. Protoc., 2019, 14, 991–1014 CAS.
- L. Egger, O. Menard, C. Delgado-Andrade, P. Alvito, R. Assuncao, S. Balance, R. Barbera, A. Brodkorb, T. Cattenoz, A. Clemente, I. Comi, D. Dupont, G. Garcia-Llatas, M. J. Lagarda, S. Le Feunteun, L. JanssenDuijghuijsen, S. Karakaya, U. Lesmes, A. R. Mackie, C. Martins, A. Meynier, B. Miralles, B. S. Murray, A. Pihlanto, G. Picariello, C. N. Santos, S. Simsek, I. Recio, N. Rigby, L. E. Rioux, H. Stoffers, A. Tavares, L. Tavares, S. Turgeon, E. K. Ulleberg, G. E. Vegarud, G. Vergeres and R. Portmann, The harmonized INFOGEST in vitro digestion method: From knowledge to action, Food Res. Int., 2016, 88, 217–225 CrossRef CAS.
- L. Egger, O. Menard, C. Baumann, D. Duerr, P. Schlegel, P. Stoll, G. Vergeres, D. Dupont and R. Portmann, Digestion of milk proteins: Comparing static and dynamic in vitro digestion systems with in vivo data, Food Res. Int., 2019, 118, 32–39 CrossRef CAS.
- S. J. Hur, B. O. Lim, E. A. Decker and D. J. McClements, In vitro human digestion models for food applications, Food Chem., 2011, 125, 1–12 CrossRef CAS.
- J. Gass, H. Vora, A. F. Hofmann, G. M. Gray and C. Khosla, Enhancement of dietary protein digestion by conjugated bile acids, Gastroenterology, 2007, 133, 16–23 CrossRef CAS.
- G. Mandalari, A. M. Mackie, N. M. Rigby, M. S. Wickham and E. N. Mills, Physiological phosphatidylcholine protects bovine beta-lactoglobulin from simulated gastrointestinal proteolysis, Mol. Nutr. Food Res., 2009, 53(Suppl 1), S131–S139 CrossRef.
- A. Macierzanka, A. Torcello-Gomez, C. Jungnickel and J. Maldonado-Valderrama, Bile salts in digestion and transport of lipids, Adv. Colloid Interface Sci., 2019, 274, 102045 CrossRef CAS.
- M. Minekus, M. Alminger, P. Alvito, S. Ballance, T. Bohn, C. Bourlieu, F. Carriere, R. Boutrou, M. Corredig, D. Dupont, C. Dufour, L. Egger, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. Mackie, S. Marze, D. J. McClements, O. Menard, I. Recio, C. N. Santos, R. P. Singh, G. E. Vegarud, M. S. Wickham, W. Weitschies and A. Brodkorb, A standardised static in vitro digestion method suitable for food - an international consensus, Food Funct., 2014, 5, 1113–1124 RSC.
- A. I. Mulet-Cabero, L. Egger, R. Portmann, O. Menard, S. Marze, M. Minekus, S. Le Feunteun, A. Sarkar, M. M. Grundy, F. Carriere, M. Golding, D. Dupont, I. Recio, A. Brodkorb and A. Mackie, A standardised semi-dynamic in vitro digestion method suitable for food - an international consensus, Food Funct., 2020, 11, 1702–1720 RSC.
- L. Sams, S. Amara, P. Mansuelle, R. Puppo, R. Lebrun, J. Paume, J. Giallo and F. Carriere, Characterization of pepsin from rabbit gastric extract, its action on beta-casein and the effects of lipids on proteolysis, Food Funct., 2018, 9, 5975–5988 RSC.
- A. Roussel, S. Canaan, M. P. Egloff, M. Riviere, L. Dupuis, R. Verger and C. Cambillau, Crystal structure of human gastric lipase and model of lysosomal acid lipase, two lipolytic enzymes of medical interest, J. Biol. Chem., 1999, 274, 16995–17002 CrossRef CAS.
- P. Capolino, C. Guérin, J. Paume, J. Giallo, J. M. Ballester, J. F. Cavalier and F. Carrière, In Vitro Gastrointestinal Lipolysis: Replacement of Human Digestive Lipases by a Combination of Rabbit Gastric and Porcine Pancreatic Extracts, Food Dig., 2011, 2, 43–51 CrossRef CAS.
- A. Macierzanka, A. I. Sancho, E. N. C. Mills, N. M. Rigby and A. R. Mackie, Emulsification alters simulated gastrointestinal proteolysis of β-casein and β-lactoglobulin, Soft Matter, 2009, 5, 538–550 RSC.
- N. H. Zangenberg, A. Mullertz, H. G. Kristensen and L. Hovgaard, A dynamic in vitro lipolysis model. I. Controlling the rate of lipolysis by continuous addition of calcium, Eur. J. Pharm. Sci., 2001, 14, 115–122 CrossRef CAS.
- E. K. Eriksen, H. Holm, E. Jensen, R. Aaboe, T. G. Devold, M. Jacobsen and G. E. Vegarud, Different digestion of caprine whey proteins by human and porcine gastrointestinal enzymes, Br. J. Nutr., 2010, 104, 374–381 CrossRef CAS.
- K. E. Aarak, B. Kirkhus, H. Holm, G. Vogt, M. Jacobsen and G. E. Vegarud, Release of EPA and DHA from salmon oil - a comparison of in vitro digestion with human and porcine gastrointestinal enzymes, Br. J. Nutr., 2013, 110, 1402–1410 CrossRef CAS.
- T. Asledottir, T. T. Le, B. Petrat-Melin, T. G. Devold, L. B. Larsen and G. E. Vegarud, Identification of bioactive peptides and quantification of β-casomorphin-7 from bovine β-casein A1, A2 and I after ex vivo gastrointestinal digestion, Int. Dairy J., 2017, 71, 98–106 CrossRef CAS.
- E. K. Ulleberg, I. Comi, H. Holm, E. B. Herud, M. Jacobsen and G. E. Vegarud, Human Gastrointestinal Juices Intended for Use in In Vitro Digestion Models, Food Dig., 2011, 2, 52–61 CrossRef CAS.
- C. Li, W. Yu, P. Wu and X. D. Chen, Current in vitro digestion systems for understanding food digestion in human upper gastrointestinal tract, Trends Food Sci. Technol., 2020, 96, 114–126 CrossRef CAS.
- J. D. Astwood, J. N. Leach and R. L. Fuchs, Stability of food allergens to digestion in vitro, Nat. Biotechnol., 1996, 14, 1269–1273 CrossRef CAS.
- K. Thomas, M. Aalbers, G. A. Bannon, M. Bartels, R. J. Dearman, D. J. Esdaile, T. J. Fu, C. M. Glatt, N. Hadfield, C. Hatzos, S. L. Hefle, J. R. Heylings, R. E. Goodman, B. Henry, C. Herouet, M. Holsapple, G. S. Ladics, T. D. Landry, S. C. MacIntosh, E. A. Rice, L. S. Privalle, H. Y. Steiner, R. Teshima, R. van Ree, M. Woolhiser and J. Zawodny, A multi-laboratory evaluation of a common in vitro pepsin digestion assay protocol used in assessing the safety of novel proteins, Regul. Toxicol. Pharmacol., 2004, 39, 87–98 CrossRef CAS.
- H. Naegeli, A. N. Birch, J. Casacuberta, A. De Schrijver, M. A. Gralak, P. Guerche, H. Jones, B. Manachini, A. Messean, E. E. Nielsen, F. Nogue, C. Robaglia, N. Rostoks, J. Sweet, C. Tebbe, F. Visioli, J. M. Wal, P. Eigenmann, M. Epstein, K. Hoffmann-Sommergruber, F. Koning, M. Lovik, C. Mills, F. J. Moreno, H. van Loveren, R. Selb, A. F. Dumont and E. P. G. Modified, Guidance on allergenicity assessment of genetically modified plants, EFSA J., 2017, 15, 4862–4911 Search PubMed.
