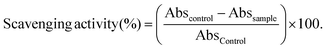Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Benefits of the ipowder® extraction process applied to Melissa officinalis L.: improvement of antioxidant activity and in vitro gastro-intestinal release profile of rosmarinic acid
Valérie
Bardot
a,
Anaïs
Escalon
a,
Isabelle
Ripoche
b,
Sylvain
Denis
c,
Monique
Alric
c,
Sandrine
Chalancon
c,
Pierre
Chalard
b,
César
Cotte
a,
Lucile
Berthomier
b,
Martin
Leremboure
b and
Michel
Dubourdeaux
 *a
*a
aPiLeJe Industrie, Naturopôle Nutrition Santé, Les Tiolans, F-03800 Saint-Bonnet-de-Rochefort, France. E-mail: m.dubourdeaux@pileje.com
bUniversité Clermont Auvergne, CNRS, SIGMA Clermont, Institut de Chimie de Clermont Ferrand, BP 10448, F-63000 Clermont Ferrand, France
cUniversité Clermont Auvergne, INRA, UMR454 MEDIS, F-63000 Clermont Ferrand, France
First published on 8th January 2020
Abstract
The objective of this study was to evaluate the benefits of a new extraction process, the ipowder® technology, applied to Melissa officinalis L. Compared to M. officinalis ground dry leaves, the ipowder® had a similar phytochemical fingerprint but contained twice the concentration of rosmarinic acid (by HPTLC and HPLC) and had a two-fold greater antioxidant activity (DPPH* method). In vitro digestion experiments (TIM-1 model) showed better availability of rosmarinic acid for intestinal absorption with the ipowder® than with ground dry leaves, manifested by a three-fold reduction in the quantity of ingested product needed for delivery of the same amount of rosmarinic acid into the upper gastro-intestinal tract. This study shows that the ipowder® technology preserves all the original plant compounds intact while making some active ingredients more accessible and available to exert their effects. To obtain a given effect, the amount of ipowder® extract to ingest will therefore be lower; a reduction in the daily dosage will be more convenient for the patient and will improve patient compliance with supplementation.
1. Introduction
Herbs have been used as therapeutic agents all over the world for thousands of years. The World Health Organization estimated that 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 plant species could potentially be used for medicinal purposes and that over 80% of people worldwide rely on herbal medicines for their everyday health care needs.1,2 It has also been estimated that herbal products account for 25% of total medicine consumption in developed countries and up to 80% in developing countries such as India or China.
000 plant species could potentially be used for medicinal purposes and that over 80% of people worldwide rely on herbal medicines for their everyday health care needs.1,2 It has also been estimated that herbal products account for 25% of total medicine consumption in developed countries and up to 80% in developing countries such as India or China.
Medicinal plants are used either directly as dry plant powder or as liquid or dry formulations of their active ingredients extracted by various methods. The amount of dry plant powder to be ingested in order to achieve a physiological effect is generally relatively high, especially since the bioavailability of the active compounds is usually low. Moreover, in the case of an extract, its quality depends greatly on the extraction method. According to the method used, different active compounds will be extracted, at varying concentrations.
Loss and degradation of certain plant ingredients often occur during the preparation of extracts and as a result, their phytochemical composition differs from that of the totum, defined as the entire set of active compounds contained in the part of the plant used for extraction. A fundamental principle of phytotherapy is that of synergy, according to which the activity of the totum is more than the sum of the effects of the individual active ingredients taken separately. It is therefore essential to design new processes for the preparation of plant extracts that preserve intact in the finished product all the active substances contained in the original medicinal plant while increasing their availability. The ipowder® technology was developed for this purpose.
The ipowder® process consists of three essential steps, namely contact between the plant material and a solvent, at least one step of extraction of the active compounds, and finally spray-drying of the resulting extract on to the same plant material as that used to produce the extract.3 This plant material is then crushed to form the ipowder®. The spray-drying procedure yields a final product enriched in active substances and also guarantees the presence of all the active compounds contained in the original plant material used. In contrast, the composition of dry plant extracts prepared by conventional methods generally differs from the totum because these extracts are dried on an inert support (such as maltodextrin, starch etc.) or without using any support.
