Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The influence of dietary conditions in the effects of resveratrol on hepatic steatosis
I.
Milton-Laskibar
 abc,
L.
Aguirre
abc,
L.
Aguirre
 *abc,
S.
Gómez-Zorita
*abc,
S.
Gómez-Zorita
 abc,
A. P.
Rolo
abc,
A. P.
Rolo
 de and
M. P.
Portillo
de and
M. P.
Portillo
 abc
abc
aNutrition and Obesity group, Department of Nutrition and Food Science, Faculty of Pharmacy, University of the Basque Country (UPV/EHU), Lucio Lascaray Research Center, Vitoria-Gasteiz, Spain. E-mail: leixuri.aguirre@ehu.eus; Tel: +34-945014298; Fax: +34-945013014
bBIOARABA Institute of Health, Vitoria-Gasteiz, Spain
cCIBERobn Physiopathology of Obesity and Nutrition, Institute of Health Carlos III (ISCIII), Vitoria-Gasteiz, Spain
dDepartment of Life Sciences, Faculty of Sciences and Technology, University of Coimbra, Calçada Martim de Freitas, 3000-456, Coimbra, Portugal
eCNC, Center for Neuroscience and Cell Biology, University of Coimbra, Coimbra, Portugal
First published on 2nd November 2020
Abstract
Non-alcoholic fatty liver disease (NAFLD) is considered the major cause for the development of chronic liver alterations. Hepatic steatosis is the most benign and common form of NAFLD, although its potential to evolve into more detrimental liver alterations makes its treatment necessary. In this regard, much attention has been paid to polyphenols, with resveratrol being one of the most studied ones. This review is aimed at studying the effects induced by resveratrol on hepatic steatosis in both preclinical studies conducted under different feeding conditions (overfeeding, normal feeding and caloric restriction), and in clinical trials. The vast majority of studies have been conducted by administering the polyphenol at the same time as an obesogenic diet. Under these experimental conditions, resveratrol has shown effectiveness improving diet-induced excessive liver lipid accumulation. Data are scarce for studies carried out by administering resveratrol under standard or energy-restricted feeding conditions. In this regard, while resveratrol retains its effectiveness, ameliorating hepatic steatosis under standard feeding conditions, such an effect has not been reported for the administration of the polyphenol under energy restriction. With regard to clinical trials, in the majority of them, resveratrol did not show its effectiveness in improving hepatic steatosis. This lack of effect could be due to significant differences in the experimental procedures (mainly the length of the experimental period). The relevance of liver fat content at the baseline should also be considered. Altogether, there is no sufficient scientific support so far for proposing resveratrol as a tool for hepatic steatosis treatment.
1. Introduction
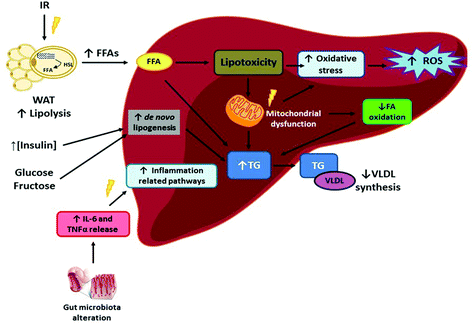
Liver diseases are among the leading mortality causes worldwide in the last few decades. By the year 2010, it was estimated that 4% of all deaths worldwide were due to major liver diseases, such as cirrhosis and hepatocellular carcinoma.1 Non-alcoholic fatty liver disease (NAFLD) comprises several hepatic pathological conditions, characterised by excessive lipid content, with or without inflammation and fibrosis (steatohepatitis and steatosis, respectively). The main concern with NAFLD is the possibility to progress towards more harmful stages (cirrhosis or hepatocellular carcinoma),2 as well as being intimately related to other cardiometabolic alterations such as type 2 diabetes, metabolic syndrome and heart failure.3 Indeed, NAFLD is widely considered as the hepatic manifestation of metabolic syndrome.4Traditionally, the “double-hit” theory has been used to explain the underlying processes resulting in NAFLD. In this regard, liver triglyceride (TG) accumulation is considered as the “first hit”, which causes damage in this organ, making it more prone to progressing towards non-alcoholic steatohepatitis (NASH). As far as the “second hit” is concerned, this would be comprised of oxidative stress, autophagy and inflammation, contributing to further NASH progression.5 However, this theory has been considered obsolete for explaining such a complex process, and thus the “multiple-hit” theory has been proposed as a more accurate one (Fig. 1). In this regard, insulin resistance (common in overweight and obese people) would be triggering the whole process. Thus, impaired adipose tissue insulin signalling, which increases adipose tissue lipolysis, and adipose tissue inflammation,6,7 results in excessive free fatty acids (FFA), which, in turn, are deposited in the liver. Along with the aforementioned hepatic lipid accumulation, the high levels of FFA, cholesterol and lipid metabolites present in the liver induce lipotoxicity. Therefore, mitochondrial dysfunction occurs, which in turn enhances oxidative stress and reactive oxygen species (ROS) production, while mechanisms of endoplasmic reticulum stress are also activated. Moreover, the alterations in gut microbiota result in greater intestinal permeability and the release of pro-inflammatory cytokines such as interleukin 6 (IL-6) and tumour necrosis factor α (TNF α).8 It is important to point out that, whereas this sequence of metabolic alterations is found in subjects who show NAFLD associated to obesity, there are also genetic types of NAFLD such as that associated to polymorphisms in PNPLA3 and TM6SF2, which are not related to altered TG and HDL-cholesterol levels and insulin resistance.9
Hepatic steatosis is the most benign form of NAFLD, as well as the most common one. This hepatic alteration can be diagnosed chemically when intrahepatic TG accumulation is greater than 5% of liver weight, or by histologic analysis of the tissue, when 5% or more of the hepatocytes show TG accumulation.5 Even though hepatic steatosis by itself does not represent a major threat to health, its potential to evolve into more detrimental liver alterations make its treatment necessary.
Since hepatic steatosis is mainly present in patients suffering from obesity and/or type-2 diabetes, hypocaloric diets, along with interventions devoted to enhancing physical activity, represent the cornerstone for its treatment. The effectiveness of energy-restricted diets in improving hepatic steatosis has been extensively described in both, preclinical studies10–13 and clinical trials.14,15 However, the main limitation of this kind of treatment is the low adherence that is often achieved, mainly due to stress, emotional factors, or difficulties in modifying dietary habits.
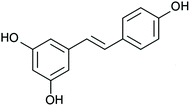
In this scenario, scientific community has been looking for alternatives that could improve the outcomes of the approaches currently used for hepatic steatosis treatment. In recent years, much attention has been paid to phenolic compounds, a wide and heterogeneous group of plant-derived molecules that are naturally present in a variety of foodstuffs within our diet. Among them, resveratrol (3,5,4′-trihydroxy-trans-stilbene) has been one of the most studied ones (Fig. 2). This phenolic compound has been proposed as an energy restriction mimetic molecule (mimicking the effects of such dietary approach, without limiting the caloric intake), and thus has been extensively studied as a potential anti-obesity16–18 and anti-hypertensive agent.19 In addition, resveratrol has also shown effectiveness in the amelioration of further health alterations, such as hepatic steatosis. In this regard, this phenolic compound has been reported to modulate and reduce hepatic lipid content in rodents under different experimental conditions,10,12,13,17,20–50 as well as in humans suffering from this liver condition;51–53 despite, in the case of humans, the effects of resveratrol are not so clear. In none of the studies included in this review significant reductions in hepatic lipid content after resveratrol administration were reported; however, in some of them, resveratrol-derived improvements in NAFLD were observed. In this regard, it is important to highlight that the studies were probably too short to show an effect on hepatic lipid composition. In some studies, the doses used may not have been the most effective ones.54
This review aims to analyze the effects of resveratrol in different models of hepatic steatosis, as well as under different feeding conditions (overfeeding, normal feeding and caloric restriction). The effectiveness of this phenolic compound in clinical studies is also revised.