- O. Menard, C. Bourlieu, S. C. De Oliveira, N. Dellarosa, L. Laghi, F. Carriere, F. Capozzi, D. Dupont and A. Deglaire, A first step towards a consensus static in vitro model for simulating full-term infant digestion, Food Chem., 2018, 240, 338–345 CrossRef CAS.
- C. S. Levi and U. Lesmes, Bi-compartmental elderly or adult dynamic digestion models applied to interrogate protein digestibility, Food Funct., 2014, 5, 2402–2409 RSC.
- C. S. Levi, N. Goldstein, R. Portmann and U. Lesmes, Emulsion and protein degradation in the elderly: Qualitative insights from a study coupling a dynamic in vitro digestion model with proteomic analyses, Food Hydrocolloids, 2017, 69, 393–401 CrossRef.
- C. Bourlieu, O. Menard, K. Bouzerzour, G. Mandalari, A. Macierzanka, A. R. Mackie and D. Dupont, Specificity of infant digestive conditions: some clues for developing relevant in vitro models, Crit. Rev. Food Sci. Nutr., 2014, 54, 1427–1457 CrossRef.
- C. S. Levi, P. Alvito, A. Andrés, R. Assunção, R. Barberá, S. Blanquet-Diot, C. Bourlieu, A. Brodkorb, A. Cilla, A. Deglaire, S. Denis, D. Dupont, A. Heredia, S. Karakaya, C. V. L. Giosafatto, L. Mariniello, C. Martins, O. Ménard, S. N. El, G. E. Vegarud, E. Ulleberg and U. Lesmes, Extending in vitro digestion models to specific human populations: Perspectives, practical tools and bio-relevant information, Trends Food Sci. Technol., 2017, 60, 52–63 CrossRef.
- T. T. P. Nguyen, B. Bhandari, J. Cichero and S. Prakash, A comprehensive review on in vitro digestion of infant formula, Food Res. Int., 2015, 76, 373–386 CrossRef CAS.
- M. Christian, C. Edwards and L. T. Weaver, Starch digestion in infancy, J. Pediatr. Gastroenterol. Nutr., 1999, 29, 116–124 CrossRef CAS.
- L. Poquet and T. J. Wooster, Infant digestion physiology and the relevance of in vitro biochemical models to test infant formula lipid digestion, Mol. Nutr. Food Res., 2016, 60, 1876–1895 CrossRef CAS.
- D. W. Piper and B. H. Fenton, pH stability and activity curves of pepsin with special reference to their clinical importance, Gut, 1965, 6, 506–508 CrossRef CAS.
- D. Kamstrup, R. Berthelsen, P. J. Sassene, A. Selen and A. Mullertz, In Vitro Model Simulating Gastro-Intestinal Digestion in the Pediatric Population (Neonates and Young Infants), AAPS PharmSciTech, 2017, 18, 317–329 CrossRef.
- J. Sarles, H. Moreau and R. Verger, Human gastric lipase: ontogeny and variations in children, Acta Paediatr., 1992, 81, 511–513 CrossRef CAS.
- E. Lebenthal, P. C. Lee and L. A. Heitlinger, Impact of development of the gastrointestinal tract on infant feeding, J Pediatr., 1983, 102, 1–9 CrossRef CAS.
- C. Bourlieu, O. Menard, A. De La Chevasnerie, L. Sams, F. Rousseau, M. N. Madec, B. Robert, A. Deglaire, S. Pezennec, S. Bouhallab, F. Carriere and D. Dupont, The structure of infant formulas impacts their lipolysis, proteolysis and disintegration during in vitro gastric digestion, Food Chem., 2015, 182, 224–235 CrossRef CAS.
- D. Bosscher, M. Van Caillie-Bertrand, H. Robberecht, K. Van Dyck, R. Van Cauwenbergh and H. Deelstra, In vitro availability of calcium, iron, and zinc from first-age infant formulae and human milk, J. Pediatr. Gastroenterol. Nutr., 2001, 32, 54–58 CrossRef CAS.
- D. E. W. Chatterton, J. T. Rasmussen, C. W. Heegaard, E. S. Sørensen and T. E. Petersen, In vitro digestion of novel milk protein ingredients for use in infant formulas: Research on biological functions, Trends Food Sci. Technol., 2004, 15, 373–383 CrossRef CAS.
- D. Dupont, G. Mandalari, D. Molle, J. Jardin, J. Leonil, R. M. Faulks, M. S. J. Wickham, E. N. C. Mills and A. R. Mackie, Comparative resistance of food proteins to adult and infant in vitro digestion models, Mol. Nutr. Food Res., 2010, 54, 767–780 CrossRef CAS.
- S. Cattaneo, M. Stuknyte, F. Masotti and I. De Noni, Protein breakdown and release of beta-casomorphins during in vitro gastro-intestinal digestion of sterilised model systems of liquid infant formula, Food Chem., 2017, 217, 476–482 CrossRef CAS.
- T. T. P. Nguyen, B. Bhandari, J. Cichero and S. Prakash, Gastrointestinal digestion of dairy and soy proteins in infant formulas: An in vitro study, Food Res. Int., 2015, 76, 348–358 CrossRef CAS.
- C. Shani-Levi, S. Levi-Tal and U. Lesmes, Comparative performance of milk proteins and their emulsions under dynamic in vitro adult and infant gastric digestion, Food Hydrocolloids, 2013, 32, 349–357 CrossRef CAS.
- Y. Joubran, A. Moscovici and U. Lesmes, Antioxidant activity of bovine alpha lactalbumin Maillard products and evaluation of their in vitro gastro-duodenal digestive proteolysis, Food Funct., 2015, 6, 1229–1240 RSC.
- C. H. Versantvoort, A. G. Oomen, E. Van de Kamp, C. J. Rompelberg and A. J. Sips, Applicability of an in vitro digestion model in assessing the bioaccessibility of mycotoxins from food, Food Chem. Toxicol., 2005, 43, 31–40 CrossRef CAS.
- C. Dall'Asta, P. Florio, A. M. Lammardo, B. Prandi, T. Mazzeo, A. Budelli and N. Pellegrini, Development of an in vitro digestive model for studying the peptide profile of breast milk, Int. J. Food Sci. Nutr., 2015, 66, 409–415 CrossRef.
- M. Klitgaard, P. J. Sassene, A. Selen, A. Mullertz and R. Berthelsen, Studying furosemide solubilization using an in vitro model simulating gastrointestinal digestion and drug solubilization in neonates and young infants, Eur. J. Pharm. Sci., 2017, 109, 191–199 CrossRef CAS.
- A. A. O. Xavier, J. E. Garrido-Lopez, J. Aguayo-Maldonado, J. Garrido-Fernandez, J. Fontecha and A. A. Perez-Galvez, In Vitro Digestion of Human Milk: Influence of the Lactation Stage on the Micellar Carotenoids Content, Antioxidants, 2019, 8, 291 CrossRef CAS.
- D. Remond, D. R. Shahar, D. Gille, P. Pinto, J. Kachal, M. A. Peyron, C. N. Dos Santos, B. Walther, A. Bordoni, D. Dupont, L. Tomas-Cobos and G. Vergeres, Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition, Oncotarget, 2015, 6, 13858–13898 CrossRef.
- M. Feldman, B. Cryer, K. E. McArthur, B. A. Huet and E. Lee, Effects of aging and gastritis on gastric acid and pepsin secretion in humans: a prospective study, Gastroenterology, 1996, 110, 1043–1052 CrossRef CAS.
- S. Denis, T. Sayd, A. Georges, C. Chambon, S. Chalancon, V. Sante-Lhoutellier and S. Blanquet-Diot, Digestion of cooked meat proteins is slightly affected by age as assessed using the dynamic gastrointestinal TIM model and mass spectrometry, Food Funct., 2016, 7, 2682–2691 RSC.