The objective of this study was to show that the ipowder® technology: (1) concentrates certain active ingredients while preserving all the active compounds contained in the original plant material without altering these; (2) enhances their biological effects; and (3) increases the availability of the active compounds for intestinal absorption during digestion. For this purpose, we applied the process to leaves of Melissa officinalis L., also known as lemon balm, a medicinal plant that has long been used in traditional medicine. M. officinalis has sedative, spasmolytic, and hypotensive properties, as well as fever-reducing, thyroid-related, antiviral and antioxidant activities, and has been shown to be of benefit against asthma, heart failure, ulcers and wounds.4–6M. officinalis is also used for the symptomatic treatment of mild gastro-intestinal complaints, including bloating and flatulence,7 and for the symptomatic treatment of digestive disorders such as minor spasms.8 Extracts of M. officinalis leaves contain substances belonging to various chemical classes, such as triterpenes, flavonoids and polyphenolic compounds.9 The major compound present in leaf extracts, identified as rosmarinic acid (RA), is commonly used as a marker.9
To show the benefits of the ipowder® technology, we determined the phytochemical fingerprint of the M. officinalis ipowder® (hereafter referred to as ipowder®), RA concentration, antioxidant activity and release of RA during in vitro digestion (using the TIM-1 model that mimics the human digestion process), and compared the characteristics and properties of this ipowder® to those of M. officinalis ground dry leaves.
2. Materials and methods
2.1. Plant collection, identification and extraction
The leaves of M. officinalis were collected in Aubiat (France) in June 2015 and identified by Gilles Thébault from the herbarium of the Museum d'Histoire Naturelle Henri-Lecoq (Clermont-Ferrand, France) in which a voucher specimen was deposited (CLF106452). This herbarium is registered with the International Association for Plant Taxonomy, the head office of which is located in the New York botanical garden.The extract of M. officinalis tested (Lemon balm ipowder®, PiLeJe Industrie, France3) is obtained by extraction of 1 kg of M. officinalis cut dry leaves in 10 L of water at 85 °C for 30 minutes. After filtration, the resulting extract is concentrated under vacuum (native extract ratio [NER]: 5 to 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), then fixed and dried on 0.5 kg of M. officinalis cut dry leaves (impregnation support) under reduced pressure (drug extract ratio [DER]: 2 to 4
1), then fixed and dried on 0.5 kg of M. officinalis cut dry leaves (impregnation support) under reduced pressure (drug extract ratio [DER]: 2 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The enriched plant material is finally crushed to form the ipowder® and filled into capsules.
1). The enriched plant material is finally crushed to form the ipowder® and filled into capsules.
2.2. Chromatographic fingerprinting
All chemicals were purchased from Sigma Aldrich (USA).Samples were obtained from the analytical extraction of 1 g of M. officinalis ground dry leaves or ipowder® in 100 mL of different solvents (water, ethanol/water: 50/50 v/v [50% ethanol] or methanol). After a 15 min sonication at room temperature, the samples were filtered. The RA standard solution was prepared from 10 mg of RA in 200 mL of 50% ethanol and sonicated for 5 min.
Test solutions (10 μL) and RA standard solution (8 μL) were applied on 8 mm bands, 8 mm from the lower edge of the plate. The mobile phase was a mixture of ethyl acetate, water, acetic acid and formic acid (100/27/11/11). Plates were developed over a distance of 70 mm from the lower edge using a twin trough glass chamber saturated for 20 min with the mobile phase under controlled humidity (RH: 33%). After development, plates were dried under a stream of cool air for 10 min. The plates were heated at 100 °C for 3 min then immersed in Natural Product (NP) reagent (1 g of 2-aminoethyl diphenylborinate in 200 mL of ethyl acetate) at a speed of 5 cm s−1 with an immersion time of 0 s, then dried under a stream of cool air. The plates were immersed in polyethylene glycol (PEG) reagent (10 g of PEG 400 in 200 mL of dichloromethane) at the same speed and immersion time then dried. Digital images were taken at 366 nm.
The same HPTLC conditions were used to detect antioxidant activity. After development, the plate was immersed in a 0.5 mM methanolic 2,2-diphenyl-1-picrylhydrazyl (DPPH) solution with a speed of 3 cm s−1 and an immersion time of 5 s. The plate was dried at room temperature in the dark for 90 s and then heated for 30 s at 60 °C. The chromatogram was analysed under white light (reflectance mode).
Chromatographic analyses using ultra-high-performance liquid chromatography (UHPLC) were performed on an Ultimate 3000 RSLC UHPLC system (Thermo Fisher Scientific Inc., MA, USA) coupled to a quaternary rapid separation pump (ultimate autosampler) and a rapid separation diode array detector. Compounds were separated on an Uptisphere Strategy C18 column (250 × 4.6 mm, 5 μm, Interchim, France), controlled at 30 °C. The mobile phase was a mixture of 0.1% (v/v) formic acid in water (phase A) and 0.1% (v/v) formic acid in acetonitrile (phase B). The gradient of phase A was 100% (0 min), 80% (10 min), 73% (35 min), 0% (40–50 min) and 100% (51–60 min). The flow rate was 0.8 mL min−1, and the injection volume was 5 μL. The UHPLC system was connected to an Orbitrap (Thermo Fisher Scientific Inc., MA, USA) mass spectrometer, operated in the negative electrospray ionization mode. Source operating conditions were: 3 kV spray voltage; 320 °C heated capillary temperature; 400 °C auxiliary gas temperature; sheath, sweep and auxiliary gas (nitrogen) flow rate 50, 10 and 2 arbitrary units, respectively; and collision cell voltage between 10 and 50 eV. Full scan data were obtained at a resolution of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 whereas MS2 data were obtained at a resolution of 17
000 whereas MS2 data were obtained at a resolution of 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500. Data were processed using Xcalibur software (Thermo Fisher Scientific Inc., MA, USA).
500. Data were processed using Xcalibur software (Thermo Fisher Scientific Inc., MA, USA).
2.3. Quantification of RA
M. officinalis ground dry leaves or ipowder® (200 mg of each) were extracted with 200 mL of 50% ethanol and the extracts were sonicated for 1 h at room temperature, protected from light. The extracts were then filtered and diluted with the same solvent to 200 mL. The resulting solutions were then filtered through 0.45 μm syringe PTFE filters.The RA standard solution was prepared from 22.4 mg of RA in 50 mL of a mixture of 50% ethanol and 50% water, acidified by the addition of 2% glacial acetic acid (ethanol/acidified water). The mixture was then sonicated for 5 min. The RA calibration curve was obtained by analysing samples containing 25 to 400 mg L−1 of RA in the same solvent. The solutions were filtered through a 0.45 μm PTFE membrane filter prior to injection. All assays to determine the quantity of RA in the ground dry leaves and ipowder® were carried out in triplicate.
2.4. DPPH* method
The DPPH* free radical scavenging method allows evaluation of the antioxidant potential of a compound or an extract. Analyses were performed using a microplate reader (Tecan M Nano – Infinite 200 pro, Lyon, France) at 515 nm and flat, transparent 96-well plates with lids (Greiner, Kremsmunster, Austria).For sample preparation, 0.5 g of M. officinalis ground dry leaves or ipowder® were extracted with 45 mL of 50% ethanol, under sonication for 30 min at room temperature. The extracts were then filtered, the volume was completed to 50 mL and finally diluted 75 times with the same solvent. Standard solutions of Trolox (2.35 to 150 μM) were prepared in methanol.
Aliquots of the standard or sample solutions (50 μL) were introduced into the wells with 250 μL of DPPH* solution (79 μM in methanol). The plates were kept for 30 min in the dark at room temperature. The absorbance (Abs) was measured at 515 nm and converted into the percentage of inhibition of DPPH* radical using the following formula:
A DPPH* solution (100 μL) in methanol (100 μL) was used as the control (Abs control). All assays were performed in triplicate and results were reported as the mean ± standard deviation (SD). DPPH* scavenging activities were expressed as mg of Trolox equivalents per g of samples using a linear regression curve.
2.5. In vitro gastro-intestinal digestion
The TNO gastro-Intestinal Model (TIM-1) is a multi-compartment, dynamic and computer-controlled system developed at TNO Nutrition and Food Research (Zeist, the Netherlands) to reproduce the digestive process occurring in the luminal part of the human upper digestive tract, as previously described.11–13 Briefly, the system consists of four successive compartments simulating the stomach, duodenum, jejunum and ileum. It enables control of the main mechanical, physical and biochemical parameters of digestion such as pH, temperature, peristaltic movements, volumes, transit times of the chyme, digestive secretions and passive absorption of water, salts and soluble small compounds through hollow fibres that continuously dialyse the jejunal and ileal contents. The TIM-1 system was set to simulate the digestion of a glass of water in a healthy human adult for a total duration of 4 h (Table 1).| Compartment | Volume (mL) at initial time | pH/time (min) | Secretions | T (min); β coefficienta |
|---|---|---|---|---|
| a Mathematical modelling of gastric and ileal deliveries with power exponential equation was used for the computer control of chyme transit: f = 1–2−(t/T)↑β where f represents the fraction of the meal delivered, t the time of delivery, T the half-time of delivery and β a coefficient describing the shape of the curve. | ||||
| Stomach | 10 gastric residue | 1.8/0; 6.0/1; 3.2/10; 2.4/20; 1.8/40; 1.6/60; 1.5/240 | Pepsin: 130 IU min−1 | 20; 1 |
| 200 water | Lipase: 7.5 IU min−1 | |||
| 0.5 M HCl when necessary | ||||
| Duodenum | 50 | Maintained at 6.4 | Bile extract (porcine): 40 mg min−1 on 0–25 min, then 20 mg min−1 | |
| Pancreatin 4USP: 22.3 mg min−1 | ||||
| 0.5 M NaHCO3 when necessary | ||||
| Jejunum | 130 | Maintained at 6.9 | 0.5 M NaHCO3 when necessary | |
| Jejunal dialysis | 10 mL min −1 | 5 mM K-phosphate buffer pH 6.9; NaCl 5 g L −1 ; CaCl 2 , 2H2O 0.2 g L−1 | ||
| Ileum | 130 | Maintained at 7.2 | 0.5 M NaHCO3 when necessary | 150; 2.4 |
| Ileal dialysis | 10 mL min −1 | 5 mM K-phosphate buffer pH 7.2; NaCl 5 g L −1 ; CaCl 2 , 2H2O 0.2 g L−1 | ||
In this model, we compared the amount of RA released from a specified quantity of ipowder® with that released from the total quantity of ground dry plant leaves required to produce this quantity of ipowder®. As described in section 2.1, the ipowder® is produced by spray-drying of an extract obtained from two parts of M. officinalis leaves on one part of M. officinalis leaves meaning that one part of ipowder® is obtained from three parts of raw plant material. HydroxyPropylMethylCellulose (HPMC) capsules size 00 were filled with 300 mg of M. officinalis ground dry leaves or ipowder®. One capsule of ipowder® or three capsules of leaves were digested with 200 mL of mineral water (Volvic®, Volvic, France) in the TIM-1 system, in triplicate. During digestion, cumulated dialysis fluids (jejunal and ileal dialysates) and cumulated ileal effluents were regularly collected. Volumes were measured and samples were stored at −20 °C for downstream analysis of RA concentration. After 4 h, the final gastro-intestinal contents of the four compartments of the TIM-1 system were pooled and a sample was also frozen for analysis.
3. Results and discussion
3.1. Fingerprint
M. officinalis ground dry leaves and ipowder® were extracted with three different solvents (methanol, water and 50% ethanol) in order to determine the best analytical extraction conditions for studying their fingerprints by HPTLC. The use of 50% ethanol allowed optimal extraction of phenolic compounds (Fig. 1a, lanes 3 and 6). The comparison between ground dry leaves and ipowder® profiles showed that the ipowder® fingerprint was equivalent to that of the ground dry leaves but that the concentration of secondary metabolites was higher in the ipowder®. The ipowder® technology therefore preserves the fingerprint of ground dry leaves and concentrates secondary metabolites.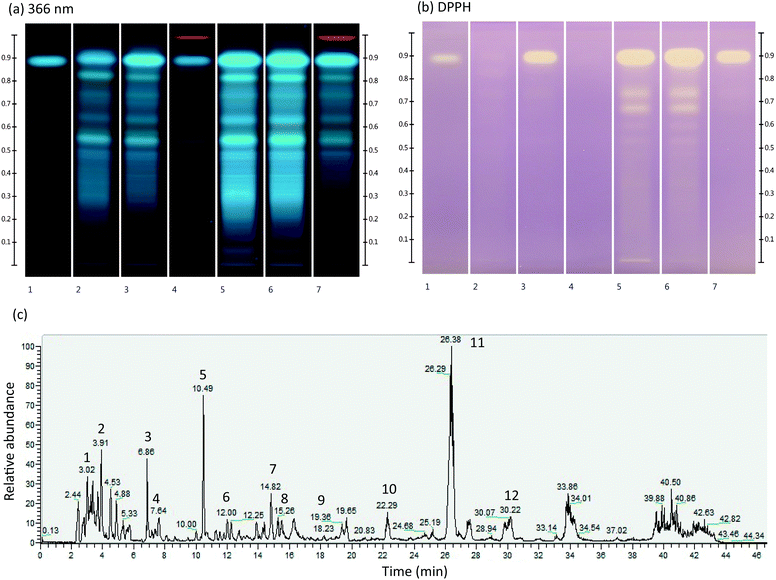 | ||
| Fig. 1 (a) and (b) Chromatographic fingerprint of analytical extracts of M. officinalis ground dry leaves (lanes 2–4) and ipowder® (lanes 5–7) with three extraction solvents (water: lanes 2 and 5; 50% ethanol: lanes 3 and 6; methanol: lanes 4 and 7) at 366 nm (a) and with DPPH* method (b). Lane 1: rosmarinic acid standard. (c). LC/MS spectrum of ipowder® (see Table 2 for signal identification). | ||
The immersion of a second HPTLC plate in DPPH* (Fig. 1b) showed that both ground dry leaves and ipowder® have anti-oxidant activities with RA as the major marker of this activity (upper white spots on lanes 1, 3, 5, 6, 7). HPTLC analysis showed the presence of various polyphenol derivatives including RA. As previously described, RA was the major compound detected in the extracts of M. officinalis analysed.9,14,15
The phytochemical profile of ipowder® was determined by LC/MS analyses in the negative ionization mode using the optimal extraction solvent (i.e. 50% ethanol; Fig. 1c and Table 2). The LC/MS spectrum showed the presence of RA (Fig. 1c, signal 11; rt: 26.38 min M–H: 359.0773). Other compounds identified included RA derivatives such as danshensu (Fig. 1c, signal 5, M–H: 197.0450), and 3′-O-(8′′-Z-caffeoyl) rosmarinic acid (Fig. 1c, signal 12, M–H: 537.1042) and flavones such as luteolin 3′-O-β-D-glucuronide (Fig. 1c, signal 9, M–H: 461.0732).
| Peak | Retention time (min) | Molecular ion [M–H]− (m/z) | Formula | MS2 (m/z) | Compounds | Ref. |
|---|---|---|---|---|---|---|
| 1 | 3.68 | 149.0076 | C4H6O6 | 149/87/72/59/103 | Tartaric acid | Standard |
| 2 | 3.91 | 191.0549 | C7H12O6 | 191/85/127 | Quinic acid | Standard |
| 3 | 6.86 | 191.0192 | C6H8O7 | 111/87/85/191 | Citric acid | Standard |
| 4 | 7.64 | 117.0182 | C4H6O4 | 73/117/99 | Succinic acid | 16 |
| 5 | 10.49 | 197.0450 | C9H10O5 | 72/135/123/179 | Danshensu | 17 |
| 6 | 12.00 | 311.0411 | C13H12O9 | 149/179/135/87 | Caftaric acid | 18 |
| 7 | 14.82 | 179.0341 | C9H8O4 | 135 | Caffeic acid | Standard |
| 8 | 16.04 | 537.1046 | C27H22O12 | 295/179/135/121/493 | Lithospermic acid A | 19 |
| 9 | 19.47 | 461.0732 | C21H18O12 | 285 | Luteolin 3′-O-β-D-glucuronide | 20 |
| 10 | 22.29 | 719.1618 | C36H32O16 | 161/359/197/179/135/341/133 | Sagerinic acid | 21 |
| 11 | 26.38 | 359.0773 | C18H16O8 | 161/197/179/135 | Rosmarinic acid | Standard |
| 12 | 30.22 | 537.1042 | C27H22O12 | 161/135/359/179/197 | 3′-O-(8′′-Z-Caffeoyl) rosmarinic acid | 22 |
3.2. Quantification of RA by HPLC and HPTLC
The quantity of RA in M. officinalis ground dry leaves and ipowder® was measured by HPLC and HPTLC (Table 3). HPTLC allows rapid and efficient quantification of markers. RA was quantified using three different plates and five standard concentrations.| M. officinalis leaves | ipowder® | |
|---|---|---|
| mg of RA per 100 mg of dried raw material | ||
| Measured by HPTLC | 1.45 ± 0.10 | 2.62 ± 0.05 |
| Measured by HPLC | 1.34 ± 0.07 | 2.76 ± 0.05 |
| % scavenging activity | 21.6 ± 0.6 | 44.9 ± 1.8 |
| Trolox equivalent (mg g −1 sample) | 291 ± 10 | 618 ± 23 |
Both HPTLC and HPLC analyses showed that the ipowder® contained a higher concentration of RA than the ground dry leaves. The concentration of RA detected in the ipowder® was 2.62 ± 0.05 mg of RA per 100 mg of dried raw material by HPTLC and 2.76 ± 0.05 mg by HPLC. These concentrations were almost twofold higher than in the ground dry leaves (1.45 ± 0.10 mg of RA per 100 mg of dried raw material by HPTLC and 1.34 ± 0.07 mg by HPLC). It is worth noting that the two quantification methods resulted in the detection of similar concentrations of RA. The ratio of 1.8 between the RA concentrations in the ipowder® and in the ground dry leaves detected with HPTLC was equivalent to the ratio of 2.1 obtained with HPLC.
3.3. Antioxidant activity
Antioxidant activity was evaluated using the DPPH* test, based on the ability of metabolites to donate a hydrogen atom or electron to the stable radical DPPH*. The assay evaluates the ability of M. officinalis extracts to scavenge free radicals in solution. We also assessed the Trolox equivalent antioxidant capacity of the extracts.The ipowder® exhibited a scavenging activity that was twofold greater than that of the ground dry leaves (Table 3). A similar result was obtained for the Trolox equivalent.
Altogether these results showed that the antioxidant activity of ground dry leaves and ipowder® was correlated with the concentration of RA: with a RA concentration twice as high as in ground dry leaves, the ipowder® had a scavenging activity two-fold greater.
3.4. Release of RA during in vitro gastro-intestinal digestion
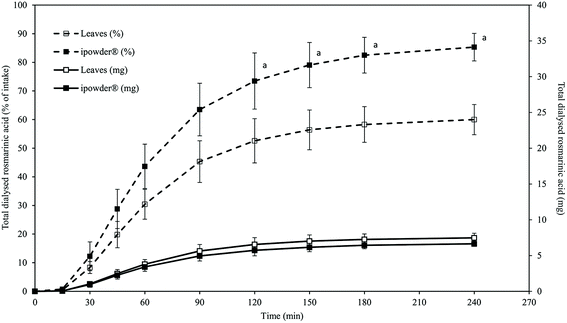
The ability of M. officinalis ipowder® to release RA into the gastro-intestinal tract (GIT) was compared to that of M. officinalis ground dry leaves (plant equivalent) using the TIM-1 model, a dynamic computer-controlled system simulating the main spatio-temporal digestive parameters occurring into the upper GIT of humans. The TIM-1 system is a relevant in vitro model previously used to study the release of chemical compounds from food matrices and drugs;23,24 it was used for the first time to investigate a plant extract in this study.RA release and dialysis from the two preparations were similar as no difference in the kinetic profiles and total quantities dialysed were observed (Fig. 2 and Table 4). This observation is not due to system saturation since other studies have shown that higher amounts of solubilized substances can be dialysed in the TIM-1 model.24 Moreover, we observed in a preliminary digestion on a dry extract of M. officinalis leaves (60–80% native extract, 30% ethanol) that about 12 mg of RA for an ingested quantity of 13.3 mg was solubilized and dialysed in the TIM-1 system (data not shown). One capsule of ipowder® (300 mg) therefore released the same amount of RA as three capsules containing M. officinalis ground dry leaves (900 mg in total). This result is of great interest as it shows that the novel ipowder® extraction process will enable reduction of the recommended daily intake of this herbal product.
| M. officinalis ground dry leaves (3 capsules) | ipowder® (1 capsule) | |||
|---|---|---|---|---|
| Total RA ± SD (mg) | % of intake ± SD | Total RA ± SD (mg) | % of intake ± SD | |
| a Statistically significant difference between ipowder® and ground dry leaves (student t-test, with a probability level of P < 0.05 considered to be statistically different). | ||||
| Intake | 12.44 ± 0.02 | 100 | 7.79 ± 0.10a | 100 |
| Cumulated dialysates | 7.5 ± 0.7 | 60.0 ± 5.3 | 6.6 ± 0.4 | 85.3 ± 4.8a |
| Cumulated ileal effluents | 0.07 ± 0.02 | 0.6 ± 0.2 | 0.18 ± 0.06a | 2.3 ± 0.7a |
| Final GIT content | 0.27 ± 0.04 | 2.2 ± 0.3 | 0.25 ± 0.24 | 3.2 ± 3.1 |
| Total release | 7.8 ± 0.6 | 62.7 ± 5.1 | 7.1 ± 0.5 | 90.8 ± 6.5a |
In both cases, RA was mainly delivered in the proximal part of the GIT since 83 and 84% of RA from the ground dry leaves and the ipowder®, respectively, were recovered in the jejunal dialysates (data not shown). Only a small quantity of free RA was recovered in the cumulative ileal effluents during the 4 h of digestion, and at the end of digestion in the residual contents of the TIM-1 compartments, suggesting that RA was rapidly released from both formulations during digestion (Table 4).
However, compared to the total amount of RA contained in each product ingested, the percentage of recovery of the free biomarker was significantly higher for the ipowder® than for the ground dry leaves (90.8 ± 6.5% for ipowder® vs. 62.7 ± 5.1% for the leaves). RA therefore seems to be more easily released from the ipowder® than from ground dry leaves during digestion and consequently more available for intestinal absorption. This is most likely due to the pre-extraction of RA (and other active compounds) in the case of the ipowder®. It should be noted that other compounds, in particular caffeic acid and danshensu with a chemical structure similar to that of RA, were also detected in the dialysates (data not shown).
4. Conclusion
The results of this study, focusing on M. officinalis ipowder®, demonstrate that the novel extraction technology based on a plant extract that is concentrated then fixed and dried on the source plant material gives a phytochemical fingerprint similar to that of the original plant material but with a higher concentration of RA, one of the main biologically active compounds of M. officinalis. We identified various chemical compounds (both phenolic and flavonoid) and quantified RA using two different methods: HPTLC and HPLC. These two methods gave comparable results, showing that the concentration of RA was two-fold higher in the ipowder® than in the ground dry leaves. Using the DPPH* method, we also demonstrated that the antioxidant properties of M. officinalis were improved in the ipowder® form due to the higher RA concentration. The absorption of RA during in vitro digestion, and possibly that of other compounds, was also improved with the ipowder® due to the higher percentage of release of the biologically active substance from this formulation. We have chosen to carry out our experiments under the simplest conditions, i.e. with water, because it is a usual way for patients to ingest food supplements. Nevertheless, it would be interesting in a further work to study the impact of a complex food matrix on the release and availability for absorption of rosmarinic acid.Altogether, this study shows that the ipowder® technology preserves all the original plant compounds intact (nonselective extraction) while making some active compounds more accessible and available to exert their effects. The quantity of ingested product required to obtain the same amount of RA available for absorption was reduced by a factor of three. To obtain a given effect, the amount of M. officinalis ipowder® extract to be ingested will therefore be lower than in the case of ground dry leaves. The consequent reduction in daily dosage will result in greater convenience for the patient and will improve patient compliance with supplementation.
Conflicts of interest
Valérie Bardot, Anaïs Escalon, César Cotte, Michel Dubourdeaux are employees of PiLeJe Industrie. Isabelle Ripoche, Pierre Chalard, Lucile Berthomier, and Martin Leremboure performed the chromatographic analyses and Sylvain Denis, Monique Alric, Sandrine Chalancon performed the experiments using the TIM-1 system for PiLeJe.Acknowledgements
The authors acknowledge writing and editorial assistance provided by Claude Blondeau and Aurélie Berlin (PiLeJe Laboratoire), Lucie Etienne-Mesmin (Université Clermont Auvergne), and Vivien Paula Harry (freelance medical writer). They also thank Mustapha Aboussif, Augusto Hubaide-Nozella and Romain Barnouin (PiLeJe Industrie) for HPTLC and HPLC analyses.References
- WHO, Guidelines on Safety Monitoring of Herbal Medicines in Pharmacovigilance Systems, 2004. http://apps.who.int/medicinedocs/documents/s7148e/s7148e.pdf Search PubMed (last access February 2019).
- WHO, National policy on traditional medicine and regulation of herbal medicines - Report of a WHO global survey, 2005. http://apps.who.int/iris/bitstream/handle/10665/43229/9241593237.pdf;jsessionid=19FF7BE926FE9C22A35179A53A602822?sequence=1 (last access February 2019) Search PubMed.
- M. Dubourdeaux, Procédé de préparation d'extraits végétaux permettant l'obtention d'une nouvelle forme galénique, EP2080436A2, 2009. https://patentimages.storage.googleapis.com/c6/89/1e/07a71ba38bd0d5/EP2080436A2.pdf (last access February 2019).
- H. Nasri and M. Rafieian-Kopaei, Oxidative stress and aging prevention, Int. J. Prev. Med., 2013, 4(9), 1101–1102 Search PubMed.
- M. Setorki, M. Rafieian-Kopaei, A. Merikhi, E. Heidarian, N. Shahinfard, R. Ansari, H. Nasri, N. Esmael and A. Baradaran, Suppressive impact of Anethum graveolens consumption on biochemical risk factors of atherosclerosis in hypercholesterolemic rabbits, Int. J. Prev. Med., 2013, 4(8), 889–895 Search PubMed.
- M. Akhlaghi, G. Shabanian, M. Rafieian-Kopaei, N. Parvin, M. Saadat and M. Akhlaghi, Citrus aurantium blossom and preoperative anxiety, Rev. Bras. Anestesiol., 2011, 61(6), 702–712 CrossRef.
- European medicine agency (EMA), Community herbal monograph on Melissa officinalis L., folium, 2013. https://www.ema.europa.eu/documents/herbal-monograph/final-community-herbal-monograph-melissa-officinalis-l-folium_en.pdf (last access February 2019) Search PubMed.
- European scientific cooperative on phytotherapy (ESCOP), ES-COP monograph on Melissae folium, Exeter, England, 2013 Search PubMed.
- A. Shakeri, A. Sahebkar and B. Javadi, A review of its traditional uses, phytochemistry and pharmacology, J. Ethnopharmacol., 2016, 188, 204–228 CrossRef CAS.
- S. A. Coran, S. Mulas and N. Mulinacci, Crucial aspects of high performance thin layer chromatog-raphy quantitative validation. The case of determination of rosmarinic acid in different matrices, J. Chromatogr. A, 2012, 1220, 156–161 CrossRef CAS.
- D. Dupont, M. Alric, S. Blanquet-Diot, G. Bornhorst, C. Cueva, A. Deglaire, S. Denis, M. Ferrua, R. Havenaar, J. Lelieveld, A. R. Mackie, M. Marzorati, O. Menard, M. Minekus, B. Miralles, I. Recio and P. van den Abbeele, Can dynamic in vitro digestion systems mimic the physiological reality?, Crit. Rev. Food Sci. Nutr., 2018, 83, 1–17 Search PubMed.
- M. Minekus, P. Marteau, R. Havenaar and J. Huis in't Veld, A multicompartmental dynamic computer-controlled model simulating the stomach and the small intestine, Altern. Lab. Anim., 1995, 23, 197–209 Search PubMed.
- L. Lvova, S. Denis, A. Barra, P. Mielle, C. Salles, C. Vergoignan, C. Di Natale, R. Paolesse, P. Temple-Boyer and G. Feron, Salt release monitoring with specific sensors in “in vitro” oral and digestive environments from soft cheeses, Talanta, 2012, 97, 171–180 CrossRef CAS PubMed.
- A. Heitz, A. Carnat, D. Fraisse, A.-P. Carnat and J.-L. Lamaison, Luteolin 3′-glucuronide, the major flavonoid from Melissa of-ficinalis subsp. officinalis, Fitoterapia, 2000, 71(2), 201–202 CrossRef CAS.
- L. Barros, M. Dueñas, M. I. Dias, M. J. Sousa, C. Santos-Buelga and I. C. Ferreira, Phenolic profiles of cultivated, in vitro cultured and commercial samples of Melissa officinalis L. infusions, Food Chem., 2013, 136(1), 1–8 CrossRef CAS PubMed.
- MassBank of North America (MoNa), Quinate. Retrieved from http://mona.fiehnlab.ucdavis.edu/spectra/browse?query=splash.splash%3D%3D%22splash10-0006-0900000000-d2ce95c3b5d8693983f3%22&text= (last access February 2019).
- X. Wang, W. Li, X. Ma, K. Yan, Y. Chu, M. Han, S. Li, H. Zhang, S. Zhou, Y. Zhu, H. Sun and C. Liu, Identification of a major metabolite of danshensu in rat urine and simultaneous determination of danshensu and its metabolite in plasma: Application to a pharmacokinetic study in rats, Drug Test. Anal., 2015, 7(8), 727–736 CrossRef CAS.
- H.-J. Chen, B. S. Inbaraj and B.-H. Chen, Determination of phenolic acids and flavonoids in Taraxacum formosanum Kitam by liquid chromatography-tandem mass spectrometry coupled with a post-column derivatization technique, Int. J. Mol. Sci., 2012, 13(1), 260–285 CrossRef CAS.
- S. Wang, L. Liu, L. Wang, Y. Hu, W. Zhang and R. Liu, Structural characterization and identification of major constituents in Jitai tablets by high-performance liquid chromatography/diode-array detection coupled with electrospray ionization tandem mass spectrometry, Molecules, 2012, 17(9), 10470–10493 CrossRef CAS.
- A. Tenfen, D. A. Siebert, D. Spudeit, C. M. Mendes de Cordova, G. A. Micke and M. Debiasi Alberton, Determination of phenolic profile by HPLC-ESI-MS/MS and antibacterial activity of Eugenia platysema against mollicutes strains, J. Appl. Pharm. Sci., 2017, 7(05), 007–011 CAS.
- MassBank of North America (MoNa), Sagerenic acid. http://mona.fiehnlab.ucdavis.edu/spectra/splash/splash10-03di-0902000000-1a1faddf80089d58bfe8 (last access February 2019).
- O. R. Pereira, A. M. Peres, A. M. Silva, M. R. Domingues and S. M. Cardoso, Simultaneous characterization and quantification of phenolic compounds in Thymus x citriodorus using a validated HPLC–UV and ESI–MS combined method, Food Res. Int., 2013, 54(2), 1773–1780 CrossRef CAS.
- S. Blanquet-Diot, M. Soufi, M. Rambeau, E. Rock and M. Alric, Digestive stability of xanthophylls exceeds that of carotenes as studied in a dynamic in vitro gastrointestinal system, J. Nutr., 2009, 139(5), 876–883 CrossRef CAS.
- S. Souliman, E. Beyssac, J.-M. Cardot, S. Denis and M. Alric, Investigation of the biopharmaceutical behavior of theophyl-line hydrophilic matrix tablets using USP methods and an arti-ficial digestive system, Drug Dev. Ind. Pharm., 2007, 33(4), 475–483 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |