Effects of flocculant-modified phosphogypsum on sludge treatment: investigation of the operating parameters, variations of the chemical groups, and heavy metals in the sludge†
Quxiu
Dai
a,
Longgui
Xie
a,
Liping
Ma
 *a,
Jie
Yang
b,
Xinbo
Yang
c,
Nanqi
Ren
d,
Guocai
Tian
e,
Zhiying
Guo
a and
Ping
Ning
*a
*a,
Jie
Yang
b,
Xinbo
Yang
c,
Nanqi
Ren
d,
Guocai
Tian
e,
Zhiying
Guo
a and
Ping
Ning
*a
aFaculty of Environmental Science and Engineering, Kunming University of Science and Technology, Kunming 650500, Yunnan, China. E-mail: lipingma_kmust@163.com; ningping1958@163.com; Fax: 86 871 5170906; Tel: 86 871 5170905
bCollege of Resources and Environment, Chengdu University of Information Technology, Chengdu 610225, Sichuan, China
cYunnan Tianlang Energy Saving & Environmental Protection Group Co. Ltd., Yunnan, China
dState Key Laboratory of Urban Water Resources and Environment, School of Environment, Harbin Institute of Technology, Harbin 150090, China
eFaculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650500, Yunnan, China
First published on 4th November 2020
Abstract
Under a modified phosphogypsum (MPG) treatment, the effective removal of bound water and risky heavy metals from sludge has been achieved. In this paper, the effects of the operating parameters on sludge treatment, variations of the active groups in the sludge, and the heavy metals' mobility risk were investigated. The results showed that adding 40–50% DS (mass of dry solid in sludge) of MPG could directly remove approximately 62% of bound water from the sludge. Moreover, about 23.96% of CdF1/F2, 39.92% of CrF1/F2, 21.21% of ZnF1/F2, 35.49% of NiF1/F2, and 78.61% of AsF1/F2 were removed from the sludge due to a transition of risky heavy metals (metal-F1/F2) to stable heavy metals (metal-F3/F4/F5). Simultaneously, organic chelation was more effective in stabilizing Cd, Cr, Zn, and As. Additionally, the flocs entrainment can significantly improve the stabilization of Ni and Pb. In addition, the risky model of heavy metals showed that a higher oxidization performance of the sludge and less loosely bound extracellular polymeric substances (LB-EPS) content was propitious to the immobilization of Cd, Cr, Zn, Ni, and As. Nevertheless, the environmental risk of Cu and Pb varied with the addition of MPG. These results indicate the potential of this method for the synergetic removal of bound water and risky heavy metals from sludge.
Water impactThis paper aims to study the co-removal of bound water and risky heavy metals in sludge, which can promote a synergistic sludge reduction–detoxification. In particular, compared with the commonly used method, the method is deemed to be cost-saving, resource-saving, and environment-friendly. This novel method may provide a late-model co-reduction/detoxification theory, and may be adopted in wastewater treatment with a new perspective. |
1. Introduction
Municipal sludge generally including more than 99% of water is a solid substance produced in the treatment process of sewage in wastewater treatment plants.1 Generally, sludge could be used to produce fertilizer and building materials after dewatering.2 Besides, sludge burning to provide heat and generate electricity is a kind of frequently used utilization method.1 However, the presence of a large amount of water in sludge has largely hindered the storage, transport, and subsequent treatment of sludge.3 Furthermore, some toxic substances, such as organics, heavy metals, and parasitic ovum, in sludge seriously hinder sludge disposal because these components cause disease, pollute groundwater, and pollute soil.4 Consequently, reducing the sludge volume and weakening its toxicity have become important pretreatment steps for the sludge disposal.5In a variety of sludge dewatering technologies, chemical agents are commonly used in sludge treatment.6 Normally, inorganic flocculants and organic polymer flocculants, which can significantly destroy the negatively charged sludge colloid network and promote the release of extracellular polymeric substances, bound water, and heavy metals wrapped in the network, are used for sludge dewatering.7 Wei and co-workers mentioned how free nitrous acid resulted in a 16–17% reduction in the volume of dewatered sludge by destroying volatile solids in an anaerobic sludge digester.7 Besides, a low frequency CaO-ultrasonic pretreatment could improve sludge dewaterability though increasing concentrations of SCOD and VFAs in treated sludge.8,9 He and co-workers reported that a combined conditioner of Fenton's reagent and a cationic surfactant may largely improve sludge dewatering by changing the electrical property of sludge particles.10 However, many drawbacks in using organic agents or inorganic agents have gradually been revealed. Polyacrylamide, which is commonly used for sludge dewatering, may naturally degrade into acrylamide, which can be easily absorbed by the body through the skin, mucous membrane, respiratory tract, and mouth, poisoning humans and other animals.11 Furthermore, Joceline and co-workers demonstrated that aluminum polychloride used for sludge will enrich the residual aluminum, which can accumulate in human tissues, such as blood, muscle, and bone, resulting in biological lesions.12
In view of sludge toxicity (i.e., heavy metal accumulation and migration, parasitic egg-borne diseases), several preparation methods for heavy metal stabilizers have been reported.1,13,14 The principles of metal stabilizers include: (1) complexing heavy metals to form stable complexes and (2) chelating heavy metals to form a precipitate.13 Chitosan was treated with carboxymethylation, then mixed with activated carbon, and then grafted with thiourea, finally producing a sludge-treating agent that could not only stabilize Cd, Ni, and Zn, but could also improve sludge dewatering.14 Besides, it was reported that a combined addition of hydrochloric acid solution, ferrous sulfate, and hydrogen peroxide solution could dissolve unstable heavy metals, reducing its content in sludge.13 Therefore, flocculants that can reduce the sludge volume and weaken the environmental risk of heavy metals can be produced by several specific methods. At present, flocculants are prepared using fresh materials such as organic polymer agents, aluminum chloride, and ferric chloride, which need larger materials consumption and incur a higher cost.13 In order to reduce the costs and weaken environmental pollution, massive wastes that can be recycled are considered for used in the preparation of sludge-treating agents, making use of its unique composition or structure to prepare highly efficient flocculants for sludge treatment. This can not only promote waste utilization, but also boost sludge reduction and sludge detoxification.