2. Effects of resveratrol on hepatic steatosis in preclinical studies conducted under overfeeding conditions
The use of overfeeding conditions is a commonly applied experimental procedure in studies in which hepatic steatosis induction is looked for. This feeding pattern is based on the usage of obesogenic diets, which generally are high-fat diets that may also contain high amounts of sugars (such as sucrose or fructose). It must be taken into account that in the studies using this experimental procedure, the polyphenol is usually administered at the same time than the obesogenic diet, and thus the preventive effect that the bioactive compound may exert on this hepatic condition is studied.In the vast majority of the studies available in the literature that have been conducted using this experimental procedure, and are included in this review article, resveratrol has been shown to be effective in reducing the hepatic lipid content, in both mice10,17,20–22,24,25,27–34,55 and rats12,35,37–41,56 (Table 1). In these studies, besides this hepatic lipid-lowering effect, resveratrol has also shown to be effective in improving other parameters related to hepatic steatosis, such as blood glucose, serum lipids and transaminase levels, as well as liver oxidative status. It is worth emphasizing that these effects have been observed when using diets with a lipid content in a broad range (from 20 to 60% of energy as fat), resveratrol doses also in a broad range (from 15 to 400 mg per kg body weight per day) and with different treatment period lengths (from 4 to 20 weeks). With regard to the dose, there is no consensus regarding the existence of a dose–response effect of resveratrol. Thus, while in some studies the high dose of the compound was the most effective one,10 in others the low dose induced greater effects20 or no differences were described among the tested doses at all.24,37
| Author | Animal model | Diet | Increase in dietary fat (vs. control) | Resveratrol dose | Improvement in hepatic steatosis |
|---|---|---|---|---|---|
| ICR: institute of cancer research. | |||||
| Baur et al. (2006)17 | 1-Year old-male C57BL/6NIA mice | 60% of energy from fat | +44% | 22.4 mg kg−1 d−1 | Yes |
| Tauriainen et al. (2011)10 | 7-Week-old male C57Bl/6J mice | 60% of energy from fat | +50% | 0.2 or 0.4% in the diet (w/w) | Yes |
| Cho et al. (2012)21 | 4-Week-old male C57BL/6J mice | 40% of energy from fat | +28% | 0.02 or 0.04% in the diet (w/w) | Yes |
| Jeon et al. (2012)21 | 4-Week-old male C57BL/6N mice | 60% of energy from fat | +50% | 200 mg kg−1 d−1 | Yes |
| Kang et al. (2012)21,22 | 6-Week-old male C57BL/6N mice | 58% of energy from fat | +45.5% | 30 mg kg−1 d−1 | Yes |
| Andrade et al. (2014)23,55 | 4-Week-old FVB/N male mice | 61% of energy from fat | +53% | 30 mg kg−1 d−1 | Yes |
| Choi et al. (2014)24,25 | Pathogen-free male ICR mice | 59% of energy from fat | No specified | 15 or 45 mg kg−1 d−1 | Yes |
| Jeon et al. (2014)25 | 4-Week-old male apoE-deficient mice | 20% of energy from fat (corn oil and lard) | No control diet | 0.2% in the diet (w/w) | Yes |
| Montero et al. (2014)26 | 3-Month-old male C57BL mice | 60% of energy from fat | +50% | 0.025% in the diet (w/w) | No |
| Qiao et al. (2014)27 | Male Kunming mice | 50% of energy from fat | +40% | 200 mg kg−1 d−1 | Yes |
| Nishikawa et al. (2015)28 | 6 and 7-week-old C57BL/6 mice | 50% of energy from fat | +46.2% | 0.2% in the diet (w/w) | Yes |
| Tian et al. (2016)29 | 4-Week-old C57BL/6 mice | 60% of energy from fat | Not specified | 30 mg kg−1 d−1 | Yes |
| Zhou et al. (2018)30 | 4–6 Weeks-old male C57BL/6 mice | 60% of energy from fat | Not specified | 400 mg kg−1 d−1 | Yes |
| Cheng et al. (2019)31 | 6-Week-old male C57BL/6 mice | 60% of energy from fat | +50% | 100 mg kg−1 d−1 | Yes |
| Teng et al. (2019)32 | 6 Weeks-old male C57BL/6J mice | 60% of energy from fat, mainly from lard | +50% | 200 mg kg−1 every 2 days | Yes |
| Hosseini et al. (2020)33 | 6 Weeks-old male C57BL/6 mice | 55.9% of energy from fat | +45.9% | 0.4% resveratrol in the diet (w/w) | Yes |
| Yin et al. (2020)34 | 8–12 Weeks-old male C57BL/6 mice | 60% of energy from fat | +50% | 0.4% resveratrol in the diet (w/w) | Yes |
| Shang et al. (2008)35 | Male Wistar rats | 59% of energy from fat | Not specified | 100 mg kg−1 d−1 | Yes |
| Poulsen et al. (2012)36 | 8-Week-old male Wistar rats | 60% of energy from fat | +50% | 100 mg d−1 | Yes |
| Xin et al. (2013)37 | Adult male Wistar rats | 54% of energy from fat | +44% | 50 or 100 mg kg−1 d−1 | Yes |
| Arias et al. (2015)57 | 6-Week-old male Wistar rats | 20% of energy from sucrose and 24% of energy from fat | No standard diet | 15 mg kg−1 d−1 | No |
| Pan et al. (2015)38 | 6-Week-old male Sprague-Dawley rats | 45% of energy from fat | +35% | 100 mg kg−1 d−1 | Yes |
| Ding et al. (2017)12 | 8-Week-old male Wistar rats | 41.3% of energy from fat | +27.6% | 200 mg kg−1 d−1 | Yes |
| Badi et al. (2019)39 | Adult Wistar rats | 60% of energy from fat | +50% | 20 mg kg−1 d−1 | Yes |
| Chen et al. (2019)40 | 2 Month-old male Sprague Dawley | 60% of energy from fat | +50% | 15 mg kg−1 d−1 | Yes |
| Huang et al. (2020)41 | 8–9 Weeks-old male Sprague-Dawley rats | 45% of energy from fat | +35% | 100 mg kg−1 d−1 | Yes |
In a reduced number of studies, resveratrol administration did not induce beneficial effects on hepatic steatosis. In one such study, conducted by Montero et al.,26 3-month-old male C57BL mice were fed a hypercaloric diet (60% of energy from fat) supplemented or not with resveratrol (0.025% w/w) for 12 months. When liver sections were analyzed by hematoxylin–eosin (H&E) staining, no differences in lipid content were found after 6 or 12 months in comparison to animals fed the hypercaloric diet alone. Although the authors did not suggest an explanation for this lack of effect, the selected dose (significantly lower in comparison to doses used in other studies) cannot be ruled out as the reason justifying this fact. In a study from our group, carried out in 6-week-old male Wistar rats fed an obesogenic diet (24% of energy from fat and 20% of energy from sucrose) supplemented or not with 15 mg per kg body weight per day of resveratrol for 6 weeks, we did not observe differences in liver weight or hepatic lipid content (TG and cholesterol) between resveratrol-treated and untreated rats. In that study, in contrast to that observed in other studies from our group, only one metabolic pathway (de novo lipogenesis) was affected by resveratrol. We considered that this effect was not enough to reduce the hepatic lipid content.57
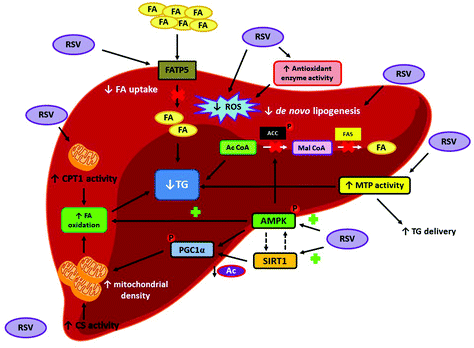
To understand the mechanisms of action involved in the beneficial effects that resveratrol exerts in hepatic steatosis under overfeeding conditions, different metabolic pathways have been studied. In this regard, several studies have reported a resveratrol-mediated reduction in hepatic de novo lipogenesis by measuring the expression and/or activation of fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC), the two main enzymes involved in this process. Other authors have also studied the effect of the polyphenol on fatty acid oxidation, a process that may also underlie the lipid-lowering effect shown by the compound. Enhanced activity of carnitine palmitoyltransferase 1 (CPT1), which is the enzyme regulating the entrance of long-chain fatty acids into the mitochondria for their subsequent β-oxidation, has been described in the animals receiving resveratrol. Several studies have also shown increased activation of 5′AMP-activated protein kinase (AMPK) along with a greater expression of sirtuin 1 (SIRT1) after resveratrol administration. In this regard, it is well known that SIRT1 is directly related to mitochondrial synthesis, which in turn may explain the enhanced hepatic CPT1 activity reported in resveratrol-treated animals. As far as AMPK is concerned, besides being involved in cell energy status regulation by activating adenosine triphosphate (ATP)-producing pathways, its activation also affects the de novo lipogenesis by inhibiting ACC (Fig. 3).
Besides the effects described for resveratrol in these pathways, some authors have also reported increased activity of antioxidant enzymes, decreased lipid peroxidation and immune cell infiltration, or enhanced autophagy in the liver of the animals treated with the polyphenol, all of them mechanisms that may contribute to alleviating the excessive hepatic lipid accumulation due to overfeeding conditions. In addition to these mechanisms of action, it has also been proposed that some of the beneficial effects described for resveratrol may occur through the modulation induced by the polyphenol in gut microbiota composition.58 In this regard, in the study conducted by Qiao et al.,27 male Kunming mice were fed a high-fat diet (50% of energy from fat) and supplemented or not with resveratrol (200 mg per kg body weight per day) for 12 weeks. At the end of the experimental period, the staining of liver sections (Oil red O staining) revealed that the severe steatosis observed in the non-treated animals was prevented in the animals receiving the polyphenol. As far as gut microbiota is concerned, lowered abundances of the Lactobacillus and Bifidobacterium groups, as well as increased abundance of Lactobacillus faecalis were found in the non-treated high-fat diet-fed animals. In the case of the group treated with resveratrol, these changes were reverted. The lowered Bacteroidetes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Firmicutes ratio values found in the non-treated animals were significantly increased in the group receiving the polyphenol. Similar results were reported in the study conducted by Yin et al.,34 in which 8–12 weeks-old male C57BL/6 mice were fed a high-fat diet (60% of energy from fat) supplemented or not with resveratrol (0.4% content in the diet, w/w) for 12 weeks. At the end of the experimental period, resveratrol effectively reduced the hepatic lipid content in the treated animals when compared to the non-treated ones. With regard to microbiota, the authors reported that at the phylum level, the mice treated with resveratrol had a greater abundance of Bacteroidetes, while the abundances of Firmicutes and Proteobacteria were significantly decreased. Consequently, the Bacteroidetes
Firmicutes ratio values found in the non-treated animals were significantly increased in the group receiving the polyphenol. Similar results were reported in the study conducted by Yin et al.,34 in which 8–12 weeks-old male C57BL/6 mice were fed a high-fat diet (60% of energy from fat) supplemented or not with resveratrol (0.4% content in the diet, w/w) for 12 weeks. At the end of the experimental period, resveratrol effectively reduced the hepatic lipid content in the treated animals when compared to the non-treated ones. With regard to microbiota, the authors reported that at the phylum level, the mice treated with resveratrol had a greater abundance of Bacteroidetes, while the abundances of Firmicutes and Proteobacteria were significantly decreased. Consequently, the Bacteroidetes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Firmicutes ratio value was significantly greater in the resveratrol-treated group when compared to the animals that only received the high-fat diet. In addition, resveratrol also reverted the increases in the abundance of some genera (such as Oscillospira, Ruminococcus and Akkermansia) observed in the non-treated high-fat diet fed animals. Interestingly, in the same study, the faecal samples of the animals in the two aforementioned groups were used to carry out faecal transplants. Briefly, the faecal transplants from the non-treated and resveratrol-treated animals were given to two additional groups of mice that were then fed a high-fat diet for 8 additional weeks. At the end of the experimental period, the hepatic lipid content in mice receiving the faecal transplant from the resveratrol-treated animals was significantly lower in comparison with animals receiving the faecal transplant from non-treated animals. The authors concluded that the hepatic lipid-lowering effects shown by resveratrol in the high-fat diet-fed mice may be related to the changes induced by the polyphenol in the gut microbiota composition.
Firmicutes ratio value was significantly greater in the resveratrol-treated group when compared to the animals that only received the high-fat diet. In addition, resveratrol also reverted the increases in the abundance of some genera (such as Oscillospira, Ruminococcus and Akkermansia) observed in the non-treated high-fat diet fed animals. Interestingly, in the same study, the faecal samples of the animals in the two aforementioned groups were used to carry out faecal transplants. Briefly, the faecal transplants from the non-treated and resveratrol-treated animals were given to two additional groups of mice that were then fed a high-fat diet for 8 additional weeks. At the end of the experimental period, the hepatic lipid content in mice receiving the faecal transplant from the resveratrol-treated animals was significantly lower in comparison with animals receiving the faecal transplant from non-treated animals. The authors concluded that the hepatic lipid-lowering effects shown by resveratrol in the high-fat diet-fed mice may be related to the changes induced by the polyphenol in the gut microbiota composition.
Based on the results reported in the studies included in this section, it seems that resveratrol administration is an effective approach for totally or partially preventing hepatic steatosis induced by a feeding pattern in which energy intake surplus occurs. Nevertheless, although beneficial effects have been obtained by using this treatment protocol, due to obvious ethical reasons, it would not be suitable for application in humans. Allowing people to maintain an unhealthy dietary pattern and to advise them to use resveratrol supplementation to avoid the development of hepatic steatosis is not advisable.
3. Effects of resveratrol on hepatic steatosis in preclinical studies conducted under standard feeding conditions
Few studies have analyzed the effects of resveratrol on liver steatosis under standard feeding conditions. The experimental procedure commonly used in these studies is as follows: animals are firstly fed an obesogenic/steatotic diet and then, once hepatic steatosis has been established, animals are switched to a standard feeding pattern (using chow or standard semi-purified diets) and treated with resveratrol. With this experimental design, the usefulness of resveratrol for hepatic steatosis treatment was analyzed. This is a scenario closer to that used in clinical studies.Under this feeding pattern, significant decreases in liver lipid content have been reported in studies carried out in mice42 and rats13,41 (Table 2). The hepatic lipid-lowering effect of resveratrol was observed when different doses of resveratrol (from 30 to 100 mg per kg body weight per day) or treatment period lengths (from 4 to 8 weeks) were used. Besides lower hepatic lipid content, decreased plasma TG and transaminase levels were also described in the animals treated with the polyphenol.
| Author | Animal model | Diet | Resveratrol dose | Improvement in hepatic steatosis |
|---|---|---|---|---|
| Zhang et al. (2015)42 | 8-Week-old 129/SvJ mice | Chow diet (10% fat) | 0.4% resveratrol in the diet (w/w) | Yes |
| Milton-Laskibar et al. (2017)13 | 6-Week-old male Wistar rats | Standard diet (16% lipid, 20% protein, 64% carbohydrates) | 30 mg kg−1 d−1 | Yes |
| Huang et al. (2020)41 | 8–9-Week-old male Sprague-Dawley rats | Chow diet (10% of energy from fat) | 100 mg kg−1 d−1 | Yes |
| Zhu et al. (2014)43 | 8-Week-old male KKAy mice | Standard diet | 2 or 4 g kg per diet | Yes |
| Rivera et al. (2009)44 | 13-Week-old male obese Zucker rats (fa/fa) | Standard diet | 10 mg kg−1 d−1 | Yes |
| Gómez-Zorita et al. (2012)45 | 6-Week-old male obese Zucker rats (fa/fa) | Standard diet | 15 or 45 mg kg−1 d−1 | Yes |
With regard to the mechanisms of action involved in the aforementioned effects, in the study carried out by our group in 6-week-old male Wistar rats fed a high-fat high-sucrose diet (45% of energy as fat and 13% of energy as sucrose) for 6 weeks, and then switched to a standard diet (16% of energy as fat) supplemented or not with 30 mg per kg body weight per day of resveratrol for 6 additional weeks, a significant reduction in the protein expression of the fatty acid transport protein 5 (FATP5) was reported in the livers of animals receiving the polyphenol. This effect suggests a resveratrol-mediated reduction in fatty acid uptake. Moreover, greater CPT1 and citrate synthase (CS) activities, as well as enhanced AMPK phosphorylation and decreased peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC1α) acetylation were observed in the rats treated with resveratrol, suggesting an increase in fatty acid oxidation. Altogether, these metabolic modifications lead to a lower fatty acid availability for TG synthesis. The enhanced activity of the microsomal triglyceride transfer protein (MTP) was also described in these same rats, which pointed towards an increased TG delivery from liver to plasma (Fig. 3).13
Similar results have been recently reported in the study conducted by Huang et al.41 in which 8–9-week-old male Sprague Dawley rats were firstly fed a high-fat diet (45% of energy from fat) for 8 weeks and then switched to a standard diet (10% of energy from fat) and treated or not with 100 mg per kg body weight per day of resveratrol for 8 additional weeks. In this case, the decrease in hepatic lipids was accompanied by enhanced CPT1a activity and greater AMPK activation. The authors also reported a decrease in both FAS protein expression and ACC activation, suggesting that under these experimental conditions, resveratrol also diminished de novo lipogenesis. Moreover, it was also observed that the reductions induced by the high-fat diet in the total anti-oxidative capability, the activities of antioxidant enzymes superoxide dismutase (SOD) and catalase (CAT), as well as in the activities of mitochondrial complexes I and IV were totally reverted by resveratrol treatment.
Based on the data reported in these studies, resveratrol is effective at reducing excessive hepatic lipid accumulation when administered under standard feeding conditions to animals that were previously fed obesogenic diets, and can consequently treat liver steatosis.
Other studies aimed at managing fatty liver have been conducted in animals with genetically induced hepatic steatosis and fed standard diets. In this regard, the excessive hepatic lipid accumulation occurs as the result of the genetic background of the animals, instead of being a matter of dietary conditions. One such animal model is the KKAy mice, which are used for research in obesity and/or metabolic disorders. This animal model was used in the study carried out by Zhu et al.,43 in which 8-week-old male KKAy were fed a standard diet supplemented or not with two resveratrol doses (2 and 4 g per kg diet), for 12 weeks. Decreased hepatic lipid content was observed at the end of the experimental period, with no differences in both doses. In contrast, serum TG and FFA levels were only decreased in the group receiving the highest dose. Besides the liver lipid-lowering effect, decreased serum malondialdehyde (MDA) and increased SOD levels were observed in the animals treated with the polyphenol. When the hepatic oxidative status was studied, the authors found that both resveratrol doses similarly decreased the levels of ROS, although enhanced antioxidant enzyme activities were only observed in the group treated with the highest dose. Finally, the results also demonstrated that resveratrol effectively enhanced the expression of SIRT1 and the activation of AMPK, suggesting that resveratrol also activated ATP-producing pathways in the livers of these animals.
Another genetic model of hepatic steatosis is the obese Zucker rat (fa/fa), which is characterized by a constant accumulation of body fat throughout their life, and thus considered as a well-characterized model of NAFLD.59 Using this experimental model, Rivera et al.44 carried out a study in which obese male Zucker rats (fa/fa) were fed a standard diet, supplemented or not with 10 mg per kg body weight per day of resveratrol (administered by oral gavage), for 8 weeks. The enhanced hepatic TG and cholesterol contents found in the non-treated animals were significantly decreased by resveratrol administration. Similarly, the blood glucose and insulin levels in the animals receiving the polyphenol were significantly lowered in comparison with the non-treated animals. When the potential mechanisms of action were analyzed, the authors found that resveratrol significantly decreased the activity of ACC, while that of AMPK increased, suggesting that the treatment not only inhibited de novo lipogenesis but also activated catabolic pathways devoted to energy production. The authors concluded that chronic resveratrol administration effectively improves the metabolic syndrome features that are present in obese Zucker rats.
Subsequently, our group addressed a study in the same animal model. Obese male Zucker (fa/fa) rats were fed a standard diet and supplemented or not with 15 or 45 mg per kg body weight per day of resveratrol (administered through an orogastric catheter) for 6 weeks. In this case, resveratrol treatment also resulted in a significant reduction in hepatic lipid content that was irrespective of the dose. Similar results were observed regarding serum levels of FFA. The activities of CPT1 and acyl-coenzyme A oxidase (ACO), both enzymes involved in fatty acid oxidation, were significantly enhanced in the livers of rats receiving resveratrol. Finally, both resveratrol doses induced a significant reduction in the hepatic levels of thiobarbituric acid reactive substances (TBARS), although the overall redox status was more markedly improved in the group receiving the highest dose.45
According to the reported results, it seems that resveratrol at a dose in the range of 10–45 mg per kg body weight per day is also effective at reducing genetically-induced excessive hepatic lipid accumulation. The effects induced by the polyphenol were at least partially driven by enhanced fatty acid oxidation and improved hepatic oxidative status.
4. Effects of resveratrol on hepatic steatosis in preclinical studies conducted under energy restriction
Energy restriction, along with increased physical activity, is the non-pharmacological strategy commonly used for obesity treatment. It is well-known that besides this anti-obesity effect, energy restriction also reduces liver lipid content, thus ameliorating hepatic steatosis, not only in rodent models showing diet-induced or genetic liver steatosis,10–12,60 but also in clinical trials.14,15 Despite the health benefits of this dietary approach for hepatic steatosis management, the low adherence of many subjects to this protocol limits its effectiveness as a therapeutic tool in humans. In this context, since resveratrol has been considered an energy restriction mimetic molecule, the combination of both approaches (energy restriction and resveratrol supplementation) could represent an alternative to overcome the aforementioned limited adherence to restricted diets because the same effect might be achieved by using less restrictive diets. Nevertheless, data regarding studies conducted under energy restriction feeding conditions aimed to determine the effects of resveratrol are really scarce.In our group, we have addressed the only two reported studies carried out under these feeding conditions. In the first one, 6-week-old male Wistar rats were fed a high-fat high-sucrose diet (HFHS) for 6 weeks to induce obesity and liver steatosis.50 Then, the animals were switched to a standard diet and submitted to a 25% energy restriction supplemented or not with resveratrol (30 mg per kg body weight per day), for 2 additional weeks. At the end of the experimental period, no differences were observed between both groups in terms of final body weight or liver parameters (weight, hepatic index and TG content). We were surprised by these results since resveratrol had shown effectiveness in improving hepatic steatosis under overfeeding and normal feeding conditions, and thus, we hypothesized that this polyphenol would also be able to increase the beneficial effects induced by energy restriction. To explain this lack of effect, we proposed that the effects produced by energy restriction were strong enough to mask those of resveratrol. It is important to point out that in this study, we chose a 25% energy restriction because it is commonly used in interventions conducted in humans. To avoid this possible bias, in a second study we used a similar experimental procedure, maintaining the dose of resveratrol but using a lower energy restriction percentage (−15%) and a longer experimental period length (6 weeks).13 As in the first study, no significant differences were appreciated between both experimental groups submitted to the restricted diet, receiving the polyphenol or not.
These results suggest that resveratrol is not an effective approach to enhancing the liver's delipidating effect induced by energy restriction. By using the data obtained in our studies, we can state that the effects induced by 30 mg resveratrol per kg per d are weaker than those induced by a 15% energy restriction.
5. Effects of resveratrol on hepatic steatosis in preclinical studies conducted using other dietary models
In addition to the models of liver steatosis described in the previous sections of this review, other models have also been used in the studies devoted to analyzing the effects of resveratrol. One of these models is that described by Delzenne et al.,61 consisting on the combination of a high carbohydrate-fat free (HCFF) feeding and fasting/refeeding periods. Based on this model, Bujanda et al.46 fed male Wistar rats a high carbohydrate-fat free diet (80% of energy from starch) for 4 days, and then they fasted the animals for 3 additional days. This procedure was repeated 4 times during the whole experimental period. The experimental group treated with resveratrol received the polyphenol at a dose of 10 mg d−1 and the control group just received the vehicle. At the end of the experimental period, H&E staining revealed lower fat infiltration in the livers of the animals treated with resveratrol. Lower serum levels of TNF-α and MDA were also found in the resveratrol-treated group. As far as liver oxidative stress is concerned, enhanced activities of SOD, CAT and glutathione peroxidase (GPx), as well as decreased activity of nitric oxide synthase (NOS), were reported by the authors in the animals receiving the polyphenol. Altogether, these results suggest that in these animals, the inflammatory and oxidative status were ameliorated by the polyphenol.Methionine choline-deficient diet (MCD) feeding is another frequently used dietary model to induce hepatic steatosis. It is based on the deficiency of methionine and choline, which are essential for liver β-oxidation and the production of very low-density lipoprotein (VLDL).62 Indeed, the histological and morphological changes driven by this dietary approach in the liver encompass steatosis, inflammation and aminotransferase elevation.63 Ali et al.48 fed male Wistar rats a MCD diet for 28 days, following the same feeding pattern than that used by Bujanda et al. (4 day ad libitum feeding followed by a 3 day fast). During this experimental period, the rats were given resveratrol (10 mg per kg body weight per day) or the vehicle daily by the oral route. At the end of the experimental period, the multifocal hepatocellular necrosis and inflammatory cell infiltration, revealed by H&E staining in the non-treated rats, were avoided in the group treated with resveratrol, which showed a preserved liver architecture. In addition, lower serum levels of transaminase, glucose, lipids and inflammatory markers were found in the group receiving the phenolic compound when compared to the non-treated steatotic rats. Similarly, hepatic levels of oxidative stress markers were also significantly decreased by the polyphenol treatment.
Another model is that in which early weaning programming is used to induce different metabolic disturbances. It has been described that this procedure causes undernutrition for a short period of time, programming the offspring for the development of different metabolic alterations in the adult life.64 Based on this model, Franco et al.,47 shortened the lactation of male Wistar rats for 3 days (18 days of lactation instead of 21). The rats were then fed a standard diet for 150 days. At this point, animals received resveratrol (30 mg per kg body weight per day) or vehicle by oral gavage for 30 days and were fed the same diet. At the end of the experimental period, the increased content of TG found in the liver was lower in rats treated with resveratrol than in the control rats. The microvesicular steatosis revealed by H&E staining in non-treated rats was significantly ameliorated by resveratrol administration. Similarly, resveratrol significantly prevented the increase in blood lipid levels found in the non-treated animals, as well as those observed in oxidative markers in both liver and blood.
Another approach commonly used to induce hepatic steatosis is high-fructose feeding. This sugar is characterized for being highly lipogenic, resulting in increased TG and free fatty acid accumulation in the liver and blood. El-Haleim et al.,49 fed two groups of adult male albino rats a normal chow diet and provided them with fructose in drinking water (10%), for 12 weeks. During the last 4 weeks of the treatment (weeks 9 to 12), a group of rats was treated with resveratrol (70 mg kg−1 d−1). Lower liver index and TG content were observed at the end of the experimental period in the animals receiving the compound when compared to the non-treated ones. Similarly, the histopathological examination of liver sections revealed that resveratrol significantly decreased the average steatosis area in comparison with the non-treated rats. The authors reported that the polyphenol prevented most of the histopathological abnormalities observed in the livers of non-treated rats. Additionally, lower serum TG levels and decreased hepatic MDA content were also observed in the animals receiving resveratrol.
The results reported in the studies included in this section demonstrate that resveratrol is also effective in improving hepatic steatosis induced by dietary models other than those based on energy intake or genetic alterations. In this regard, the beneficial effects exerted by the polyphenol are consistent in a range of doses from 10 to 30 mg per kg body weight per day (Table 3). Regarding the proposed mechanisms of action underlying these beneficial effects, it seems that the polyphenol mainly acts by reducing the hepatic oxidative stress induced in these experimental models.
| Author | Animal model | Diet | Resveratrol dose | Improvement in hepatic steatosis |
|---|---|---|---|---|
| HCFF: high carbohydrate fat-free, MCDD: methionine choline-deficient diet. | ||||
| Bujanda et al. (2008)46 | Male Wistar rats | HCFF diet (80% of energy from starch) | 10 mg d−1 | Yes |
| Franco et al. (2013)47 | Male Wistar rats (early weaned) | Standard diet | 30 mg kg−1 d−1 | Yes |
| Ali et al. (2016)48 | Male Wistar rats (weanling) | MCDD | 10 mg kg−1 d−1 | Yes |
| El-Haleim et al. (2016)49 | Adult male Albino rats | Normal chow diet +10% fructose in drinking water | 70 mg kg−1 d−1 | Yes |
6. Effects of resveratrol in hepatic steatosis in clinical studies
Several studies have been conducted in humans, which were aimed at determining the effectiveness of resveratrol for hepatic steatosis treatment (Table 4). In the eight reported studies that have been included in this review52,53,65–70 all the patients had diagnosed NAFLD at the beginning of the study. Besides this common feature, the experimental procedures used in these studies were significantly different among them, not only regarding the used resveratrol dose (ranging from 50 to 3000 mg d−1), but also regarding the selected experimental period length (from 8 weeks to 6 months) or patient characteristics (age, sex or body mass index). In some of the studies, the experimental groups received nutritional/lifestyle modification advice besides resveratrol administration, making it more difficult to identify the effects induced by the polyphenol itself.| Author | Subject | Additional interventions | Resveratrol dose | Improvement in hepatic steatosis |
|---|---|---|---|---|
| NAFLD: non-alcoholic fatty liver disease. | ||||
| Chachay et al. (2014)65 | Obese or overweight men with NAFLD | No | 3000 mg day−1 | No |
| Faghihzadeh et al. (2014)52 | Subjects with NAFLD | Yes (dietary and physical activity advice) | 500 mg day−1 | Yes |
| Chen et al. (2015)53 | Subjects with NAFLD | No | 600 mg day−1 | Yes |
| Heebøll et al. (2016)66 | Overweight subjects with NAFLD | No | 1.5 g day−1 | No |
| Poulsen et al. (2018)67 | Obese or overweight, non-diabetic subjects with NAFLD | No | 1.5 g day−1 | No |
| Kantartzis et al. (2018)68 | Obese or overweight men with NAFLD | No | 150 mg day−1 | No |
| Asghari et al. (2018)69 | Overweight subjects with NAFLD | Yes (dietary advice) | 600 mg day−1 | No |
| Theodotou et al. (2019)70 | Subjects with NAFLD | Yes (dietary advice) | 50 and 200 mg day−1 | No |
Compared to the results described in preclinical studies, the effects of resveratrol in steatosis in humans are rather weak. None of the eight studies included in this review showed significant reductions in the hepatic lipid content after resveratrol administration. In this regard, it should be considered that the baseline liver fat content can play an important role in subject responsiveness. Thus, in the study reported by Kantartzis et al., where a large variability in this parameter was observed (ranging from 0.09% to 37.55%), subjects with a very high liver fat content at baseline showed a significant reduction in hepatic steatosis after resveratrol treatment. In contrast, this effect was not observed in subjects with a low or moderately elevated baselina liver fat content.68 Despite the lack of effect on liver lipid content, in some studies, resveratrol-derived improvements in NAFLD have been reported, even though liver lipid content was not diminished. In this regard, decreased levels of serum transaminases,52,53 lipids,53 glucose53 and inflammatory markers52 were observed. A reduction in hepatic fibrosis was also described in one study,52 although without reaching statistical significance when compared to the non-treated group.
The apparent discrepancies among the results obtained in animal studies and those reported in clinical trials may be due to differences in the experimental procedures. There are 3 variables that may explain this lack of consensus, which are the used resveratrol doses, the selected experimental period length and the additional interventions (besides resveratrol administration) that were used in some of the clinical trials. Concerning the resveratrol doses, based on the Reagan-Shaw formula,71 which is commonly used to translate the doses used in animal studies to humans, it can be demonstrated that the doses selected in the clinical trials are in the range of those shown to be effective in ameliorating hepatic steatosis in rodent models. Thus, it seems that the lack of effect reported in humans is not due to the use of lower doses of resveratrol. As far as the experimental period length is concerned, it must be taken into account that the lifespan in rodents is shorter than in humans. Consequently, the influence of several weeks of treatment in rodents can be more effective in these small animals than in humans. As such, it has been reported that in adult rats one day is equivalent to 34.8 human days,72 a correlation that is even greater for young rats. Based on the aforementioned equivalence, the experimental periods used to test the usefulness of resveratrol for hepatic steatosis treatment in rats would range from 1044 to 4385 days in humans. This observation may justify the lack of correlation between results obtained using rodent models and those obtained in human studies. Indeed, resveratrol-derived hepatic lipid content reductions in humans cannot be ruled out if such treatment periods were used. Finally, in some studies, lifestyle modification interventions were introduced to the patients of the treated and placebo groups: dietary advice, encouraging participants to acquire/maintain healthy dietary habits52,69 and/or encouraging the practice of regular physical exercise.52 In one study, participants maintained a low-fat diet under the supervision of a nutritionist throughout the whole experimental period.70 These lifestyle modifications could have masked the potential effects derived from resveratrol administration, as occurred in rats fed an energy-restricted diet and supplemented with resveratrol.13,50
8. Concluding remarks
After analyzing the reported results, it can be stated that preclinical studies demonstrate the ability of resveratrol to prevent liver steatosis induced by different unbalanced dietary patterns. Moreover, when liver steatosis is already established, this phenolic compound is useful for reducing this lipid alteration, meaning that it could represent a new tool for fatty liver treatment, although its effectiveness is lower than that of energy restriction. These beneficial effects are mainly due to the reduction in de novo lipogenesis, the increase in fatty acid oxidation, the increase in autophagy and the decrease in oxidative stress. The results reported concerning the effects of resveratrol on gut microbiota composition should also be taken into account, since the modulation induced by the polyphenol (restoration of the diversity and the equilibrium of gut microbiota), especially in animals that have been fed obesogenic diets causing dysbiosis, seems to be related to the aforementioned beneficial effects.In contrast, resveratrol administration seems not to be effective when provided under energy restriction conditions, showing that it cannot be considered as a complementary tool to hypocaloric diets in the treatment of liver steatosis. The reason for this lack of effect is not clear but it could be hypothesized that the strong effect of energy restriction masks the milder effect of resveratrol.
After determining the beneficial effects of resveratrol on liver steatosis in rodents, the next step was to check whether these effects are reproduced in humans. Unfortunately, this is not the case since despite resveratrol treatment improving several parameters related to this hepatic alteration, the hepatic TG content did not decrease in the reported studies. Several aspects can be considered to explain the lack of effectiveness of this compound in humans, and among them, the treatment duration seems to be a crucial aspect. Nevertheless, more clinical studies are needed to definitely discard this treatment option.
Author contribution
All the authors contributed to revise the literature and to write the manuscript and MPP revised the final version.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This study has been supported by grants from Ministerio de Economía y Competitividad-Fondo Europeo de Desarrollo Regional, under grant AGL-2015-65719-R MINECO/FEDER, (UE), Instituto de Salud Carlos III (CIBERobn) under Grant CB12/03/30007 and University of the Basque Country, under Grant GIU18-173.References
- P. Byass, The global burden of liver disease: a challenge for methods and for public health, BMC Med., 2014, 12, 159 CrossRef.
- WHO, Global health sector strategy on viral hepatitis 2016–2021. Towards ending viral hepatitis 2016, 2016 Search PubMed.
- E. Fabbrini, S. Sullivan and S. Klein, Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications, Hepatology, 2010, 51, 679–689 CrossRef CAS.
- Z. Younossi, Q. M. Anstee, M. Marietti, T. Hardy, L. Henry, M. Eslam, J. George and E. Bugianesi, Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention, Nat. Rev. Gastroenterol. Hepatol., 2018, 15, 11–20 CrossRef.
- A. Engin, Non-Alcoholic Fatty Liver Disease, Adv. Exp. Med. Biol., 2017, 960, 443–467 CrossRef CAS.
- T. Eigentler, D. Lomberg, J. Machann and N. Stefan, Lipodystrophic Nonalcoholic Fatty Liver Disease Induced by Immune Checkpoint Blockade, Ann. Intern. Med., 2020, 172, 836–837 CrossRef.
- N. Stefan, Causes, consequences, and treatment of metabolically unhealthy fat distribution, Lancet Diabetes Endocrinol., 2020, 8, 616–627 CrossRef.
- E. Buzzetti, M. Pinzani and E. A. Tsochatzis, The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD), Metabolism, 2016, 65, 1038–1048 CrossRef CAS.
- N. Stefan, H. U. Häring and K. Cusi, Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies, Lancet Diabetes Endocrinol., 2019, 7, 313–324 CrossRef.
- E. Tauriainen, M. Luostarinen, E. Martonen, P. Finckenberg, M. Kovalainen, A. Huotari, K. H. Herzig, A. Lecklin and E. Mervaala, Distinct effects of calorie restriction and resveratrol on diet-induced obesity and Fatty liver formation, J. Nutr. Metab., 2011, 2011, 525094 Search PubMed.
- M. A. Linden, J. A. Fletcher, G. M. Meers, J. P. Thyfault, M. H. Laughlin and R. S. Rector, A return to ad libitum feeding following caloric restriction promotes hepatic steatosis in hyperphagic OLETF rats, Am. J. Physiol.: Gastrointest. Liver Physiol., 2016, 311, G387–G395 CrossRef.
- S. Ding, J. Jiang, G. Zhang, Y. Bu and X. Zhao, Resveratrol and caloric restriction prevent hepatic steatosis by regulating SIRT1-autophagy pathway and alleviating endoplasmic reticulum stress in high-fat diet-fed rats, PLoS One, 2017, 12, e0183541 CrossRef.
- I. Milton-Laskibar, L. Aguirre, A. Fernández-Quintela, A. P. Rolo, J. Soeiro Teodoro, C. M. Palmeira and M. P. Portillo, Lack of Additive Effects of Resveratrol and Energy Restriction in the Treatment of Hepatic Steatosis in Rats, Nutrients, 2017, 9(7), 737 CrossRef.
- D. E. Larson-Meyer, B. R. Newcomer, L. K. Heilbronn, J. Volaufova, S. R. Smith, A. J. Alfonso, M. Lefevre, J. C. Rood, D. A. Williamson, E. Ravussin and P. C. Team, Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function, Obesity, 2008, 16, 1355–1362 CrossRef CAS.
- M. C. Elias, E. R. Parise, L. de Carvalho, D. Szejnfeld and J. P. Netto, Effect of 6-month nutritional intervention on non-alcoholic fatty liver disease, Nutrition, 2010, 26, 1094–1099 CrossRef.
- J. L. Barger, T. Kayo, J. M. Vann, E. B. Arias, J. Wang, T. A. Hacker, Y. Wang, D. Raederstorff, J. D. Morrow, C. Leeuwenburgh, D. B. Allison, K. W. Saupe, G. D. Cartee, R. Weindruch and T. A. Prolla, A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice, PLoS One, 2008, 3, e2264 CrossRef.
- J. A. Baur, K. J. Pearson, N. L. Price, H. A. Jamieson, C. Lerin, A. Kalra, V. V. Prabhu, J. S. Allard, G. Lopez-Lluch, K. Lewis, P. J. Pistell, S. Poosala, K. G. Becker, O. Boss, D. Gwinn, M. Wang, S. Ramaswamy, K. W. Fishbein, R. G. Spencer, E. G. Lakatta, D. Le Couteur, R. J. Shaw, P. Navas, P. Puigserver, D. K. Ingram, R. de Cabo and D. A. Sinclair, Resveratrol improves health and survival of mice on a high-calorie diet, Nature, 2006, 444, 337–342 CrossRef CAS.
- E. M. Mercken, B. A. Carboneau, S. M. Krzysik-Walker and R. de Cabo, Of mice and men: the benefits of caloric restriction, exercise, and mimetics, Ageing Res. Rev., 2012, 11, 390–398 CrossRef.
- F. Fogacci, G. Tocci, V. Presta, A. Fratter, C. Borghi and A. F. G. Cicero, Effect of resveratrol on blood pressure: A systematic review and meta-analysis of randomized, controlled, clinical trials, Crit. Rev. Food Sci. Nutr., 2019, 59, 1605–1618 CrossRef CAS.
- S. J. Cho, U. J. Jung and M. S. Choi, Differential effects of low-dose resveratrol on adiposity and hepatic steatosis in diet-induced obese mice, Br. J. Nutr., 2012, 108, 2166–2175 CrossRef CAS.
- B. T. Jeon, E. A. Jeong, H. J. Shin, Y. Lee, D. H. Lee, H. J. Kim, S. S. Kang, G. J. Cho, W. S. Choi and G. S. Roh, Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet, Diabetes, 2012, 61, 1444–1454 CrossRef CAS.
- W. Kang, H. J. Hong, J. Guan, D. G. Kim, E. J. Yang, G. Koh, D. Park, C. H. Han, Y. J. Lee and D. H. Lee, Resveratrol improves insulin signaling in a tissue-specific manner under insulin-resistant conditions only: in vitro and in vivo experiments in rodents, Metabolism, 2012, 61, 424–433 CrossRef CAS.
- J. M. Andrade, A. F. Paraíso, M. V. de Oliveira, A. M. Martins, J. F. Neto, A. L. Guimarães, A. M. de Paula, M. Qureshi and S. H. Santos, Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation, Nutrition, 2014, 30, 915–919 CrossRef CAS.
- Y. J. Choi, H. R. Suh, Y. Yoon, K. J. Lee, D. G. Kim, S. Kim and B. H. Lee, Protective effect of resveratrol derivatives on high-fat diet induced fatty liver by activating AMP-activated protein kinase, Arch. Pharmacal Res., 2014, 37, 1169–1176 CrossRef CAS.
- S. M. Jeon, S. A. Lee and M. S. Choi, Antiobesity and vasoprotective effects of resveratrol in apoE-deficient mice, J. Med. Food, 2014, 17, 310–316 CrossRef CAS.
- M. Montero, S. de la Fuente, R. I. Fonteriz, A. Moreno and J. Alvarez, Effects of long-term feeding of the polyphenols resveratrol and kaempferol in obese mice, PLoS One, 2014, 9, e112825 CrossRef.
- Y. Qiao, J. Sun, S. Xia, X. Tang, Y. Shi and G. Le, Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity, Food Funct., 2014, 5, 1241–1249 RSC.
- K. Nishikawa, K. Iwaya, M. Kinoshita, Y. Fujiwara, M. Akao, M. Sonoda, S. Thiruppathi, T. Suzuki, S. Hiroi, S. Seki and T. Sakamoto, Resveratrol increases CD68+ Kupffer cells colocalized with adipose differentiation-related protein and ameliorates high-fat-diet-induced fatty liver in mice, Mol. Nutr. Food Res., 2015, 59, 1155–1170 CrossRef CAS.
- Y. Tian, J. Ma, W. Wang, L. Zhang, J. Xu, K. Wang and D. Li, Resveratrol supplement inhibited the NF-κB inflammation pathway through activating AMPKα-SIRT1 pathway in mice with fatty liver, Mol. Cell. Biochem., 2016, 422, 75–84 CrossRef CAS.
- R. Zhou, L. Yi, X. Ye, X. Zeng, K. Liu, Y. Qin, Q. Zhang and M. Mi, Resveratrol Ameliorates Lipid Droplet Accumulation in Liver Through a SIRT1/ATF6-Dependent Mechanism, Cell. Physiol. Biochem., 2018, 51, 2397–2420 CrossRef CAS.
- K. Cheng, Z. Song, H. Zhang, S. Li, C. Wang, L. Zhang and T. Wang, The therapeutic effects of resveratrol on hepatic steatosis in high-fat diet-induced obese mice by improving oxidative stress, inflammation and lipid-related gene transcriptional expression, Med. Mol. Morphol., 2019, 52, 187–197 CrossRef CAS.
- W. Teng, L. Zhao, S. Yang, C. Zhang, M. Liu, J. Luo, J. Jin, M. Zhang, C. Bao, D. Li, W. Xiong, Y. Li and F. Ren, The hepatic-targeted, resveratrol loaded nanoparticles for relief of high fat diet-induced nonalcoholic fatty liver disease, J. Controlled Release, 2019, 307, 139–149 CrossRef CAS.
- H. Hosseini, M. Teimouri, M. Shabani, M. Koushki, R. Babaei Khorzoughi, F. Namvarjah, P. Izadi and R. Meshkani, Resveratrol alleviates non-alcoholic fatty liver disease through epigenetic modification of the Nrf2 signaling pathway, Int. J. Biochem. Cell Biol., 2020, 119, 105667 CrossRef CAS.
- X. Yin, W. Liao, Q. Li, H. Zhang, Z. Liu, X. Zheng, L. Zheng and X. Feng, Interactions between resveratrol and gut microbiota affect the development of hepatic steatosis: A fecal microbiota transplantation study in high-fat diet mice, J. Funct. Foods, 2020, 67, 103883 CrossRef CAS.
- J. Shang, L. L. Chen, F. X. Xiao, H. Sun, H. C. Ding and H. Xiao, Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase, Acta Pharmacol. Sin., 2008, 29, 698–706 CrossRef CAS.
- M. M. Poulsen, J. Ø. Larsen, S. Hamilton-Dutoit, B. F. Clasen, N. Jessen, S. K. Paulsen, T. N. Kjær, B. Richelsen and S. B. Pedersen, Resveratrol up-regulates hepatic uncoupling protein 2 and prevents development of nonalcoholic fatty liver disease in rats fed a high-fat diet, Nutr. Res., 2012, 32, 701–708 CrossRef CAS.
- P. Xin, H. Han, D. Gao, W. Cui, X. Yang, C. Ying, X. Sun and L. Hao, Alleviative effects of resveratrol on nonalcoholic fatty liver disease are associated with up regulation of hepatic low density lipoprotein receptor and scavenger receptor class B type I gene expressions in rats, Food Chem. Toxicol., 2013, 52, 12–18 CrossRef CAS.
- Q. R. Pan, Y. L. Ren, W. X. Liu, Y. J. Hu, J. S. Zheng, Y. Xu and G. Wang, Resveratrol prevents hepatic steatosis and endoplasmic reticulum stress and regulates the expression of genes involved in lipid metabolism, insulin resistance, and inflammation in rats, Nutr. Res., 2015, 35, 576–584 CrossRef CAS.
- R. M. Badi, D. G. Mostafa, E. F. Khaleel and H. H. Satti, Resveratrol protects against hepatic insulin resistance in a rat's model of non-alcoholic fatty liver disease by down-regulation of GPAT-1 and DGAT2 expression and inhibition of PKC membranous translocation, Clin. Exp. Pharmacol. Physiol., 2019, 46, 545–555 CrossRef CAS.
- X. X. Chen, Y. Y. Xu, R. Wu, Z. Chen, K. Fang, Y. X. Han, Y. Yu, L. L. Huang, L. Peng and J. F. Ge, Resveratrol Reduces Glucolipid Metabolic Dysfunction and Learning and Memory Impairment in a NAFLD Rat Model: Involvement in Regulating the Imbalance of Nesfatin-1 Abundance and Copine 6 Expression, Front. Endocrinol., 2019, 10, 434 CrossRef.
- Y. Huang, H. Lang, K. Chen, Y. Zhang, Y. Gao, L. Ran, L. Yi, M. Mi and Q. Zhang, Resveratrol protects against nonalcoholic fatty liver disease by improving lipid metabolism and redox homeostasis via the PPARα pathway, Appl. Physiol., Nutr., Metab., 2020, 45, 227–239 CrossRef CAS.
- Y. Zhang, M. L. Chen, Y. Zhou, L. Yi, Y. X. Gao, L. Ran, S. H. Chen, T. Zhang, X. Zhou, D. Zou, B. Wu, Y. Wu, H. Chang, J. D. Zhu, Q. Y. Zhang and M. T. Mi, Resveratrol improves hepatic steatosis by inducing autophagy through the cAMP signaling pathway, Mol. Nutr. Food Res., 2015, 59, 1443–1457 CrossRef CAS.
- W. Zhu, S. Chen, Z. Li, X. Zhao, W. Li, Y. Sun, Z. Zhang, W. Ling and X. Feng, Effects and mechanisms of resveratrol on the amelioration of oxidative stress and hepatic steatosis in KKAy mice, Nutr. Metab., 2014, 11, 35 CrossRef.
- L. Rivera, R. Morón, A. Zarzuelo and M. Galisteo, Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats, Biochem. Pharmacol., 2009, 77, 1053–1063 CrossRef CAS.
- S. Gómez-Zorita, A. Fernández-Quintela, M. T. Macarulla, L. Aguirre, E. Hijona, L. Bujanda, F. Milagro, J. A. Martínez and M. P. Portillo, Resveratrol attenuates steatosis in obese Zucker rats by decreasing fatty acid availability and reducing oxidative stress, Br. J. Nutr., 2012, 107, 202–210 CrossRef.
- L. Bujanda, E. Hijona, M. Larzabal, M. Beraza, P. Aldazabal, N. García-Urkia, C. Sarasqueta, A. Cosme, B. Irastorza, A. González and J. I. Arenas, Resveratrol inhibits nonalcoholic fatty liver disease in rats, BMC Gastroenterol., 2008, 8, 40 CrossRef.
- J. G. Franco, P. C. Lisboa, N. S. Lima, T. A. Amaral, N. Peixoto-Silva, A. C. Resende, E. Oliveira, M. C. Passos and E. G. Moura, Resveratrol attenuates oxidative stress and prevents steatosis and hypertension in obese rats programmed by early weaning, J. Nutr. Biochem., 2013, 24, 960–966 CrossRef CAS.
- M. H. Ali, B. A. Messiha and H. A. Abdel-Latif, Protective effect of ursodeoxycholic acid, resveratrol, and N-acetylcysteine on nonalcoholic fatty liver disease in rats, Pharm. Biol., 2016, 54, 1198–1208 CAS.
- E. A. Abd El-Haleim, A. K. Bahgat and S. Saleh, Resveratrol and fenofibrate ameliorate fructose-induced nonalcoholic steatohepatitis by modulation of genes expression, World J. Gastroenterol., 2016, 22, 2931–2948 CrossRef CAS.
- G. Alberdi, M. T. Macarulla, M. P. Portillo and V. M. Rodríguez, Resveratrol does not increase body fat loss induced by energy restriction, J. Physiol. Biochem., 2014, 70, 639–646 CrossRef CAS.
- S. Timmers, E. Konings, L. Bilet, R. H. Houtkooper, T. van de Weijer, G. H. Goossens, J. Hoeks, S. van der Krieken, D. Ryu, S. Kersten, E. Moonen-Kornips, M. K. Hesselink, I. Kunz, V. B. Schrauwen-Hinderling, E. E. Blaak, J. Auwerx and P. Schrauwen, Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans, Cell Metab., 2011, 14, 612–622 CrossRef CAS.
- F. Faghihzadeh, P. Adibi, R. Rafiei and A. Hekmatdoost, Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease, Nutr. Res., 2014, 34, 837–843 CrossRef CAS.
- S. Chen, X. Zhao, L. Ran, J. Wan, X. Wang, Y. Qin, F. Shu, Y. Gao, L. Yuan, Q. Zhang and M. Mi, Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: a randomized controlled trial, Dig. Liver Dis., 2015, 47, 226–232 CrossRef CAS.
- A. F. G. Cicero, A. Colletti and S. Bellentani, Nutraceutical Approach to Non-Alcoholic Fatty Liver Disease (NAFLD): The Available Clinical Evidence, Nutrients, 2018, 10(9), 1153 CrossRef.
- J. M. Andrade, A. C. Frade, J. B. Guimarães, K. M. Freitas, M. T. Lopes, A. L. Guimarães, A. M. de Paula, C. C. Coimbra and S. H. Santos, Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet, Eur. J. Nutr., 2014, 53, 1503–1510 CrossRef CAS.
- M. M. Poulsen, P. F. Vestergaard, B. F. Clasen, Y. Radko, L. P. Christensen, H. Stødkilde-Jørgensen, N. Møller, N. Jessen, S. B. Pedersen and J. O. Jørgensen, High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition, Diabetes, 2013, 62, 1186–1195 CrossRef CAS.
- N. Arias, M. T. Macarulla, L. Aguirre, J. Miranda and M. P. Portillo, Liver delipidating effect of a combination of resveratrol and quercetin in rats fed an obesogenic diet, J. Physiol. Biochem., 2015, 71, 569–576 CrossRef CAS.
- S. Gómez-Zorita, L. Aguirre, I. Milton-Laskibar, A. Fernández-Quintela, J. Trepiana, N. Kajarabille, A. Mosqueda-Solís, M. González and M. P. Portillo, Relationship between Changes in Microbiota and Liver Steatosis Induced by High-Fat Feeding-A Review of Rodent Models, Nutrients, 2019, 11(9), 2156 CrossRef.
- G. Palladini, L. G. Di Pasqua, C. Berardo, V. Siciliano, P. Richelmi, S. Perlini, A. Ferrigno and M. Vairetti, Animal Models of Steatosis (NAFLD) and Steatohepatitis (NASH) Exhibit Hepatic Lobe-Specific Gelatinases Activity and Oxidative Stress, Can. J. Gastroenterol. Hepatol., 2019, 2019, 5413461 Search PubMed.
- K. E. Kim, Y. Jung, S. Min, M. Nam, R. W. Heo, B. T. Jeon, D. H. Song, C. O. Yi, E. A. Jeong, H. Kim, J. Kim, S. Y. Jeong, W. Kwak, d. H. Ryu, T. L. Horvath, G. S. Roh and G. S. Hwang, Caloric restriction of db/db mice reverts hepatic steatosis and body weight with divergent hepatic metabolism, Sci. Rep., 2016, 6, 30111 CrossRef CAS.
- N. M. Delzenne, N. A. Hernaux and H. S. Taper, A new model of acute liver steatosis induced in rats by fasting followed by refeeding a high carbohydrate-fat free diet. Biochemical and morphological analysis, J. Hepatol., 1997, 26, 880–885 CrossRef CAS.
- S. C. Sanches, L. N. Ramalho, M. J. Augusto, D. M. da Silva and F. S. Ramalho, Nonalcoholic Steatohepatitis: A Search for Factual Animal Models, BioMed Res. Int., 2015, 2015, 574832 Search PubMed.
- M. E. Rinella, M. S. Elias, R. R. Smolak, T. Fu, J. Borensztajn and R. M. Green, Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet, J. Lipid Res., 2008, 49, 1068–1076 CrossRef CAS.
- N. a. S. Lima, E. G. de Moura, M. C. Passos, F. J. Nogueira Neto, A. M. Reis, E. de Oliveira and P. C. Lisboa, Early weaning causes undernutrition for a short period and programmes some metabolic syndrome components and leptin resistance in adult rat offspring, Br. J. Nutr., 2011, 105, 1405–1413 CrossRef CAS.
- V. S. Chachay, G. A. Macdonald, J. H. Martin, J. P. Whitehead, T. M. O'Moore-Sullivan, P. Lee, M. Franklin, K. Klein, P. J. Taylor, M. Ferguson, J. S. Coombes, G. P. Thomas, G. J. Cowin, C. M. Kirkpatrick, J. B. Prins and I. J. Hickman, Resveratrol does not benefit patients with nonalcoholic fatty liver disease, Clin. Gastroenterol. Hepatol., 2014, 12, 2092–2103 CrossRef CAS.
- S. Heebøll, M. Kreuzfeldt, S. Hamilton-Dutoit, M. Kjær Poulsen, H. Stødkilde-Jørgensen, H. J. Møller, N. Jessen, K. Thorsen, Y. Kristina Hellberg, S. Bønløkke Pedersen and H. Grønbæk, Placebo-controlled, randomised clinical trial: high-dose resveratrol treatment for non-alcoholic fatty liver disease, Scand. J. Gastroenterol., 2016, 51, 456–464 CrossRef.
- M. K. Poulsen, B. Nellemann, B. M. Bibby, H. Stødkilde-Jørgensen, S. B. Pedersen, H. Grønbaek and S. Nielsen, No effect of resveratrol on VLDL-TG kinetics and insulin sensitivity in obese men with nonalcoholic fatty liver disease, Diabetes, Obes. Metab., 2018, 20, 2504–2509 CrossRef CAS.
- K. Kantartzis, L. Fritsche, M. Bombrich, J. Machann, F. Schick, H. Staiger, I. Kunz, R. Schoop, A. Lehn-Stefan, M. Heni, A. Peter, A. Fritsche, H. U. Häring and N. Stefan, Effects of resveratrol supplementation on liver fat content in overweight and insulin-resistant subjects: A randomized, double-blind, placebo-controlled clinical trial, Diabetes, Obes. Metab., 2018, 20, 1793–1797 CrossRef CAS.
- S. Asghari, M. Asghari-Jafarabadi, M. H. Somi, S. M. Ghavami and M. Rafraf, Comparison of Calorie-Restricted Diet and Resveratrol Supplementation on Anthropometric Indices, Metabolic Parameters, and Serum Sirtuin-1 Levels in Patients With Nonalcoholic Fatty Liver Disease: A Randomized Controlled Clinical Trial, J. Am. Coll. Nutr., 2018, 37, 223–233 CrossRef CAS.
- M. Theodotou, K. Fokianos, D. Moniatis, R. Kadlenic, A. Chrysikou, A. Aristotelous, A. Mouzouridou, J. Diakides and E. Stavrou, Effect of resveratrol on non-alcoholic fatty liver disease, Exp. Ther. Med., 2019, 18, 559–565 CAS.
- S. Reagan-Shaw, M. Nihal and N. Ahmad, Dose translation from animal to human studies revisited, FASEB J., 2008, 22, 659–661 CrossRef CAS.
- P. Sengupta, The Laboratory Rat: Relating Its Age With Human's, Int. J. Prev. Med., 2013, 4, 624–630 Search PubMed.
| This journal is © The Royal Society of Chemistry 2020 |