- M. A. Peyron, V. Santé-Lhoutellier, D. Dardevet, M. Hennequin, D. Rémond, O. François and A. Woda, Addressing various challenges related to food bolus and nutrition with the AM2 mastication simulator, Food Hydrocolloids, 2019, 97, 105229 CrossRef CAS.
- J.-R. Malagelada, V. L. Go and W. Summerskill, Different gastric, pancreatic, and biliary responses to solid-liquid or homogenized meals, Dig. Dis. Sci., 1979, 24, 101–110 CrossRef CAS.
- J. D. Gardner, A. A. Ciociola and M. Robinson, Measurement of meal-stimulated gastric acid secretion by in vivo gastric autotitration, J. Appl. Physiol., 2002, 92, 427–434 CrossRef CAS.
- B. L. Dekkers, E. Kolodziejczyk, S. Acquistapace, J. Engmann and T. J. Wooster, Impact of gastric pH profiles on the proteolytic digestion of mixed betalg-Xanthan biopolymer gels, Food Funct., 2016, 7, 58–68 RSC.
- W. Liu, D. Fu, X. Zhang, J. Chai, S. Tian and J. Han, Development and validation of a new artificial gastric digestive system, Food Res. Int., 2019, 122, 183–190 CrossRef CAS.
- A. I. Mulet-Cabero, A. Torcello-Gomez, S. Saha, A. R. Mackie, P. J. Wilde and A. Brodkorb, Impact of caseins and whey proteins ratio and lipid content on in vitro digestion and ex vivo absorption, Food Chem., 2020, 319, 126514 CrossRef CAS.
- Y. Boirie, M. Dangin, P. Gachon, M.-P. Vasson, J.-L. Maubois and B. Beaufrère, Slow and fast dietary proteins differently modulate postprandial protein accretion, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 14930–14935 CrossRef CAS.
- A.-I. Mulet-Cabero, A. R. Mackie, P. J. Wilde, M. A. Fenelon and A. Brodkorb, Structural mechanism and kinetics of in vitro gastric digestion are affected by process-induced changes in bovine milk, Food Hydrocolloids, 2019, 86, 172–183 CrossRef CAS.
- C. C. M. van den Braak, M. Klebach, E. Abrahamse, M. Minor, Z. Hofman, J. Knol and T. Ludwig, A novel protein mixture containing vegetable proteins renders enteral nutrition products non-coagulating after in vitro gastric digestion, Clin. Nutr., 2013, 32, 765–771 CrossRef CAS.
- F. Nau, K. Nyemb-Diop, V. Lechevalier, J. Floury, C. Serrière, N. Stroebinger, T. Boucher, C. Guérin-Dubiard, M. J. Ferrua and D. Dupont, Spatial-temporal changes in pH, structure and rheology of the gastric chyme in pigs as influenced by egg white gel properties, Food Chem., 2019, 280, 210–220 CrossRef CAS.
- M. Vardakou, A. Mercuri, T. A. Naylor, D. Rizzo, J. M. Butler, P. C. Connolly, M. S. J. Wickham and R. M. Faulks, Predicting the human in vivo performance of different oral capsule shell types using a novel in vitro dynamic gastric model, Int. J. Pharm., 2011, 419, 192–199 CrossRef CAS.
- F. B. Kong and R. P. Singh, A Human Gastric Simulator (HGS) to Study Food Digestion in Human Stomach, J. Food Sci., 2010, 75, E627–E635 CrossRef CAS.
- A.-I. Mulet-Cabero, N. M. Rigby, A. Brodkorb and A. R. Mackie, Dairy food structures influence the rates of nutrient digestion through different in vitro gastric behaviour, Food Hydrocolloids, 2017, 67, 63–73 CrossRef CAS.
- C. Feinle, M. Christen, D. Grundy, H. Faas, O. Meier, B. Otto and M. Fried, Effects of duodenal fat, protein or mixed-nutrient infusions on epigastric sensations during sustained gastric distension in healthy humans, Neurogastroenterol. Motil., 2002, 14, 205–213 CrossRef CAS.
- Y. Cohen, M. Levi, U. Lesmes, M. Margier, E. Reboul and Y. D. Livney, Re-assembled casein micelles improve in vitro bioavailability of vitamin D in a Caco-2 cell model, Food Funct., 2017, 8, 2133–2141 RSC.
- L. Fahoum, A. Moscovici, S. David, R. Shaoul, G. Rozen, E. G. Meyron-Holtz and U. Lesmes, Digestive fate of dietary carrageenan: Evidence of interference with digestive proteolysis and disruption of gut epithelial function, Mol. Nutr. Food Res., 2017, 61, 1600545 CrossRef.
- A.-S. Stübler, U. Lesmes, V. Heinz, C. Rauh, A. Shpigelman and K. Aganovic, Digestibility, antioxidative activity and stability of plant protein-rich products after processing and formulation with polyphenol-rich juices: kale and kale–strawberry as a model, Eur. Food Res. Technol., 2019, 245, 2499–2514 CrossRef.
- G. A. van Aken, E. Bomhof, F. D. Zoet, M. Verbeek and A. Oosterveld, Differences in in vitro gastric behaviour between homogenized milk and emulsions stabilised by Tween 80, whey protein, or whey protein and caseinate, Food Hydrocolloids, 2011, 25, 781–788 CrossRef CAS.
- A. Ferreira-Lazarte, A. Montilla, A. I. Mulet-Cabero, N. Rigby, A. Olano, A. Mackie and M. Villamiel, Study on the digestion of milk with prebiotic carbohydrates in a simulated gastrointestinal model, J. Funct. Foods, 2017, 33, 149–154 CrossRef CAS.
- Y. C. Luiking, E. Abrahamse, T. Ludwig, Y. Boirie and S. Verlaan, Protein type and caloric density of protein supplements modulate postprandial amino acid profile through changes in gastrointestinal behaviour: A randomized trial, Clin. Nutr., 2016, 35, 48–58 CrossRef CAS.
- A. Woda, A. Mishellany-Dutour, L. Batier, O. François, J. Meunier, B. Reynaud, M. Alric and M.-A. Peyron, Development and validation of a mastication simulator, J. Biomech., 2010, 43, 1667–1673 CrossRef CAS.
- A. N. Payne, A. Zihler, C. Chassard and C. Lacroix, Advances and perspectives in in vitro human gut fermentation modeling, Trends Biotechnol., 2012, 30, 17–25 CrossRef CAS.
- A. Tharakan, I. Norton, P. Fryer and S. Bakalis, Mass transfer and nutrient absorption in a simulated model of small intestine, J. Food Sci., 2010, 75, E339–E346 CrossRef CAS.
- N. D. Wright, F. Kong, B. S. Williams and L. Fortner, A human duodenum model (HDM) to study transport and digestion of intestinal contents, J. Food Eng., 2016, 171, 129–136 CrossRef CAS.
- M. Wickham, R. Faulks, J. Mann and G. Mandalari, The design, operation, and application of a dynamic gastric model, Dissolution Technol., 2012, 19, 15–22 CrossRef CAS.
- M. Vardakou, A. Mercuri, S. A. Barker, D. Q. Craig, R. M. Faulks and M. S. Wickham, Achieving antral grinding forces in biorelevant in vitro models: comparing the USP dissolution apparatus II and the dynamic gastric model with human in vivo data, AAPS PharmSciTech, 2011, 12, 620–626 CrossRef.
- L. Marciani, P. A. Gowland, A. Fillery-Travis, P. Manoj, J. Wright, A. Smith, P. Young, R. Moore and R. C. Spiller, Assessment of antral grinding of a model solid meal with echo-planar imaging, Am. J. Physiol.: Gastrointest. Liver Physiol., 2001, 280, G844–G849 CrossRef CAS.
- G. E. Fraser, H. W. Bennett, K. B. Jaceldo and J. Sabaté, Effect on body weight of a free 76 kilojoule (320 calorie) daily supplement of almonds for six months, J. Am. Coll. Nutr., 2002, 21, 275–283 CrossRef.