Phosphogypsum (PG), which is widely used as a building material in the industrial field, is a solid by-product from the wet production process of phosphoric acid.14,15 In agriculture, phosphogypsum is used as a soil fertilizer due to its high levels of Ca, Al, and Si, and its porous structure.16 In recent studies, several minerals with a porous structure are frequently prepared for sludge treatment.17 According to Horpibulsuk and co-workers, clinoptilolite modified by the cationic surfactant cetyl trimethyl ammonium bromide could produce a sludge-treating agent that was recognized to be efficient for sludge dewatering.18 In view of the mineral structure and components of phosphogypsum, it is feasible to use it for sludge treatment.
In our previous work, phosphogypsum was used to produce a sludge-treating chemical (noted as MPG).19,20 Given the effects of the operating parameters on sludge treatment, systematic investigations are needed on the migration rules of heavy metals in sludge, and also the co-reduction of sludge volume and toxicity with the addition of MPG are not yet completely understood; thus the objectives of this research were: (1) to investigate the parameters for sludge treatment; (2) to analyze the effects of the variation of the active groups in the treated sludge filtrate and filter cake; (3) to reveal the effect of MPG on the migration rules of heavy metals in sludge; (4) to further evaluate the mobility risk of heavy metals in sludge, and to identify the model of heavy metal pollutants transport; and finally to explore the effects of MPG on the co-reduction of the sludge volume and toxicity.
2. Materials and methods
2.1 Materials
Sludge samples used in this study were collected from the Kunming domestic sewage treatment plant (Kunming, China). Sludge samples were kept in refrigerated bucket at 277.15 K and used within three days. The properties of raw sludge were as follows: the moisture content was 99 ± 0.5% (weight ratio), the specific resistance of filtration (SRF) was 2.248 × 1013 m kg−1, the zeta potential was −16 mA, and the content of volatile suspended solids was 82%. PG was supplied by Furui chemical of YUNTIANHUA (Kunming, China). In this paper, MPG was produced from PG and the modifier octadecyl dimethyl benzyl ammonium chloride, and its main mineral components were CaSO4, Al2O3, and SiO2.2.2 Experiments
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.004. Besides, variations of the chemical groups and positive charge of the product before and after modification were investigated to evaluate the necessity for modification.
0.004. Besides, variations of the chemical groups and positive charge of the product before and after modification were investigated to evaluate the necessity for modification.
Type 1#: the sludge pretreatments were performed in glass barrels at ambient temperature with variable operating parameters: (1) agitation speed (250, 500, 750, 1000, 1250, 1500 rpm), (2) reaction time (10, 15, 20, 25, 30, 35, 40, 50, 60 min), and (3) MPG dose (20% DS, 30% DS, 40% DS, 50% DS, 80% DS, 100% DS, 150% DS, 200% DS). The MPG dose was expressed by the mass percentage of dry solids (% DS) in sludge. When the reaction was stopped, the suspensions were filtrated immediately at −0.035 MPa for 20 min to detect the SRF, and the filter cake was used for the moisture content and moisture removal rate.
Type 2#: the sludge samples were treated under the optimum experimental conditions (agitation speed, 500 rpm; reaction time, 35 min; and DS dose, 40%), and then the suspension was filtered, and the filtrate and filter cake were used for Fourier transform-infrared (FT-IR) analysis.
Type 3#: the sludge samples were treated by different MPG doses (0% DS, 10% DS, 20% DS, 30% DS, 40% DS, 50% DS, 60% DS) at 500 rpm for 35 min. Then the suspensions were used for heavy metals extraction and detection.
Type 4#: the sludge samples were treated by different MPG doses (0% DS, 10% DS, 20% DS, 30% DS, 40% DS, 50% DS, 60% DS, 80% DS, 100% DS, 150% DS) at 500 rpm for 35 min. The sludge samples were used for thermogravimetric-differential thermal analysis (TG-DTA) to explore the water variation in sludge before and after treatment.
2.3 Analytical methods
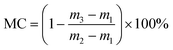 | (1) |
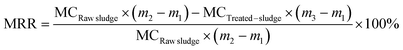 | (2) |
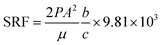 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; scan speed, 40 sheets/s; resolution, 2 cm−1; wavenumber range, 400 to 4000 cm−1; integral number, 10 times; and measurement time, 10 s.
1; scan speed, 40 sheets/s; resolution, 2 cm−1; wavenumber range, 400 to 4000 cm−1; integral number, 10 times; and measurement time, 10 s.
(1) Heavy metals' detection. The content of heavy metals (Cd, Cr, Cu, Pb, and Zn) were detected by atomic absorption spectrophotometry (Varian AA240FS, America). Besides, Ni and As were detected using inductively coupled plasma atomic emission spectrometry (ICP-AES) (Prodigy, Leeman Labs, America).
Furthermore, digestion tests of the heavy metals in the filtrate and filter cake were performed by using nitric acid, perchloric acid, sulfuric acid, hydrochloric acid, and hydrofluoric acid.13 The digestion testing of heavy metals in the filtrate proceeded as follows: 50 mL of filtrate was added into a beaker (100 mL), mixed with 5 mL of HNO3, and evaporated at low temperature to a total volume of 10 mL. Subsequently, 5 mL HNO3 and 2 mL HClO4 were added into the above beaker, and the mixture was continuously digested to about 1 mL. Then, the residual solution was cooled, and distilled water was added to 50 mL. Moreover, heavy metals in the sludge filter cake were digested as follows: 0.5 g of solid sludge was added into the beaker, mixed with 5 mL of HCl, 5 mL of HF, 5 mL of HNO3, and 3 mL of HClO4, and evaporated to an approximately dry state. Then, the mixture was cooled at room temperature, and nitric acid (4%, mass ratio) was dissolved in the mixture to a total volume of 25 mL. Finally, the solution was filtered using a filter membrane (0.45 μm). The obtained filtrate was used for detecting heavy metals.
(2) Extraction of heavy metals. In order to investigate the environmental risk properties of heavy metals in sludge, fractions of heavy metals were divided into five types: exchangeable fraction (F1), weak acid soluble fraction (F2), reducible fraction (F3), oxidizable fraction (F4), and residual fraction (F5).22 As mentioned by Tessier and co-workers, a multi-step extraction method was used to extract different fractions of heavy metals, as follows.22
Step 1: in a 50 mL centrifuge tube, 1 g sludge was mixed with 8 mL MgCl2 solution (1 mol L−1), and centrifugally separated at 8000 rpm for 20 min. The supernatant was used for F1.
Step 2: the sediment from step 1 was mixed with 8 mL NaAc solution (1 mol L−1), and centrifugally separated at 3000 g for 20 min. The supernatant was used for F2.
Step 3: to the sediment produced in step 2, 20 mL HONH3Cl solution (0.04 mol L−1) and 25% HAC (by mass) were added. After shaking the mixture at a constant temperature of 85 °C (water bath) for 5 h, the mixture was then centrifuged at 3000 g for 20 min. The supernatant was collected for F3.
Step 4: into the sediment produced in step 3, 3 mL HNO3 (0.02 mol L−1) and 5 mL H2O2 (30%) were added, and the mixture was digested at 85 °C (water bath) for 2 h. Then, 3 mL H2O2 (30%) was added into the mixture, and further digested at 85 °C for 3 h. After cooling, 5 mL NH4Ac solution (3.2 mol L−1) was added into the mixture, and then, deionized water was added to 20 mL. The mixture was shaken at 20 °C for 30 min, then centrifuged at 3000 rpm for 20 min. The supernatant was used for F4, and the sediment was used for F5.
3. Results and discussion
3.1 Properties of PG before and after modification
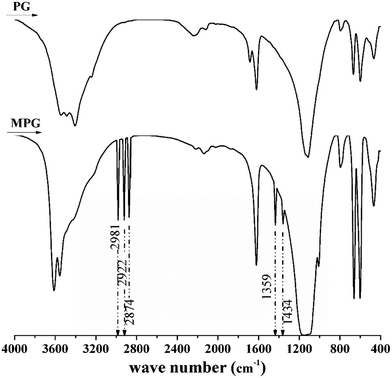
Strong absorption peaks at 2874, 2933, and 2981 cm−1 (which were assigned to the stretching vibration of –C–H) and the characteristic absorption peaks at 1434 and 1359 cm−1 (which were ascribed to the bending vibration of –C–H) were found in MPG after modification (Fig. 1).4 These indicated that –C–H from the modifier was loaded onto MPG through the modification process. After detection, it was found that the content of positive charges in MPG increased from 0 to 65% after modification. This further showed that the electrical property of the product was completely changed from negative to positive. According to Nawrocka and co-workers, those positively charged chemicals are vital to sludge dewatering.24 Thus, it was deemed that changing the charge characteristic of PG is important and essential.3.2 Effects of the operating parameters on sludge dewatering
The operating parameters, including the agitation speed, reaction time, and MPG dose, were investigated to reveal the influence of MPG on sludge dewatering, and the results are shown in Fig. 2. At 500 rpm, the moisture content of the treated sludge decreased by 35.57%, and the moisture removal rate increased by more than 11 times (Fig. 2(a)). This was mainly because MPG addition could largely destroy the stable colloid network in the sludge, and promote the release of bound water wrapped in the network, finally removing it from the sludge.5 Besides, the positive charges from MPG could neutralize those negative charges wrapped around the colloid network, and then could destroy the LB-EPS layer outside the network.19 With the destruction of LB-EPS, the stable colloid network disintegrated and released bound water to the supernatant.25 With the increase in the agitation speed, the moisture content clearly increased (Fig. 2(a)). This can be explained as due to the higher agitation speed, which will increase the viscosity of the sludge flocs by breaking the flocs into small pieces, leading to a fairly stable suspension system in the liquid phase.13 This was also mainly because the higher agitation speed will largely stretch the zooglea and polymeric organics in sludge flocs, forcing them to produce higher shear stresses, ultimately increasing the viscosity and deteriorating the sludge dewatering.26 The same results can be found from the SRF curve, which shows the filterability of the sludge.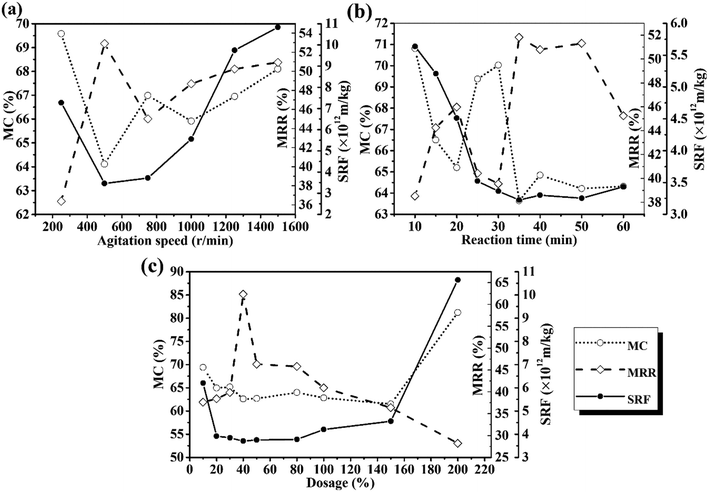 | ||
| Fig. 2 Effects of (a) agitation speed; (b) reaction time; (c) dosage on the moisture content (MC), moisture removal rate (MRR), and the SRF. | ||
Fig. 2(b) shows that, when the reaction time reached 35 min, the moisture content varied rapidly, being decreased to 63.5%. However, only minor changes occurred when increasing the reaction time. This was probably because the separation and flocculation between solid particles was quite unstable within a short time.27 Thus, 35 min was needed to complete the reaction process. Furthermore, an optimum value of the SRF of less than 3.25 × 1012 m kg−1 was also found when reacting for 35 min (Fig. 2(b)).
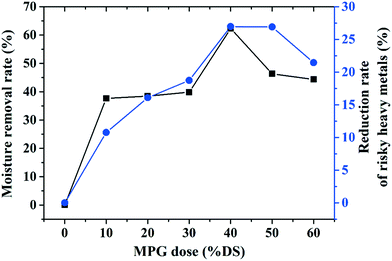
With a 40% DS MPG dosage, the moisture content and the SRF decreased by 37.11% and 83.56%, respectively. Moreover, it can be seen from the moisture removal rate curve (Fig. 2(c)) that positively charged MPG could reduce approximately 62% of the water in the sludge with the 40% DS dose. However, excessive MPG will hamper sludge dewatering (Fig. 2(c)). Improving the sludge dewatering relies on charge neutralization between the positively charged MPG and electronegative sludge flocs. Excess MPG may release additional cations, which would completely change the electrical property of the sludge.10 This will lead to re-stabilization of the sludge network, and thus make it difficult to agglomerate into larger flocs, which is crucial for sludge dewatering.3
3.3 TG-DTG analysis of the sludge dewatering
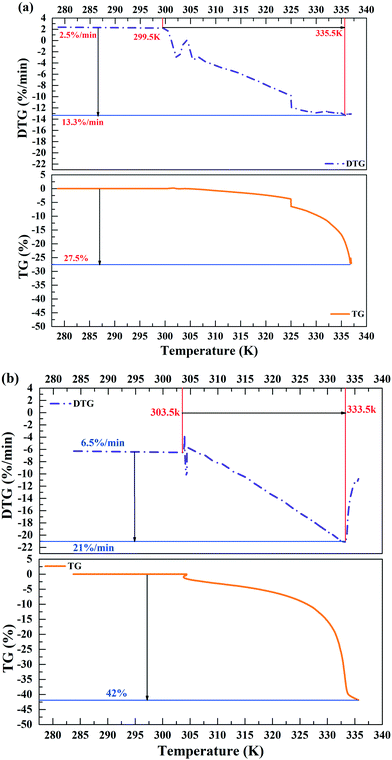
TG-DTG analysis was performed to investigate the water loss in the sludge samples before and after treatment. For raw sludge, the weight loss rate was about 27.5% when the temperature changed from 299.5 K to 335.5 K (Fig. 3(a)). However, it increased to 42% when the temperature varied from 303.5 K to 333.5 K after treatment (Fig. 3(b)). Besides, with 40% DS MPG additive, the weight loss rate was enhanced from 13.3% min−1 to 27.5% min−1 at 335.5 K (Fig. 3). These results demonstrated that MPG conditioning could change the distribution of bound water to make it easier for it to be removed from sludge.3.4 Variations of the active groups in sludge before and after treatment
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –COOH, –CHO, –C
O, –COOH, –CHO, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH, O
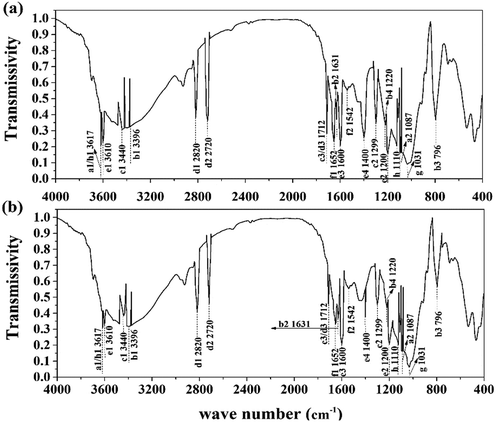
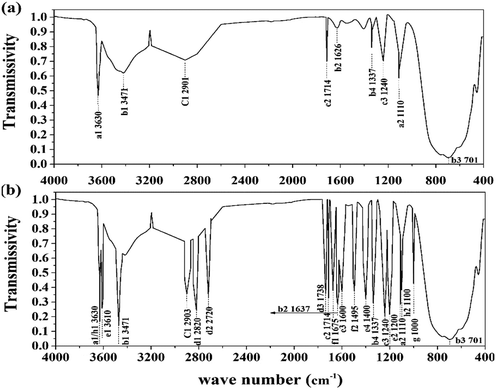
C–OH, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N, –PO4, and –SO3 were detected in the supernatant. This result verified the existence of humus, phosphates, and sulfates in the treated sludge filtrate. This was probably caused by the collapse of the LB-EPS layer in which humic substances, phosphates, and sulfates were contained. As shown in Fig. 5, the height and area for the infrared absorption peak was increased after treatment, indicating that the content of active groups in the supernatant was enhanced after treatment. Besides, as the active ligand, active groups including COO–, –NH2, –PO4, –S–, –C
C–N, –PO4, and –SO3 were detected in the supernatant. This result verified the existence of humus, phosphates, and sulfates in the treated sludge filtrate. This was probably caused by the collapse of the LB-EPS layer in which humic substances, phosphates, and sulfates were contained. As shown in Fig. 5, the height and area for the infrared absorption peak was increased after treatment, indicating that the content of active groups in the supernatant was enhanced after treatment. Besides, as the active ligand, active groups including COO–, –NH2, –PO4, –S–, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, O
O, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N can chelate risky heavy metals in the supernatant, forming an unmovable precipitate, thus further weakening its environmental risk.14 According to Sobikszołtysek and co-workers, –PO4 and –SO3 could provide a substantial number of specific and non-specific active sites for risky heavy metals to form stable complexes and organic ligands, ultimately immobilizing those unstable metals.28 Therefore, it was suggested that the massive active groups released from the sludge network to the supernatant may play an important role in the stabilization of unstable heavy metals.
C–N can chelate risky heavy metals in the supernatant, forming an unmovable precipitate, thus further weakening its environmental risk.14 According to Sobikszołtysek and co-workers, –PO4 and –SO3 could provide a substantial number of specific and non-specific active sites for risky heavy metals to form stable complexes and organic ligands, ultimately immobilizing those unstable metals.28 Therefore, it was suggested that the massive active groups released from the sludge network to the supernatant may play an important role in the stabilization of unstable heavy metals.
3.5 Migration and transformation of heavy metals
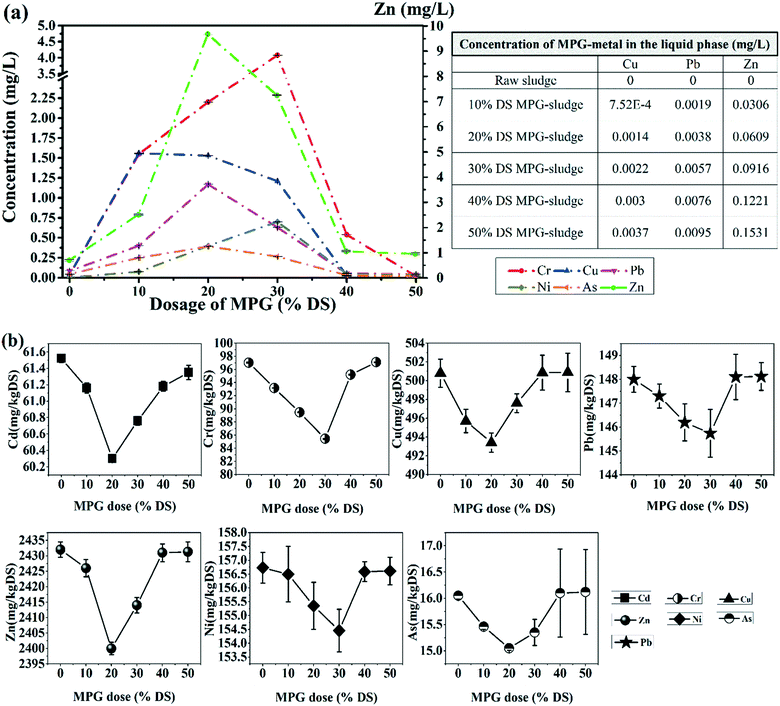
As shown in Fig. 7(a), after treatment, all the heavy metals except Cd were found in the filtrate. Besides, the content of heavy metals in the filtrate increased first and then decreased. However, the heavy metals' content in the sludge filter cake showed an opposite trend. This was probably caused by: (1) chelation between the active groups and risky heavy metals, and (2) another adsorption process in sludge flocs' flocculation.3 It can be suggested that the addition of MPG could largely immobilize heavy metals from the supernatant to the sludge filter cake.
After detection, we found that MPG would bring Cu, Pb, and Zn into the sludge (Fig. 7; Table 1). However, in the supernatant, the contents of Cu, Pb, and Zn released from MPG was tiny. In the sludge filter cake, the increment rates for the Cu, Pb, and Zn content caused by MPG were 0.48%, 3.56%, and 28.21%, respectively. Thus, MPG addition may obviously increase the Zn content in the filter cake. Nevertheless, in the preparation process of MPG, unstable heavy metals were removed from MPG due to a thorough washing operation. Thus, it can be deemed that the Cu, Pb, and Zn left in MPG were stable after modification. In other words, the Cu, Pb, and Zn brought by MPG will stably exist in the filter cake, and the mobility risk of these heavy metals caused by MPG would be negligible.
| MPG dose (% DS) | Cu | Pb | Zn |
|---|---|---|---|
| 0 | 0.0000 | 0.0000 | 0.0000 |
| 10 | 0.8076 | 1.7600 | 228.5714 |
| 20 | 1.5418 | 3.3600 | 436.3636 |
| 30 | 2.0352 | 4.4352 | 576.0000 |
| 40 | 2.4228 | 5.2800 | 685.7142 |
| 50 | 2.7354 | 5.9612 | 774.1935 |
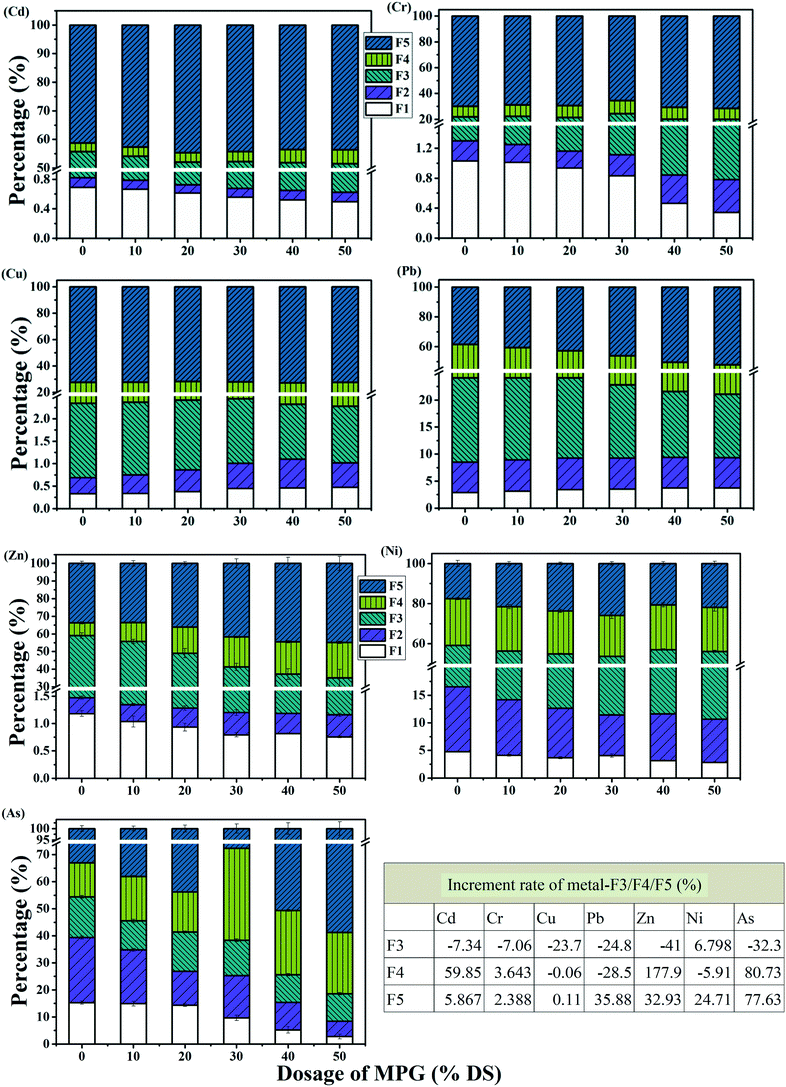
In raw sludge, Cd was presented mainly in the form of a reducible fraction (noted as Cd-F5), accounting for 54.95% of the total Cd (Fig. 8). With increasing the MPG addition, Cd-F1 and Cd-F3 decreased obviously, and moreover, Cd-F4 and Cd-F5 increased significantly (Fig. 8). The speciation distribution of Pb, Zn, and Cr showed a similar change trend to that of Cd. Obviously, MPG could change the heavy metals fraction in sludge. This was probably because the cationic MPG could partially release unstable heavy metals (F1 and F2) into the supernatant from the sludge colloid network. Furthermore, MPG could also break the LB-EPS layer wrapped in the colloid network. Additionally, several active groups in LB-EPS, such as alcohol hydroxyl, amidogen, carboxyl, and aldehyde groups, would chelate those unstable heavy metals, forming stable metal precipitates (noted as metal-F4, being produced from organics' chelating immobilization).28 Besides, in the re-accumulation of sludge flocs, those unstable heavy metals in the supernatant may be adsorbed and can then enter into the structure of sludge particles through an entrainment and bridging process, thus forming residual metals (metal-F5, being produced from flocs' entrainment immobilization).28 In particular, risky heavy metals may gather into the porous structure of MPG, which could adsorb heavy metals under electrostatic force, forming reducible metals (metal-F3, being produced from MPG adsorption immobilization).20 Thus, the MPG additive would turn metal-F1/F2 into metal-F3/F4/F5, which are environment-friendly.
However, before and after treatment, Cu still primarily presented in the form of Cu-F4 (the content of which changed from 72.38% to 72.46%). In raw sludge, Cr-F5 accounted for 69.98% of the total Cr, and As-F5 accounted for 33.08% of the total As (Fig. 8). After treatment with 50% DS MPG, Cr-F5 increased to 71.66%, and As-F5 increased to 58.77%, respectively. Furthermore, the increase in Ni-F3 (which increased from 42.61% to 45.50%) and in Ni-F5 (which increased from 17.59% to 21.93%) demonstrated that the percentage of Ni-F3/F5 was improved by treatment.
Besides, with MPG addition, the increment rate of metal-F3/F4/F5 clearly varied (Fig. 8(table)). The fractions of Cd, Cr, Zn, and As showed the same change trend, gathering massive metal-F4 in the filter cake. This showed that organics chelation may play a more important role in stabilizing Cd, Cr, Zn, and As. Ni-F3 and Ni-F5 were, respectively, enhanced by 6.80% and 24.71%; however, Ni-F4 was reduced by 5.91%. These results illustrated that the entrainment of flocs was more effective in weakening the risk from Ni. Simultaneously, Pb-F5 increased by 35.88%, indicating that risky Pb was easier to be immobilized in the inner structure of the sludge flocs. Nevertheless, irregular changes were observed in Cu-F3/F4/F5. This result may suggest that risky Cu cannot be stabilized with a MPG dose of less than 50% DS. These results may demonstrate this as a more suitable method for the stabilization of different heavy metals.
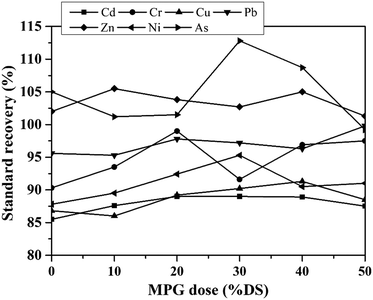
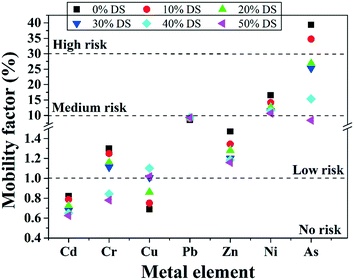
3.5.3.1 Environmental risk of heavy metals. The mobility factor represents the risk level of heavy metals: the higher the mobility factor value, the higher the risk level. With increasing the MPG dosage, the mobility factor value of all the metals except Cu obviously decreased (Fig. 9). With regard to Cr and As, the risk level decreased from low risk to no risk, while it decreased from high risk to low risk under pretreatment with 50% DS MPG. These results indicated that MPG could significantly reduce the content of metal-F1 and metal-F2, thus weakening the migration risk of the metals. Under pretreatment with 50% DS MPG, Cu-F1 and Cu-F2 increased from 0.33% to 0.48% and from 0.35% to 0.54%, respectively (Fig. 9). Accordingly, the content of the other three fractions decreased compared to that of raw sludge. Thus, with the increase in the MPG dosage, the risk level of Cu increased from no risk to low risk. Moreover, in comparison with Cd, Cr, Zn, Ni, and As, very small changes were found in the environmental risk of Pb. This was mainly because the stabilization on Pb was not only affected by the additive addition, but also by other factors, such as releasing unstable Pb from colloid network, and the immobilization efficiency of Pb-F1/F2. In addition, the risk reduction rates of Cd, Cr, Zn, Ni, and As were 23.96%, 39.92%, 21.21%, 35.49%, and 78.61%, respectively. These results demonstrated that MPG made a significant contribution to weakening the environmental risk of unstable heavy metals in sludge, especially for As, Cr, and Ni.
3.5.3.2 Environmental risk model of heavy metals. For a better understanding of the migration rules of heavy metals, the correlation between the heavy metals mobility factor (Mf) and oxidation–reduction potential (ORP), and LB-EPS content was investigated. As shown in Table 2, Mf–Cd, Mf–Cr, Mf–Cu, Mf–Pb, Mf–Zn, Mf–Ni, and Mf–As were negatively correlated with the ORP value of sludge (p < 0.05), and was found to be positively correlated with the LB-EPS content in sludge (p < 0.05).
| ORP | LB-EPS content | ||
|---|---|---|---|
| Mf–Cd | Pearson correlation | −0.995 | 0.968 |
| Significance | 0.000 | 0.001 | |
| M f–Cr | Pearson correlation | −0.944 | 0.962 |
| Significance | 0.002 | 0.001 | |
| M f–Cu | Pearson correlation | −0.953 | 0.891 |
| Significance | 0.002 | 0.009 | |
| M f–Pb | Pearson correlation | −0.917 | 0.785 |
| Significance | 0.005 | 0.032 | |
| M f–Zn | Pearson correlation | −0.970 | 0.908 |
| Significance | 0.001 | 0.006 | |
| M f–Ni | Pearson correlation | −0.958 | 0.893 |
| Significance | 0.001 | 0.008 | |
| M f–As | Pearson correlation | −0.977 | 0.982 |
| Significance | 0.000 | 0.000 |
The model summary of Mf showed that the regression coefficient (R2) of all the models was greater than 0.9. This indicated that more than 90% of changes could be explained by those models (Table 3). Meantime, the significance (p) of all the models was less than 0.05, showing that the original hypothesis that R2 was zero in this model was rejected, and the fitting effect of the model was good.
| Modela | Model summaryb | Linear regression model | ||
|---|---|---|---|---|
| Adjusted R square | Std. error of the estimate | Sig. | ||
| a Dependent variable: mobility factor. b Predictive variable: (constant), LB-EPS content, ORP. | ||||
| Mf–Cd | 0.985 | 0.00964 | 0.039 | 0.198 − 0.003 × ORP + 0.001 × CLB-EPS |
| Mf–Cr | 0.884 | 0.07314 | 0.018 | 0.320 − 0.003 × ORP + 0.021 × CLB-EPS |
| Mf–Cu | 0.865 | 0.06001 | 0.023 | 1.897 − 0.011 × ORP − 0.008 × CLB-EPS |
| Mf–Pb | 0.960 | 0.06899 | 0.004 | 10.971 − 0.039 × ORP − 0.062 × CLB-EPS |
| Mf–Zn | 0.919 | 0.03370 | 0.011 | 0.537 − 0.008 × ORP + 0.006 × CLB-EPS |
| Mf–Ni | 0.880 | 0.75291 | 0.019 | −0.447 – 0.147 × ORP + 0.114 × CLB-EPS |
| Mf–As | 0.964 | 2.20002 | 0.003 | −52.097 – 0.251 × ORP + 0.869 × CLB-EPS |
Table 3 showed that Mf of all the heavy metals decreased with increasing the ORP. The result proposed that a higher oxidation capacity of sludge may promote the stabilization of risky heavy metals. Simultaneously, in comparison with other metals, Ni and As may be more sensitive to sludge oxidizability (Table 3). This was probably caused by the formation of Ni-F3 and As-F3 with the increasing ORP. This result might provide an effective method to reduce the environmental risk of heavy metals, especially for Ni and As.
Generally, LB-EPS reduction in sludge signifies the destruction of the sludge colloid network.28 As mentioned before, under treatment, several active groups, such as –COH, –C–NH2, –COOH, –CHO, –CCOH, –CCO, –PO, and –S(O)2–OH released from LB-EPS could chelate unstable heavy metals. Moreover, the precipitate produced in the chelation process is generally metal-F4, which could stably exist in sludge filter cake. This result could be found from the mobility model of Cd, Cr, Zn, Ni, and As. However, the mobility model of Cu and Pb showed exactly opposite results. This further indicated that the immobilizations of Cu-F1/F2 and Pb-F1/F2 were mainly controlled by the improvement of the sludge oxidizability, other than LB-EPS destruction. In summary, improving the oxidation performance as well as reducing the LB-EPS content are recommended for the immobilization of Cd, Cr, Zn, Ni, and As in sludge. Moreover, the variations of the environmental risk of Cu and Pb were irregular and need further investigation.
3.6 Effects of MPG on the co-reduction of the sludge volume and toxicity
As mentioned before, with MPG addition, a massive amount of bound water and risky heavy metals were obviously removed from the sludge. Fig. 10 shows that adding 40% DS MPG could remove about 62% of the bound water, and moreover, approximately 27% of the risky heavy metals were removed. These results showed that MPG could not only reduce the sludge volume, but could also weaken the heavy metals' environmental risk. This confirmed that MPG could produce a synergistic effect on reducing the sludge volume and sludge toxicity. This was mainly because adding positive MPG could break the negative colloidal network through electrostatic attraction, releasing bound water, organics, and unstable heavy metals from the sludge network.28 Thus, the volume and toxicity of the sludge were greatly reduced. However, with an excessive dose (more than 50% DS), the removal of bound water and risky heavy metals will be hindered. This may be caused by the supersaturation effect of positive charges from MPG. Those redundant positive charges will lead to a re-stabilization of the sludge network, finally impeding the removal of bound water and risky heavy metals from the network.In particular, with MPG addition, removing a massive amount water and risky heavy metals from the sludge will greatly promote the subsequent use of the sludge. Besides, after treatment, the treated sludge sample consisting of dry sludge and MPG became an organic–inorganic mixture. Additionally, the heavy metals in the treated sludge existed mainly in the form of a stable fraction, the effect of which on the environment was largely reduced. Currently speaking, improving the soil fertility may be a suitable use for the treated sludge. In one respect, the organic nutrients, such as N, P, and K, and massive amount of microelements, including Ca, Mg, Zn, Fe, B, and Mo, in sludge will largely enhance the soil fertility. On the other hand, the inorganic mineral structure of MPG could improve the soil permeability and further enhance its ability to transport nutrients.16 Thus, using treated sludge as a soil conditioner to optimize the structure distribution and fertility of soil may be an appropriate method for its subsequent disposal. However, this concept also requires further study.
Conclusions
Through the above analyses, some conclusions could be drawn as follows:(1) An effective removal of the bound water and unstable heavy metals from sludge was achieved using MPG addition.
(2) With MPG addition, active groups, such as –COH, –C–NH2, –COOH, –CHO, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) COH, –C
COH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –P
O, –P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and –S(
O, and –S(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)2–OH, were released from the LB-EPS layer wrapped in the colloid network. Those active groups played an important role in the chelating metal-F1/F2.
O)2–OH, were released from the LB-EPS layer wrapped in the colloid network. Those active groups played an important role in the chelating metal-F1/F2.
(3) Organics' chelating immobilization was more effective in stabilizing Cd, Cr, Zn, and As. Additionally, flocs' entrainment immobilization may make a more significant improvement to the stabilization of Ni and Pb.
(4) Under MPG treatment, the risk reduction rates of Cd, Cr, Zn, Ni, and As were 23.96%, 39.92%, 21.21%, 35.49%, and 78.61%, indicating that MPG made a significant contribution to weakening the environmental risk of the unstable heavy metals in the sludge, especially for As, Cr, and Ni. Nevertheless, very little changes were found in the risk level of Cu and Pb with MPG addition.
(5) A risky model of heavy metals showed that sludge oxidizability and the LB-EPS content in sludge may have a great effect on weakening the environmental risk of Cd, Cr, Zn, Ni, and As.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21666016), China Postdoctoral Science Foundation (No. 2019M663578), and Yunnan Postdoctoral Science Foundation. And we also thanks for the support from the State Key Laboratory of Complex non-ferrous Metal Resource Clean Utilization, and National Key R&D Program of China (2018YFC1900200).References
- K. Ignatowicz, The impact of sewage sludge treatment on the content of selected heavy metals and their fractions, Environ. Res., 2017, 156, 19 CrossRef CAS.
- S. J. Skinner, L. J. Studer and D. R. Dixon, Quantification of wastewater sludge dewatering, Water Res., 2015, 82, 2 CrossRef CAS.
- J. Olivier, J. B. Conrardy and A. Mahmoud, Electro-dewatering of wastewater sludge: An investigation of the relationship between filtrate flow rate and electric current, Water Res., 2015, 82, 66 CrossRef CAS.
- R. Consonni, S. A. Ordoudi and L. R. Cagliani, On the Traceability of Commercial Saffron Samples Using 1H-NMR and FT-IR Metabolomics, Molecules, 2016, 3, 286 CrossRef.
- S. Lv, T. Sun and Q. Zhou, Modification of starch-g-p(DMDAAC) using HRP initiation and the correlation of its structure and sludge dewaterability, Carbohydr. Polym., 2014, 1, 285 CrossRef.
- P. Lewkowska, B. Cieślik, T. Dymerski, P. Konieczka and J. Namieśnik, Characteristics of odors emitted from municipal wastewater treatment plant and methods for their identification and deodorization techniques, Environ. Res., 2016, 151, 573 CrossRef CAS.
- W. Wei, Q. Wang, L. Zhang, A. Laloo, H. Duan, D. J. Batstone and Z. G. Yuan, Free nitrous acid pre-treatment of waste activated sludge enhances volatile solids destruction and improves sludge dewaterability in continuous anaerobic digestion, Water Res., 2018, 130, 13 CrossRef CAS.
- H. Yuan, R. Guan, A. Chufo Wachemo, C. Zhu, D. Zou, Y. Li, Y. P. Li, X. Y. Zuo and X. J. Li, Enhancing methane production of excess sludge and dewatered sludge with combined low frequency CaO-ultrasonic pretreatment, Bioresour. Technol., 2019, 273, 425 CrossRef CAS.
- H. Li, L. Song and B. H. Han, Improved sludge dewaterability using persulfate activated by humic acid supported nanoscale zero-valent iron: effect on sludge characteristics and reaction mechanisms, Environ. Sci.: Water Res. Technol., 2018, 4, 12 Search PubMed.
- D. Q. He, J. Y. Chen, B. Bao, X. L. Pan, J. Li, C. Qian and H. Q. Yu, Optimizing sludge dewatering with a combined conditioner of fenton's reagent and cationic surfactant, J. Environ. Sci., 2020, 88, 21 CrossRef.
- Q. Lin, H. Peng and S. Zhong, Synthesis, characterization, and secondary sludge dewatering performance of a novel combined silicon-aluminum-iron-starch flocculant, J. Hazard. Mater., 2015, 285, 199 CrossRef CAS.
- S. B. Joceline, M. Koné and O. Yacouba, Reed Beds for Sludge Dewatering and Stabilization, J. Environ. Prot., 2015, 06, 341 CrossRef.
- Y. Xu, C. Zhang, M. Zhao, H. Rong, K. Zhang and Q. Chen, Comparison of bioleaching and electrokinetic remediation processes for removal of heavy metals from wastewater treatment sludge, Chemosphere, 2016, 168, 1152 CrossRef.
- A. M. Rashad, Potential use of phosphogypsum in alkali-activated fly ash under the effects of elevated temperatures and thermal shock cycles, J. Cleaner Prod., 2014, 1, 717 Search PubMed.
- X. L. Wang, Z. Y. Zhang and X. S. Yang, Analysis on new approaches for utilization of phosphogypsum in China, Xiandai Huagong, 2011, 5, 1 Search PubMed.
- G. You, J. Hou, P. Wang, Y. Xu, C. Wang and L. Miao, Effects of CeO2, nanoparticles on sludge aggregation and the role of extracellular polymeric substances – explanation based on extended dlvo, Environ. Res., 2016, 151, 698 CrossRef CAS.
- X. L. Huang, X. M. Li and Q. Yang, Conditioning effect of CTAB modified clinoptilolite on waste activated sludge, Huanjing Gongcheng Xuebao, 2016, 6, 3193 Search PubMed.
- S. Horpibulsuk, C. Suksiripattanapong and W. Samingthong, Durability against Wetting–Drying Cycles of Water Treatment Sludge–Fly Ash Geopolymer and Water Treatment Sludge–Cement and Silty Clay–Cement Systems, J. Mater. Civ. Eng., 2016, 1, 04015078(1) Search PubMed.
- Q. X. Dai, L. P. Ma, N. Q. Ren, P. Ning, Z. Y. Guo and L. G. Xie, Investigation on extracellular polymeric substances, sludge flocs morphology, bound water release and dewatering performance of municipal sludge under conditioning with modified phosphogypsum, Water Res., 2018, 142, 337 CrossRef CAS.
- Q. X. Dai, L. P. Ma, N. Q. Ren, P. Ning and Z. Y. Guo, Research on the variations of organics and heavy metals in municipal sludge with additive acetic acid and modified phosphogypsum, Water Res., 2019, 15, 42 CrossRef.
- C. Y. Chen, Effectiveness and Mechanisms of Sewage Sludge Conditioning with Chitosan and Modified Coal Fly Ash, PhD dissertation, Hunan University, China, 2013 Search PubMed.
- A. Tessier, P. G. Campbell and M. Bisson, Sequential extraction procedure for the speciation of particulate trace metals, Anal. Chem., 1979, 51, 844 CrossRef CAS.
- H. Ou, N. Y. Gao, Y. Deng, J. L. Qiao, K. J. Zhang, T. Li and L. Dong, Mechanistic studies of Microcystis aeruginosa inactivation and degradation by UV-C irradiation and chlorination with poly-synchronous analyses, Desalination, 2011, 272, 107 CrossRef CAS.
- A. Nawrocka, A. Miś and Z. Niewiadomski, Dehydration of gluten matrix as a result of dietary fibre addition – A study on model flour with application of FT-IR spectroscopy, J. Cereal Sci., 2017, 74, 86 CrossRef CAS.
- C. Park, D. H. Chon and A. Brennan, Investigation of sludge reduction and biogas generation in high-rate anaerobic side-stream reactors for wastewater treatment, Environ. Sci.: Water Res. Technol., 2018, 10, 2053 Search PubMed.
- D. Ki, R. Kupferer and C. I. Torres, High-rate stabilization of primary sludge in a single-chamber microbial hydrogen peroxide producing cell, Environ. Sci.: Water Res. Technol., 2019, 5, 1124 RSC.
- U. Jayakrishnan, D. Deka and G. Das, Enhancing the volatile fatty acid production from agro-industrial waste streams through sludge pretreatment, Environ. Sci.: Water Res. Technol., 2019, 5, 334 RSC.
- J. Sobikszołtysek, K. Wystalska and A. Grobelak, Effect of addition of sewage sludge and coal sludge on bioavailability of selected metals in the waste from the zinc and lead industry, Environ. Res., 2017, 156, 588 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ew00805b |
| This journal is © The Royal Society of Chemistry 2021 |