- G. Mandalari, M. M.-L. Grundy, T. Grassby, M. L. Parker, K. L. Cross, S. Chessa, C. Bisignano, D. Barreca, E. Bellocco and G. Lagana, The effects of processing and mastication on almond lipid bioaccessibility using novel methods of in vitro digestion modelling and micro-structural analysis, Br. J. Nutr., 2014, 112, 1521–1529 CrossRef CAS.
- T. Grassby, G. Mandalari, M. M.-L. Grundy, C. H. Edwards, C. Bisignano, D. Trombetta, A. Smeriglio, S. Chessa, S. Ray and J. Sanderson, In vitro and in vivo modeling of lipid bioaccessibility and digestion from almond muffins: The importance of the cell-wall barrier mechanism, J. Funct. Foods, 2017, 37, 263–271 CrossRef CAS.
- G. Mandalari, N. M. Rigby, C. Bisignano, R. B. L. Curto, F. Mulholland, M. Su, M. Venkatachalam, J. M. Robotham, L. N. Willison and K. Lapsley, Effect of food matrix and processing on release of almond protein during simulated digestion, LWT – Food Sci. Technol., 2014, 59, 439–447 CrossRef CAS.
- S. Ballance, S. Sahlstrøm, P. Lea, N. E. Nagy, P. V. Andersen, T. Dessev, S. Hull, M. Vardakou and R. Faulks, Evaluation of gastric processing and duodenal digestion of starch in six cereal meals on the associated glycaemic response using an adult fasted dynamic gastric model, Eur. J. Nutr., 2013, 52, 799–812 CrossRef CAS.
- G. Mandalari, C. Bisignano, A. Filocamo, S. Chessa, M. Sarò, G. Torre, R. M. Faulks and P. Dugo, Bioaccessibility of pistachio polyphenols, xanthophylls, and tocopherols during simulated human digestion, Nutrition, 2013, 29, 338–344 CrossRef CAS.
- A. L. Curto, I. Pitino, G. Mandalari, J. R. Dainty, R. M. Faulks and M. S. J. Wickham, Survival of probiotic lactobacilli in the upper gastrointestinal tract using an in vitro gastric model of digestion, Food Microbiol., 2011, 28, 1359–1366 CrossRef.
- M. Kamba, Y. Seta, A. Kusai, M. Ikeda and K. Nishimura, A unique dosage form to evaluate the mechanical destructive force in the gastrointestinal tract, Int. J. Pharm., 2000, 208, 61–70 CrossRef CAS.
- M. J. Vassallo, M. Camilleri, C. M. Prather, R. Hanson and G. Thomforde, Measurement of axial forces during emptying from the human stomach, Am. J. Physiol.: Gastrointest. Liver Physiol., 1992, 263, G230–G239 CrossRef CAS.
- F. Kong, M. H. Oztop, R. P. Singh and M. J. McCarthy, Physical changes in white and brown rice during simulated gastric digestion, J. Food Sci., 2011, 76, E450–E457 CrossRef CAS.
- L. N. Panlasigui and L. U. Thompson, Blood glucose lowering effects of brown rice in normal and diabetic subjects, Int. J. Food Sci. Nutr., 2006, 57, 151–158 CrossRef CAS.
- Q. Guo, A. Ye, M. Lad, D. Dalgleish and H. Singh, Effect of gel structure on the gastric digestion of whey protein emulsion gels, Soft Matter, 2014, 10, 1214–1223 RSC.
- Q. Guo, A. Ye, M. Lad, M. Ferrua, D. Dalgleish and H. Singh, Disintegration kinetics of food gels during gastric digestion and its role on gastric emptying: an in vitro analysis, Food Funct., 2015, 6, 756–764 RSC.
- A. Ye, J. Cui, D. Dalgleish and H. Singh, Formation of a structured clot during the gastric digestion of milk: Impact on the rate of protein hydrolysis, Food Hydrocolloids, 2016, 52, 478–486 CrossRef CAS.
- A. Ye, J. Cui, D. Dalgleish and H. Singh, The formation and breakdown of structured clots from whole milk during gastric digestion, Food Funct., 2016, 7, 4259–4266 RSC.
- A. Ye, J. Cui, D. Dalgleish and H. Singh, Effect of homogenization and heat treatment on the behavior of protein and fat globules during gastric digestion of milk, J. Dairy Sci., 2017, 100, 36–47 CrossRef CAS.
- G. Mandalari, M. Vardakou, R. Faulks, C. Bisignano, M. Martorana, A. Smeriglio and D. Trombetta, Food matrix effects of polyphenol bioaccessibility from almond skin during simulated human digestion, Nutrients, 2016, 8, 568 CrossRef.
- A. Lopez de Lacey, B. Giménez, E. Pérez-Santín, R. Faulks, G. Mandalari, M. López-Caballero and P. Montero, Bioaccessibility of green tea polyphenols incorporated into an edible agar film during simulated human digestion, Food Res. Int., 2012, 48, 462–469 CrossRef CAS.
- N. López-Barón, D. Sagnelli, A. Blennow, M. Holse, J. Gao, L. Saaby, A. Müllertz, B. Jespersen and T. Vasanthan, Hydrolysed pea proteins mitigate in vitro wheat starch digestibility, Food Hydrocolloids, 2018, 79, 117–126 CrossRef.
- G. Mandalari, Z. Merali, P. Ryden, S. Chessa, C. Bisignano, D. Barreca, E. Bellocco, G. Laganà, R. M. Faulks and K. W. Waldron, Durum wheat particle size affects starch and protein digestion in vitro, Eur. J. Nutr., 2018, 57, 319–325 CrossRef CAS.
- X. Wang, A. Ye, Q. Lin, J. Han and H. Singh, Gastric digestion of milk protein ingredients: Study using an in vitro dynamic model, J. Dairy Sci., 2018, 101, 6842–6852 CrossRef CAS.
- X. Wang, Q. Lin, A. Ye, J. Han and H. Singh, Flocculation of oil-in-water emulsions stabilised by milk protein ingredients under gastric conditions: Impact on in vitro intestinal lipid digestion, Food Hydrocolloids, 2019, 88, 272–282 CrossRef CAS.
- A. Ye, X. Wang, Q. Lin, J. Han and H. Singh, Dynamic gastric stability and in vitro lipid digestion of whey-protein-stabilised emulsions: Effect of heat treatment, Food Chem., 2020, 318, 126463 CrossRef CAS.
- E. Barroso, C. Cueva, C. Peláez, M. C. Martínez-Cuesta and T. Requena, Development of human colonic microbiota in the computer-controlled dynamic SIMulator of the GastroIntestinal tract SIMGI, LWT – Food Sci. Technol., 2015, 61, 283–289 CrossRef CAS.
- T. Van de Wiele, P. Van den Abbeele, W. Ossieur, S. Possemiers and M. Marzorati, in The impact of food bioactives on health, Springer, Cham, 2015, pp. 305–317 Search PubMed.
- M. Minekus, P. Marteau and R. Havenaar, Multicompartmental dynamic computer-controlled model simulating the stomach and small intestine, ATLA, Altern. Lab. Anim., 1995, 23(2), 197–209 CrossRef.
- C. Villemejane, R. Wahl, P. Aymard, S. Denis and C. Michon, In vitro digestion of short-dough biscuits enriched in proteins and/or fibres, using a multi-compartmental and dynamic system (1): Viscosity measurement and prediction, Food Chem., 2015, 182, 55–63 CrossRef CAS.
- C. Villemejane, S. Denis, A. Marsset-Baglieri, M. Alric, P. Aymard and C. Michon, In vitro digestion of short-dough biscuits enriched in proteins and/or fibres using a multi-compartmental and dynamic system (2): Protein and starch hydrolyses, Food Chem., 2016, 190, 164–172 CrossRef CAS.
- F. M. AlHasawi, D. Fondaco, K. Ben-Elazar, S. Ben-Elazar, Y. Y. Fan, M. G. Corradini, R. D. Ludescher, D. Bolster, G. Carder and Y. Chu, In vitro measurements of luminal viscosity and glucose/maltose bioaccessibility for oat bran, instant oats, and steel cut oats, Food Hydrocolloids, 2017, 70, 293–303 CrossRef CAS.
- C. Nimalaratne, P. Savard, S. F. Gauthier, A. Schieber and J. Wu, Bioaccessibility and digestive stability of carotenoids in cooked eggs studied using a dynamic in vitro gastrointestinal model, J. Agric. Food Chem., 2015, 63, 2956–2962 CrossRef CAS.
- S. Blanquet-Diot, M. Soufi, M. Rambeau, E. Rock and M. Alric, Digestive stability of xanthophylls exceeds that of carotenes as studied in a dynamic in vitro gastrointestinal system, J. Nutr., 2009, 139, 876–883 CrossRef CAS.
- E. Deat, S. Blanquet-Diot, J.-F. Jarrige, S. Denis, E. Beyssac and M. Alric, Combining the dynamic TNO-gastrointestinal tract system with a Caco-2 cell culture model: Application to the assessment of lycopene and α-tocopherol bioavailability from a whole food, J. Agric. Food Chem., 2009, 57, 11314–11320 CrossRef CAS.
- M. Verwei, M. Minekus, E. Zeijdner, R. Schilderink and R. Havenaar, Evaluation of two dynamic in vitro models simulating fasted and fed state conditions in the upper gastrointestinal tract (TIM-1 and tiny-TIM) for investigating the bioaccessibility of pharmaceutical compounds from oral dosage forms, Int. J. Pharm., 2016, 498, 178–186 CrossRef CAS.
- A. Oosterveld, M. Minekus, E. Bomhof, F. D. Zoet and G. A. van Aken, Effects of inhomogeneity on triglyceride digestion of emulsions using an in vitro digestion model (Tiny TIM), Food Funct., 2016, 7, 2979–2995 RSC.
- A. Helbig, E. Silletti, G. A. van Aken, A. Oosterveld, M. Minekus, R. J. Hamer and H. Gruppen, Lipid Digestion of Protein Stabilized Emulsions Investigated in a Dynamic In Vitro Gastro-Intestinal Model System, Food Dig., 2013, 4, 58–68 CrossRef CAS.
- O. Ménard, T. Cattenoz, H. Guillemin, I. Souchon, A. Deglaire, D. Dupont and D. Picque, Validation of a new in vitro dynamic system to simulate infant digestion, Food Chem., 2014, 145, 1039–1045 CrossRef.
- S. C. de Oliveira, A. Deglaire, O. Ménard, A. Bellanger, F. Rousseau, G. Henry, E. Dirson, F. Carrière, D. Dupont and C. Bourlieu, Holder pasteurization impacts the proteolysis, lipolysis and disintegration of human milk under in vitro dynamic term newborn digestion, Food Res. Int., 2016, 88(Part B), 263–275 CrossRef CAS.
- L. Sánchez-Rivera, O. Ménard, I. Recio and D. Dupont, Peptide mapping during dynamic gastric digestion of heated and unheated skimmed milk powder, Food Res. Int., 2015, 77, 132–139 CrossRef.
- F. Barbé, O. Ménard, Y. Le Gouar, C. Buffière, M.-H. Famelart, B. Laroche, S. Le Feunteun, D. Dupont and D. Rémond, The heat treatment and the gelation are strong determinants of the kinetics of milk proteins digestion and of the peripheral availability of amino acids, Food Chem., 2013, 136, 1203–1212 CrossRef.
- M. N. Corstens, C. C. Berton-Carabin, K. Schroën, M. Viau and A. Meynier, Emulsion encapsulation in calcium-alginate beads delays lipolysis during dynamic in vitro digestion, J. Funct. Foods, 2018, 46, 394–402 CrossRef CAS.
- D. Freitas and S. Le Feunteun, Oro-gastro-intestinal digestion of starch in white bread, wheat-based and gluten-free pasta: unveiling the contribution of human salivary α-amylase, Food Chem., 2019, 274, 566–573 CrossRef CAS.
- L. Barros, C. Retamal, H. Torres, R. N. Zuniga and E. Troncoso, Development of an in vitro mechanical gastric system (IMGS) with realistic peristalsis to assess lipid digestibility, Food Res. Int., 2016, 90, 216–225 CrossRef CAS.
- J. J. Wang, P. Wu, M. H. Liu, Z. K. Liao, Y. Wang, Z. Z. Dong and X. D. Chen, An advanced near real dynamic in vitro human stomach system to study gastric digestion and emptying of beef stew and cooked rice, Food Funct., 2019, 10, 2914–2925 RSC.
- Y. W. Li, L. Fortner and F. B. Kong, Development of a Gastric Simulation Model (GSM) incorporating gastric geometry and peristalsis for food digestion study, Food Res. Int., 2019, 125, 108598 CrossRef CAS.
- M. L. Alminger, C. Eklund-Jonsson, S. Kidman and M. Langton, Starch microstructure and starch hydrolysis in barley and oat tempe during in vitro digestion, Food Dig., 2012, 3, 53–62 CrossRef CAS.
- D. P. Johansson, J. L. V. Gutiérrez, R. Landberg, M. Alminger and M. Langton, Impact of food processing on rye product properties and their in vitro digestion, Eur. J. Nutr., 2018, 57, 1651–1666 CrossRef CAS.
- J. Karthikeyan, D. Salvi, M. G. Corradini, R. D. Ludescher and M. V. Karwe, Effect of bolus viscosity on carbohydrate digestion and glucose absorption processes: An in vitro study, Phys. Fluids, 2019, 31, 111905 CrossRef.
- F. M. AlHasawi, D. Fondaco, M. G. Corradini, R. D. Ludescher, D. Bolster, Y. Chu, Y. Chung, J. Johnson and M. A. Rogers, Gastric viscosity and sugar bioaccessibility of instant and steel cut oat/milk protein blends, Food Hydrocolloids, 2018, 82, 424–433 CrossRef CAS.
- A. Speranza, M. Corradini, T. Hartman, D. Ribnicky, A. Oren and M. Rogers, Influence of emulsifier structure on lipid bioaccessibility in oil–water nanoemulsions, J. Agric. Food Chem., 2013, 61, 6505–6515 CrossRef CAS.
- B. Framroze, P. Savard, D. Gagnon, V. Richard and S. F. Gauthier, Comparison of nitrogen bioaccessibility from salmon and whey protein hydrolysates using a human gastrointestinal model (TIM-1), Funct. Foods Health Dis., 2014, 4, 222–231 CrossRef CAS.
- N. M. Anson, R. Havenaar, A. Bast and G. R. Haenen, Antioxidant and anti-inflammatory capacity of bioaccessible compounds from wheat fractions after gastrointestinal digestion, J. Cereal Sci., 2010, 51, 110–114 CrossRef.
- M. Larsson, M. Minekus and R. Havenaar, Estimation of the bioavailability of iron and phosphorus in cereals using a dynamic in vitro gastrointestinal model, J. Sci. Food Agric., 1997, 74, 99–106 CrossRef CAS.
- C. A. Van Loo-Bouwman, T. H. Naber, M. Minekus, R. B. Van Breemen, P. J. Hulshof and G. Schaafsma, Food matrix effects on bioaccessibility of β-carotene can be measured in an in vitro gastrointestinal model, J. Agric. Food Chem., 2014, 62, 950–955 CrossRef CAS.
- M. A. Lila, D. M. Ribnicky, L. E. Rojo, P. Rojas-Silva, A. Oren, R. Havenaar, E. M. Janle, I. Raskin, G. G. Yousef and M. H. Grace, Complementary approaches to gauge the bioavailability and distribution of ingested berry polyphenolics, J. Agric. Food Chem., 2012, 60, 5763–5771 CrossRef CAS.
- M. Verwei, K. Arkbåge, R. Havenaar, H. van den Berg, C. Witthöft and G. Schaafsma, Folic acid and 5-methyltetrahydrofolate in fortified milk are bioaccessible as determined in a dynamic in vitro gastrointestinal model, J. Nutr., 2003, 133, 2377–2383 CrossRef CAS.
- O. Ménard, M.-H. Famelart, A. Deglaire, Y. Le Gouar, S. Guérin, C.-H. Malbert and D. Dupont, Gastric Emptying and Dynamic In Vitro Digestion of Drinkable Yogurts: Effect of Viscosity and Composition, Nutrients, 2018, 10, 1308 CrossRef.
- D. Freitas and S. Le Feunteun, Inhibitory effect of black tea, lemon juice, and other beverages on salivary and pancreatic amylases: What impact on bread starch digestion? A dynamic in vitro study, Food Chem., 2019, 297, 124885 CrossRef CAS.
- A. Deglaire, S. C. De Oliveira, J. Jardin, V. Briard-Bion, M. Emily, O. Ménard, C. Bourlieu and D. Dupont, Impact of human milk pasteurization on the kinetics of peptide release during in vitro dynamic term newborn digestion, Electrophoresis, 2016, 37, 1839–1850 CrossRef CAS.
- R. Holmes and R. W. Lobley, Intestinal brush border revisited, Gut, 1989, 30, 1667–1678 CrossRef CAS.
- R. E. McConnell, A. E. Benesh, S. Mao, D. L. Tabb and M. J. Tyska, Proteomic analysis of the enterocyte brush border, Am. J. Physiol.: Gastrointest. Liver Physiol., 2011, 300, G914–G926 CrossRef CAS.
- J. F. Woodley, Enzymatic barriers for GI peptide and protein delivery, Crit. Rev. Ther. Drug Carrier Syst., 1994, 11, 61–95 CAS.
- G. Picariello, P. Ferranti and F. Addeo, Use of brush border membrane vesicles to simulate the human intestinal digestion, Food Res. Int., 2016, 88, 327–335 CrossRef CAS.
- F. Waldeck, in Human Physiology, ed. R. F. Schmidt and G. Thews, Springer-Verlag, Berlin-Heidelberg-New York, 1983, ch. 26 Search PubMed.
- L. Shan, O. Molberg, I. Parrot, F. Hausch, F. Filiz, G. M. Gray, L. M. Sollid and C. Khosla, Structural basis for gluten intolerance in celiac sprue, Science, 2002, 297, 2275–2279 CrossRef CAS.
- G. Picariello, B. Miralles, G. Mamone, L. Sanchez-Rivera, I. Recio, F. Addeo and P. Ferranti, Role of intestinal brush border peptidases in the simulated digestion of milk proteins, Mol. Nutr. Food Res., 2015, 59, 948–956 CrossRef CAS.
- J. K. Patterson, X. G. Lei and D. D. Miller, The pig as an experimental model for elucidating the mechanisms governing dietary influence on mineral absorption, Exp. Biol. Med., 2008, 233, 651–664 CrossRef CAS.
- N. Mach, M. Berri, D. Esquerre, C. Chevaleyre, G. Lemonnier, Y. Billon, P. Lepage, I. P. Oswald, J. Dore, C. Rogel-Gaillard and J. Estelle, Extensive expression differences along porcine small intestine evidenced by transcriptome sequencing, PLoS One, 2014, 9, e88515 CrossRef.
- S. P. Shirazi-Beechey, A. G. Davies, K. Tebbutt, J. Dyer, A. Ellis, C. J. Taylor, P. Fairclough and R. B. Beechey, Preparation and properties of brush-border membrane vesicles from human small intestine, Gastroenterology, 1990, 98, 676–685 CrossRef CAS.
- M. Claude, R. Lupi, G. Picariello, M. Drouet, C. Larre, S. Denery-Papini and C. Brossard, Digestion differently affects the ability of native and thermally aggregated ovalbumin to trigger basophil activation, Food Res. Int., 2019, 118, 108–114 CrossRef CAS.
- L. Di Stasio, O. Tranquet, G. Picariello, P. Ferranti, M. Morisset, S. Denery-Papini and G. Mamone, Comparative analysis of eliciting capacity of raw and roasted peanuts: the role of gastrointestinal digestion, Food Res. Int., 2020, 127, 108758 CrossRef CAS.
- C. Gianfrani, A. Camarca, G. Mazzarella, L. Di Stasio, N. Giardullo, P. Ferranti, G. Picariello, V. R. Aufiero, S. Picascia, R. Troncone, N. Pogna, S. Auricchio and G. Mamone, Extensive in vitro gastrointestinal digestion markedly reduces the immune-toxicity of Triticum monococcum wheat: implication for celiac disease, Mol. Nutr. Food Res., 2015, 59, 1844–1854 CrossRef CAS.
- J. L. Piper, G. M. Gray and C. Khosla, Effect of prolyl endopeptidase on digestive-resistant gliadin peptides in vivo, J. Pharmacol. Exp. Ther., 2004, 311, 213–219 CrossRef CAS.
- A. H.-M. Lin, B.-H. Lee and W.-J. Chang, Small intestine mucosal α-glucosidase: A missing feature of in vitro starch digestibility, Food Hydrocolloids, 2016, 53, 163–171 CrossRef CAS.
- O. Hernandez-Hernandez, A. Olano, R. A. Rastall and F. J. Moreno, In vitro Digestibility of Dietary Carbohydrates: Toward a Standardized Methodology Beyond Amylolytic and Microbial Enzymes, Front. Nutr., 2019, 6, 61 CrossRef.
- S. Howell, A. J. Kenny and A. J. Turner, A survey of membrane peptidases in two human colonic cell lines, Caco-2 and HT-29, Biochem. J., 1992, 284(Pt 2), 595–601 CrossRef CAS.
- G. Picariello, G. Iacomino, G. Mamone, P. Ferranti, O. Fierro, C. Gianfrani, A. Di Luccia and F. Addeo, Transport across Caco-2 monolayers of peptides arising from in vitro digestion of bovine milk proteins, Food Chem., 2013, 139, 203–212 CrossRef CAS.
- S. Howell, I. A. Brewis, N. M. Hooper, A. J. Kenny and A. J. Turner, Mosaic expression of membrane peptidases by confluent cultures of Caco-2 cells, FEBS Lett., 1993, 317, 109–112 CrossRef CAS.
- K. Gunathilake, K. Ranaweera and H. P. V. Rupasinghe, Change of phenolics, carotenoids, and antioxidant capacity following simulated gastrointestinal digestion and dialysis of selected edible green leaves, Food Chem., 2018, 245, 371–379 CrossRef CAS.
- S. Blanquet, E. Zeijdner, E. Beyssac, J. P. Meunier, S. Denis, R. Havenaar and M. Alric, A dynamic artificial gastrointestinal system for studying the behavior of orally administered drug dosage forms under various physiological conditions, Pharm. Res., 2004, 21, 585–591 CrossRef CAS.
- I. J. Hidalgo, T. J. Raub and R. T. Borchardt, Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability, Gastroenterology, 1989, 96, 736–749 CrossRef CAS.
- J. M. Gamboa and K. W. Leong, In vitro and in vivo models for the study of oral delivery of nanoparticles, Adv. Drug Delivery Rev., 2013, 65, 800–810 CrossRef CAS.
- M. Pinto, S. Robineleon and M. D. Appay, Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture, Biol. Cell, 1983, 47, 323–330 Search PubMed.
- K. Verhoeckx, P. Cotter, I. Lopez-Exposito, C. Kleiveland, T. Lea, A. Mackie, T. Requena, D. Swiatecka and H. Wichers, The Impact of Food Bioactives on Health: in vitro and ex vivo models, Springer International Publishing, Cham (CH), 2015 Search PubMed.
- C. Vors, P. Capolino, C. Guerin, E. Meugnier, S. Pesenti, M. A. Chauvin, J. Monteil, N. Peretti, M. Cansell, F. Carriere and M. C. Michalski, Coupling in vitro gastrointestinal lipolysis and Caco-2 cell cultures for testing the absorption of different food emulsions, Food Funct., 2012, 3, 537–546 RSC.
- V. D. Felice, D. M. O’Gorman, N. M. O’Brien and N. P. Hyland, Bioaccessibility and Bioavailability of a Marine-Derived Multimineral, Aquamin-Magnesium, Nutrients, 2018, 10, 912 CrossRef.
- H. J. Kim, D. Huh, G. Hamilton and D. E. Ingber, Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow, Lab Chip, 2012, 12, 2165–2174 RSC.
- N. Gunness, J. Michiels, S. De Smet, L. Vanhaecke, O. Kravchuk and M. Gidley, Blood lipids – soluble dietary fibres: Study of bile salts diffusion across intestinal mucosa using the Ussing chamber system, J. Nutr. Intermed. Metab, 2016, 4, 35 CrossRef.
- D. A. Norris and P. J. Sinko, Effect of size, surface charge, and hydrophobicity on the translocation of polystyrene microspheres through gastrointestinal mucin, J. Appl. Polym. Sci., 1997, 63, 1481–1492 CrossRef CAS.
- K. M. Hillgren, A. Kato and R. T. Borchardt, In vitro systems for studying intestinal drug absorption, Med. Res. Rev., 1995, 15, 83–109 CrossRef CAS.
- A. Trapani, A. Lopedota, M. Franco, N. Cioffi, E. Ieva, M. Garcia-Fuentes and M. J. Alonso, A comparative study of chitosan and chitosan/cyclodextrin nanoparticles as potential carriers for the oral delivery of small peptides, Eur. J. Pharm. Biopharm., 2010, 75, 26–32 CrossRef CAS.
- S. M. Kalman and H. H. Ussing, Active sodium uptake by the toad and its response to the antidiuretic hormone, J. Gen. Physiol., 1955, 38, 361–370 CrossRef CAS.
- P. B. Persson, Did you know? The Ussing chamber origin, Acta Physiol., 2016, 218, 67–67 CrossRef CAS.
- L. L. Clarke, A guide to Ussing chamber studies of mouse intestine, Am. J. Physiol.: Gastrointest. Liver Physiol., 2009, 296, G1151–G1166 CrossRef CAS.
- P. Lundquist and P. Artursson, Oral absorption of peptides and nanoparticles across the human intestine: Opportunities, limitations and studies in human tissues, Adv. Drug Delivery Rev., 2016, 106, 256–276 CrossRef CAS.
- A. M. Rowan, P. J. Moughan, M. N. Wilson, K. Maher and C. Tasman-Jones, Comparison of the ileal and faecal digestibility of dietary amino acids in adult humans and evaluation of the pig as a model animal for digestion studies in man, Br. J. Nutr., 1994, 71, 29–42 CrossRef CAS.
- J. García, D. Méndez, M. Álvarez, B. Sanmartin, R. Vázquez, L. Regueiro and M. Atanassova, Design of novel functional food products enriched with bioactive extracts from holothurians for meeting the nutritional needs of the elderly, LWT – Food Sci. Technol., 2019, 109, 55–62 CrossRef.
- A. Mackie, S. Gourcy, N. Rigby, J. Moffat, I. Capron and B. Bajka, The fate of cellulose nanocrystal stabilised emulsions after simulated gastrointestinal digestion and exposure to intestinal mucosa, Nanoscale, 2019, 11, 2991–2998 RSC.
- M. Foltz, A. Cerstiaens, A. van Meensel, R. Mols, P. C. van der Pijl, G. S. Duchateau and P. Augustijns, The angiotensin converting enzyme inhibitory tripeptides Ile-Pro-Pro and Val-Pro-Pro show increasing permeabilities with increasing physiological relevance of absorption models, Peptides, 2008, 29, 1312–1320 CrossRef CAS.
- V. Rozehnal, D. Nakai, U. Hoepner, T. Fischer, E. Kamiyama, M. Takahashi, S. Yasuda and J. Mueller, Human small intestinal and colonic tissue mounted in the Ussing chamber as a tool for characterizing the intestinal absorption of drugs, Eur. J. Pharm. Sci., 2012, 46, 367–373 CrossRef CAS.
- A. Sjoberg, M. Lutz, C. Tannergren, C. Wingolf, A. Borde and A. L. Ungell, Comprehensive study on regional human intestinal permeability and prediction of fraction absorbed of drugs using the Ussing chamber technique, Eur. J. Pharm. Sci., 2013, 48, 166–180 CrossRef.
- S. M. Harrison, G. Eyres, P. W. Cleary, M. D. Sinnott, C. Delahunty and L. Lundin, Computational Modeling of Food Oral Breakdown Using Smoothed Particle Hydrodynamics, J. Texture Stud., 2014, 45, 97–109 CrossRef.
- C. A. M. Fois, T. Y. L. Le, A. Schindeler, S. Naficy, D. D. McClure, M. N. Read, P. Valtchev, A. Khademhosseini and F. Dehghani, Models of the Gut for Analyzing the Impact of Food and Drugs, Adv. Healthcare Mater., 2019, 8, 1900968 CrossRef CAS.
- N. Parrott, C. Stillhart, M. Lindenberg, B. Wagner, K. Kowalski, E. Guerini, N. Djebli and G. Meneses-Lorente, Physiologically Based Absorption Modelling to Explore the Impact of Food and Gastric pH Changes on the Pharmacokinetics of Entrectinib, AAPS J., 2020, 22, 13 CrossRef.
- K. C. Drechsler and M. J. Ferrua, Modelling the breakdown mechanics of solid foods during gastric digestion, Food Res. Int., 2016, 88, 181–190 CrossRef CAS.
- S. M. Harrison, P. W. Cleary and M. D. Sinnott, Investigating mixing and emptying for aqueous liquid content from the stomach using a coupled biomechanical-SPH model, Food Funct., 2018, 9, 3202–3219 RSC.
- T. E. Moxon, O. Gouseti and S. Bakalis, In silico modelling of mass transfer & absorption in the human gut, J. Food Eng., 2016, 176, 110–120 CrossRef CAS.
- R. G. Lentle and C. de Loubens, A review of mixing and propulsion of chyme in the small intestine: fresh insights from new methods, J. Comp. Physiol., B, 2015, 185, 369–387 CrossRef CAS.
- M. D. Sinnott, P. W. Cleary, J. W. Arkwright and P. G. Dinning, Modeling Colonic Motility: How Does Descending Inhibition Influence the Transport of Fluid?, Gastroenterology, 2011, 140, S865–S866 CrossRef.
- S. Marze, Refining in silico simulation to study digestion parameters affecting the bioaccessibility of lipophilic nutrients and micronutrients, Food Funct., 2015, 6, 115–124 Search PubMed.
- T. Sayd, C. Dufour, C. Chambon, C. Buffiere, D. Remond and V. Sante-Lhoutellier, Combined in vivo and in silico approaches for predicting the release of bioactive peptides from meat digestion, Food Chem., 2018, 249, 111–118 CrossRef CAS.
- S. Bellmann, S. Krishnan, A. de Graaf, R. A. de Ligt, W. J. Pasman, M. Minekus and R. Havenaar, Appetite ratings of foods are predictable with an in vitro advanced gastrointestinal model in combination with an in silico artificial neural network, Food Res. Int., 2019, 122, 77–86 CrossRef.
- FAO, Dietary protein quality evaluation in human nutrition: report of an FAO expert consulation, 2013, vol. 92 Search PubMed.
- M. Dangin, C. Guillet, C. Garcia-Rodenas, P. Gachon, C. Bouteloup-Demange, K. Reiffers-Magnani, J. Fauquant, O. Ballèvre and B. Beaufrère, The rate of protein digestion affects protein gain differently during aging in humans, J. Physiol., 2003, 549, 635–644 CrossRef CAS.
- L. Egger, P. Schlegel, C. Baumann, H. Stoffers, D. Guggisberg, C. Brugger, D. Durr, P. Stoll, G. Vergeres and R. Portmann, Physiological comparability of the harmonized INFOGEST in vitro digestion method to in vivo pig digestion, Food Res. Int., 2017, 102, 567–574 CrossRef CAS.
- M. Yvon, S. Beucher, P. Scanff, S. Thirouin and J. P. Pelissier, In vitro simulation of gastric digestion of milk proteins: comparison between in vitro and in vivo data, J. Agric. Food Chem., 1992, 40, 239–244 CrossRef CAS.
- A. Maathuis, R. Havenaar, T. He and S. Bellmann, Protein digestion and quality of goat and cow milk infant formula and human milk under simulated infant conditions, J. Pediatr. Gastroenterol. Nutr., 2017, 65, 661 CrossRef CAS.
- M. Golding, T. J. Wooster, L. Day, M. Xu, L. Lundin, J. Keogh and P. Clifton, Impact of gastric structuring on the lipolysis of emulsified lipids, Soft Matter, 2011, 7, 3513–3523 RSC.
- A. Steingoetter, T. Radovic, S. Buetikofer, J. Curcic, D. Menne, M. Fried, W. Schwizer and T. J. Wooster, Imaging gastric structuring of lipid emulsions and its effect on gastrointestinal function: a randomized trial in healthy subjects, Am. J. Clin. Nutr., 2015, 101, 714–724 CrossRef CAS.
- A. R. Mackie, H. Rafiee, P. Malcolm, L. Salt and G. van Aken, Specific food structures supress appetite through reduced gastric emptying rate, Am. J. Physiol.: Gastrointest. Liver Physiol., 2013, 304, G1038–G1043 CrossRef CAS.
- D. Jenkins, T. Wolever, R. H. Taylor, H. Barker, H. Fielden, J. M. Baldwin, A. C. Bowling, H. C. Newman, A. L. Jenkins and D. V. Goff, Glycemic index of foods: a physiological basis for carbohydrate exchange, Am. J. Clin. Nutr., 1981, 34, 362–366 CrossRef CAS.
- A. W. Barclay, P. Petocz, J. McMillan-Price, V. M. Flood, T. Prvan, P. Mitchell and J. C. Brand-Miller, Glycemic index, glycemic load, and chronic disease risk—a meta-analysis of observational studies, Am. J. Clin. Nutr., 2008, 87, 627–637 CrossRef CAS.
- H. N. Englyst, S. Kingman and J. Cummings, Classification and measurement of nutritionally important starch fractions, Eur. J. Clin. Nutr., 1992, 46, S33–S50 Search PubMed.
- K. N. Englyst, H. N. Englyst, G. J. Hudson, T. J. Cole and J. H. Cummings, Rapidly available glucose in foods: an in vitro measurement that reflects the glycemic response, Am. J. Clin. Nutr., 1999, 69, 448–454 CrossRef CAS.
- I. Goñi, A. Garcia-Alonso and F. Saura-Calixto, A starch hydrolysis procedure to estimate glycemic index, Nutr. Res., 1997, 17, 427–437 CrossRef.
- J. W. Woolnough, J. A. Monro, C. S. Brennan and A. R. Bird, Simulating human carbohydrate digestion in vitro: a review of methods and the need for standardisation, Int. J. Food Sci. Technol., 2008, 43, 2245–2256 CrossRef CAS.
- K. A. Germaine, S. Samman, C. G. Fryirs, P. J. Griffiths, S. K. Johnson and K. J. Quail, Comparison of in vitro starch digestibility methods for predicting the glycaemic index of grain foods, J. Sci. Food Agric., 2008, 88, 652–658 CrossRef CAS.
- M. Alminger and C. Eklund-Jonsson, Whole-grain cereal products based on a high-fibre barley or oat genotype lower post-prandial glucose and insulin responses in healthy humans, Eur. J. Nutr., 2008, 47, 294 CrossRef CAS.
- S. Bellmann, M. Minekus, E. Zeijdner, M. Verwei, P. Sanders, W. Basten and R. Havenaar, TIM-carbo: a rapid, cost-efficient and reliable in vitro method for glycaemic response after carbohydrate ingestion, Dietary fibre: new frontiers for food and health, Wageningen Academic Publishers, Wageningen, 2010, pp. 467–473 Search PubMed.
- D. Gentilcore, R. Chaikomin, K. L. Jones, A. Russo, C. Feinle-Bisset, J. M. Wishart, C. K. Rayner and M. Horowitz, Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes, J. Clin. Endocrinol. Metab., 2006, 91, 2062–2067 CrossRef CAS.
- E. Reboul, M. Richelle, E. Perrot, C. Desmoulins-Malezet, V. Pirisi and P. Borel, Bioaccessibility of carotenoids and vitamin E from their main dietary sources, J. Agric. Food Chem., 2006, 54, 8749–8755 CrossRef CAS.
- V. Tyssandier, E. Reboul, J.-F. Dumas, C. Bouteloup-Demange, M. Armand, J. Marcand, M. Sallas and P. Borel, Processing of vegetable-borne carotenoids in the human stomach and duodenum, Am. J. Physiol.: Gastrointest. Liver Physiol., 2003, 284, G913–G923 CrossRef CAS.
- C. Vetrani, A. A. Rivellese, G. Annuzzi, M. Adiels, J. Borén, I. Mattila, M. Orešič and A.-M. Aura, Metabolic transformations of dietary polyphenols: comparison between in vitro colonic and hepatic models and in vivo urinary metabolites, J. Nutr. Biochem., 2016, 33, 111–118 CrossRef CAS.
- M. Verwei, A. P. Freidig, R. Havenaar and J. P. Groten, Predicted serum folate concentrations based on in vitro studies and kinetic modeling are consistent with measured folate concentrations in humans, J. Nutr., 2006, 136, 3074–3078 CrossRef CAS.
- P. Etcheverry, M. A. Grusak and L. E. Fleige, Application of in vitro bioaccessibility and bioavailability methods for calcium, carotenoids, folate, iron, magnesium, polyphenols, zinc, and vitamins B6, B12, D, and E, Front. Physiol., 2012, 3, 317 CAS.
- T. J. Wooster, L. Day, M. Xu, M. Golding, S. Oiseth, J. Keogh and P. Clifton, Impact of different biopolymer networks on the digestion of gastric structured emulsions, Food Hydrocolloids, 2014, 36, 102–114 CrossRef CAS.
- D. Jenkins, H. Ghafari, T. Wolever, R. Taylor, A. Jenkins, H. Barker, H. Fielden and A. Bowling, Relationship between rate of digestion of foods and post-prandial glycaemia, Diabetologia, 1982, 22, 450–455 CrossRef CAS.
- A. Ferrer-Mairal, C. Penalva-Lapuente, I. Iglesia, L. Urtasun, P. De Miguel-Etayo, S. Remón, E. Cortés and L. Moreno, In vitro and in vivo assessment of the glycemic index of bakery products: influence of the reformulation of ingredients, Eur. J. Nutr., 2012, 51, 947–954 CrossRef CAS.
- C. H. Edwards, M. M. Grundy, T. Grassby, D. Vasilopoulou, G. S. Frost, P. J. Butterworth, S. E. Berry, J. Sanderson and P. R. Ellis, Manipulation of starch bioaccessibility in wheat endosperm to regulate starch digestion, postprandial glycemia, insulinemia, and gut hormone responses: a randomized controlled trial in healthy ileostomy participants, Am. J. Clin. Nutr., 2015, 102, 791–800 CrossRef CAS.
- T. Walter, F. Pizarro and M. Olivares, Iron bioavailability in corn-masa tortillas is improved by the addition of disodium EDTA, J. Nutr., 2003, 133, 3158–3161 CrossRef CAS.
- R. J. de Jong, M. Verwei, C. E. West, T. van Vliet, E. Siebelink, H. van den Berg and J. J. Castenmiller, Bioavailability of folic acid from fortified pasteurised and UHT-treated milk in humans, Eur. J. Clin. Nutr., 2005, 59, 906–913 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |
