The synthesis of porous ultrathin graphitic carbon nitride for the ultrasensitive fluorescence detection of 2,4,6-trinitrophenol in environmental water†
Binhong
Qu
a,
Zhiyuan
Mu
a,
Yang
Liu
a,
Yingsha
Liu
a,
Rui
Yan
a,
Jianhui
Sun
ab,
Zishu
Zhang
a,
Peng
Li
*ab and
Liqiang
Jing
 *a
*a
aKey Laboratory of Functional Inorganic Materials Chemistry (Ministry of Education), International Joint Research Center for Catalytic Technology, School of Chemistry and Materials Science, Heilongjiang University, Harbin, 150080, P. R. China. E-mail: lipenghit@126.com; jinglq@hlju.edu.cn
bCollege of Physical Science and Technology, Heilongjiang University, Harbin, 150080, P.R. China
First published on 26th November 2019
Abstract
The ultrasensitive detection of 2,4,6-trinitrophenol (TNP) for environmental security is important but challenging. Graphitic carbon nitride (g-C3N4) is promising for the fluorescence sensing of TNP. It is highly desired to greatly promote the adsorption of TNP onto g-C3N4 for improving the fluorescence detection sensitivity. Herein, porous ultrathin g-C3N4 (∼1.3 nm) nanosheets were successfully synthesized via combining second calcination with HNO3 treating processes. Under the optimized conditions of the excitation wavelength (Ex = 350 nm) and solution pH value (pH = 3), the wide detection range of TNP from 4 μM to 54 μM was confirmed by the widely adopted normal Stern–Volmer equation (SVE). Interestingly, the limit of detection was as low as 0.04 nM according to the linear range from 0.1 nM to 4 μM by the generally neglected double logarithmic (DL) SVE, which is much competitive for TNP detection compared with previously reported results. Based on the isothermal adsorption curves and the time-resolved fluorescence and surface photovoltage spectra, it was confirmed that the multilayer adsorption of TNP on the resulting g-C3N4 at low concentrations mainly led to fluorescence quenching by the inner filter effect (IFE), while the monolayer adsorption at ultralow concentrations resulted in fluorescence quenching by the combined effects of IFE and photoinduced electron transfer. It was suggested that normal and DL SVEs are applicable to the fluorescence quenching processes determined by single and double factors, respectively. The obtained ultrasensitive detection was attributed to the greatly promoted adsorption of TNP via hydrogen bonding by enlarging the surface area from the porous nanosheet structure and by increasing the surface –OH sites from the HNO3 treatment. This work helps to deeply understand SVE related to the fluorescence quenching processes and provides a feasible route to develop a method for the ultrasensitive detection of TNP with g-C3N4 nanosheets for environmental water monitoring and security inspection.
Environmental significance2,4,6-Trinitrophenol (TNP) has strong biological toxicity and explosive risks. Thus, it is very important to ultra-sensitively detect TNP on demand for ensuring environmental security. Herein, we successfully synthesized porous ultrathin g-C3N4 nanosheets by self-assembly, second calcination and HNO3 treatment processes, which exhibited strong adsorption ability toward TNP so as to develop an ultrasensitive fluorescence detection method of TNP by the frequently neglected double-logarithmic Stern–Volmer equation. The detection limit of the resulting g-C3N4 toward TNP was as low as 0.04 nM with obvious competitiveness based on the combined effects of electron transfer and inner filter on fluorescence quenching. This work provides a feasible route for developing an efficient g-C3N4-based fluorescence method for detecting TNP in complicated water environmental systems. |
1. Introduction
2,4,6-Trinitrophenol (TNP) is an important nitroaromatic explosive with high blasting power, strong electron-withdrawing groups and good coloring ability. These features help realize wide applications of TNP in munitions, explosives, dye industries, medical examination, the pharmaceutical field and agricultural fungicides. Noticeably, with its extensive use, the gradual accumulation of TNP occurs easily and it does not readily degrade in the environment, which threatens the national security and public safety and also endangers human health due to its highly violent energy and toxic effects even at ultralow concentrations.1,2 Therefore, it is important to develop a simple, rapid-response, non-toxic, and ultrasensitive method to detect TNP. A number of analytical methods, including gas chromatography (GC), high-performance liquid chromatography (HPLC),3 and electrochemistry,4,5 have been extensively investigated to detect TNP. However, GC generally requires sophisticated preprocessing involving the treatment with oxidized chloride and organic solvent extraction. The limit of detection (LOD) of HPLC to TNP is not low enough. It is difficult for electrochemical methods to distinguish other nitroaromatic analogues like 2,4-dinitrotoluene and 2,4,6-trinitrotoluene. In comparison, fluorescence methods that possess simplicity, low cost, rapid response, and high sensitivity and selectivity can be effectively applied for the sensing of TNP in environmental monitoring. Various new fluorescent materials have been designed and prepared for detecting TNP, such as MoS2 quantum dots,6 water-soluble silicon nanoparticles,7 pyrenyl probes,8 Cd-metal–organic frameworks,9 and terbium-doped carbon dots.10–12 However, complicated synthesis, complex operation, high toxicity and utilization of organic media have limited the practical applications of these fluorescent materials for the detection of TNP in aqueous solutions.13As a new fluorescent non-metallic material, many graphitic carbon nitride (g-C3N4)-based fluorescent sensors have been fabricated for detecting inorganic ions,14 heavy metals,15,16 nitroaromatic explosives,17 and pesticide residues18,19 as well as for disease diagnosis20,21 owing to their high photoluminescence intensity, good stability, and biocompatibility. In particular, Rong et al. reported a non-toxic fluorescence sensing approach to detect TNP with LOD of 8.2 nM using g-C3N4 nanosheets, in which the fluorescence of g-C3N4 was effectively quenched by TNP through the strong inner filter effect (IFE) according to the widely adopted normal Stern–Volmer equation (SVE). However, the performance of g-C3N4-based fluorescence detection is still not satisfactory, which is mainly attributed to the limited adsorption. Also, the related mechanisms are not clearly demonstrated or are frequently ignored. Hence, it is meaningful to improve the fluorescence detectability of g-C3N4 by promoting the adsorption of TNP on g-C3N4 and to reveal the related mechanisms.
In general, increasing the specific surface area by introducing porosity into g-C3N4 is often taken as a feasible route for efficiently improving the adsorption capacity. Some efforts have been made including the use of templates,22–24 solvent-assisted methods25 and thermal oxidation etching methods.26 However, these methods are often complicated and time consuming and require environmentally hazardous reagents. Differently, the use of the cyanuric acid–melamine complex for the preparation of g-C3N4 by molecular self-assembly can simply form a structure with continuous porosity so as to increase the surface area.27 Although some unstable amorphous residues on g-C3N4 still exist, they can be removed easily by washing with acidic solutions; then, new micro/nano pores can be possibly created by a second calcination process. In addition, an ultrasonically assisted liquid exfoliation method can be employed to further reduce the size and thickness to enlarge the surface area of g-C3N4. Moreover, the acid treatment can increase the amount of surface hydroxyl (–OH) sites on g-C3N4, which would improve the connectivity between g-C3N4 and TNP by facilitating the formation of hydrogen bonds. From the above discussion, it is conceivable to develop effective synthetic procedures to achieve g-C3N4 with strong adsorption capacity of TNP for its ultrasensitive detection. On the other hand, the surface charge is also affected by the pH value of the solution, which would further influence the adsorption of TNP. Meanwhile, TNP exhibits a broad optical absorption band, which overlaps with the excitation band of g-C3N4 and then influences the fluorescence intensity. Thus, it is meaningful to optimize the conditions of the solution pH value and excitation wavelength to develop high-sensitivity fluorescence detection for TNP.
It is well-known that the fluorescence quenching process is described by the widely adopted normal SVE (F0/F = 1 + KSV[C]),6–12 where F0 and F are the fluorescence intensities in the absence and presence of a quencher, respectively, [C] is the concentration of the quencher, and KSV is the SVE constant. Normally, (F0/F − 1) is expected to be linearly dependent upon the concentration of the quencher ([C]). Notably, it is expected that normal SVE might result from F0/F = 1 + KSV[C]n, where n is a key indicator determined by the fluorescence quenching factors, which is mainly involved with multiple fluorescence quenching pathways possibly dependent on the related adsorption model at different concentrations of the quencher. A normal linear SVE plot generally indicates a single factor of fluorescence quenching like a static or a dynamic one, in which n is equal to 1. If multiple fluorescence quenching factors co-exist, n is not equal to 1 and thus, the SVE plot is not linear. The quenching data can be expressed by the double logarithmic (DL) SVE plot as lg[(F0/F) − 1] = lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) KSV + n
KSV + n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) lg[C].28–30 Obviously, there is a linear relationship between lg[(F0/F) − 1] and lg[C]. This has been used to describe the fluorescence quenching of albumin, indicating the emergence of multiple factors, like protein conformational changes and the appearance of new binding sites. Thus, it is possible to improve the detection sensitivity of fluorescence by using the DL SVE plot. Unfortunately, it is frequently neglected.
lg[C].28–30 Obviously, there is a linear relationship between lg[(F0/F) − 1] and lg[C]. This has been used to describe the fluorescence quenching of albumin, indicating the emergence of multiple factors, like protein conformational changes and the appearance of new binding sites. Thus, it is possible to improve the detection sensitivity of fluorescence by using the DL SVE plot. Unfortunately, it is frequently neglected.
As for the quencher (TNP) detected by the fluorophore (g-C3N4), the mechanism of quenching is mostly IFE, in which it obeys the normal SVE plot at low concentrations of TNP.17 In this case, the adsorption model of TNP on g-C3N4 is mainly multilayer adsorption; thus, the photoinduced electron transfer (PET) factor can be neglected. In fact, TNP at ultralow concentrations mainly exhibits monolayer adsorption on g-C3N4. As expected, the fluorescence quenching is determined by the IFE and PET factors. Thus, it is possible to apply the frequently neglected DL SVE plot for quantitatively analyzing TNP at ultralow concentrations after promoting the adsorption of TNP. Therefore, it is very feasible to develop a method for the ultrasensitive detection of TNP in aqueous solutions based on the fluorescence quenching of g-C3N4 with strong adsorption capacity.
Herein, an ultrasensitive fluorescence detection method for TNP was developed based on the fluorescence quenching of porous ultrathin g-C3N4 nanosheets. Through combining second calcination with HNO3 treating processes, the as-prepared g-C3N4 showed strong adsorption of TNP because of the enlarged surface areas and more –OH adsorption sites. The detection mechanism of TNP via g-C3N4 was investigated by the decrease in fluorescence intensity and lifetime as well as the surface photovoltage spectra and isothermal adsorption curves. These results clearly suggested that the multilayer adsorption of TNP on g-C3N4 at low concentrations mainly led to a fluorescence quenching by single IFE, while the monolayer adsorption at ultralow concentrations resulted in fluorescence quenching by dual factors (IFE and PET). Owing to more and stronger monolayer adsorption sites interacting with TNP via hydrogen bonding at ultralow concentrations, the PET factor played a significant role in the fluorescence quenching process. The frequently neglected DL SVE is applicable to the fluorescence detection process. This study will provide new insights on developing an efficient g-C3N4-based analytical method for detecting TNP, which is of great importance for environmental water monitoring and security inspection.
2. Experimental section
2.1 Reagents and materials
Cyanuric acid, melamine, 2,4-dinitrotoluene (DNT) and 2,4,6-trinitrotoluene (TNT) were purchased from Aladdin. 4-Nitrophenol (NP), nitrobenzene (NB), 4-nitrotoluene (NT), methylbenzene (MB), phenol (PHE) and nitric acid were obtained from Sinopharm Chemical Reagent Co. Ltd. 2,4,6-Trinitrophenol (TNP) was purchased from Taishan Chemical Factory. 2,4-Dinitrophenol (DNP) was purchased from Shanghai Titan Scientific Co. Ltd. All chemicals were analytical grade and used as received without further purification. Ultrapure water (resistance 18.2 MΩ cm) was obtained from a pure water system and used for all the experiments. Water phase microporous membrane filters (50 mm × 0.22 μm) were purchased from Xiamen Green Reagent Glass Instrument.2.2 Instrumentation
Atomic force microscopy (AFM) was performed on 5100 Agilent with a silicon detector. Transmission electron microscopy (TEM) was performed using a Model JEM-1400 microscopy system (JEOL, Japan) at an acceleration voltage of 120 kV. The Brunauer–Emmett–Teller (BET) surface area was measured with an ASAP2020Plus 1.03 apparatus (Micromeritics Instrument Corp.). Zeta potential was tested with an Anton Paar Sur PASS automatic analyzer.The X-ray powder diffraction (XRD) patterns of the samples were measured with a Bruker D8 Advance diffractometer using Cu Kα radiation. Fluorescence spectra were collected using a PerkinElmer LS-55 photoluminescence spectrometer with a standard 15 mm path length quartz cuvette. The fluorescence lifetimes were obtained using a Horiba Jobin Yvon Fluorolog-3 spectrometer. Fourier transform infrared (FT-IR) spectra were obtained using a Bruker Equinox 55 Spectrometer (Thermo Electron Corp., USA) with KBr diluents. Ultraviolet-visible light (UV-vis) absorption was measured by a Shimadzu Model UV-2550 spectrophotometer. The steady-state surface photovoltage spectroscopy (SS-SPS) measurements were carried out using a home-built apparatus equipped with a lock-in amplifier (SR830) and synchronized with a light chopper (SR540). The sample was sandwiched between two indium tin oxide (ITO) glass electrodes and irradiated through a 500 W xenon lamp to obtain a monochromatic light by passing through a double prism monochromator (SBP300). The transient-state surface photovoltage (TS-SPV) responses of the samples were measured in N2, air or O2 atmosphere at room temperature. In a typical measurement, the sample chamber was connected to an ITO glass at the top electrode and a steel substrate at the bottom electrode, and a thick mica spacer was placed between the ITO glass and the sample to decrease the space charge region at the ITO-sample interface. The samples were excited by a radiation pulse of 355 nm with 10 nm width from the second harmonic of a neodymium-doped yttrium aluminum garnet laser (Lab-130-10H, Newport, Co.). The signals were amplified with a preamplifier and registered with a 1 GHz digital phosphor oscilloscope (DPO 4104B, Tektronix). Adsorption experience curves were measured from a DGU-20A3R liquid chromatograph (SHIMADZU, Japan). The chromatographic separations of the analytes were carried out on a XDB-C18 column (5 μm, 4.6 mm × 250 mm). The injection volume of the sample solution was 20 μL. The mobile phase consisted of water (A) and methyl alcohol (B). 1H solid-state NMR (1H-NMR) spectra were collected using a Bruker Avance III 400 WB spectrometer with 4 mm zirconia rotor, and the chemical shifts were measured with adamantine as the internal reference. Organic elemental analysis (EA) was obtained from Elementar vario MICRO cube.
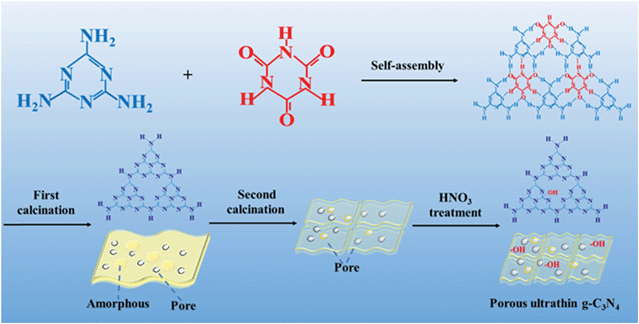
2.3 Synthesis of porous ultrathin g-C3N4
Scheme 1 illustrates the fabrication procedure of porous ultrathin g-C3N4. First, melamine (10 g) and cyanuric acid (4 g) were dispersed in 500 mL deionized water and vigorously stirred with heating power at 80 °C until dissolved. The mixed suspension of cyanuric acid and melamine was stirred for 3 h at room temperature; then, it was centrifuged at 4000 rpm for 15 min, washed with deionized water and ethanol several times, and dried in a vacuum oven. The obtained dried powder was transferred into a closed quartz boat and heated from room temperature to 520 °C with a heating rate of 1 °C min−1 in the presence of an N2 bubbled system. The primary porous g-C3N4 nanosheet (PCN) samples were obtained after calcination for 4 h. Second, 1.0 g primary PCN was added into 100 mL of 5 M HNO3 and refluxed at 115 °C for 6 h. After cooling, centrifuging, washing to neutral and drying, PCNs treated with HNO3 were prepared. The second-calcined porous g-C3N4 nanosheets (SC-PCNs) were obtained by heating the primary PCNs in a semi-closed porcelain boat at 500 °C for 2 h in the presence of air. After cooling, the obtained fluffy g-C3N4 was treated with HNO3 following the same procedure. Finally, the pure SC-PCN samples were obtained for further experiments.2.4 Fluorescence determination of TNP
After ultrasonic reaction for 12 h, filtering processes and collecting the supernatant, the stock solution of porous ultrathin g-C3N4 with a concentration of 20 mg L−1 was prepared. The pH values of the solution could be adjusted by adding HNO3 or NaOH. Before the spectroscopic measurements, TNP samples were dried at 80 °C overnight and confected into stock solutions with a final concentration (540 μM). Different concentrations of TNP testing solutions were prepared by diluting the stock solution. The detection of TNP was performed as follows: 9.0 mL porous ultrathin g-C3N4 solution was mixed with 1 mL of various concentrations of TNP, and the final volume of the solution was kept at 10 mL. The fluorescence spectrum was measured after 5 min at room temperature. The selectivity of porous ultrathin g-C3N4 toward TNP was evaluated by adding nitroaromatic explosive solutions instead of TNP in a similar way. To obtain the LOD of this method, the blank sample was analyzed 13 times. LODs were calculated with the following equation: LOD = 3S/N; here, S represents the standard deviation of the blank sample and N represents the slope of the standard curve.2.5 Analysis of water samples
The environmental water samples were centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min twice and then, the supernatant was filtered through the 0.22 μm water phase membrane. The spiked sample was prepared by adding an appropriate volume of standard solution of TNP in the water sample. A specific procedure was followed: 9 mL of the as-synthesized stock solution of porous ultrathin g-C3N4 was injected into treated water samples with different concentrations of TNP, and the fluorescence spectra of the solutions were collected after reaction for 5 min at room temperature.
000 rpm for 20 min twice and then, the supernatant was filtered through the 0.22 μm water phase membrane. The spiked sample was prepared by adding an appropriate volume of standard solution of TNP in the water sample. A specific procedure was followed: 9 mL of the as-synthesized stock solution of porous ultrathin g-C3N4 was injected into treated water samples with different concentrations of TNP, and the fluorescence spectra of the solutions were collected after reaction for 5 min at room temperature.
3. Results and discussion
3.1 Characterization of PCN and SC-PCN
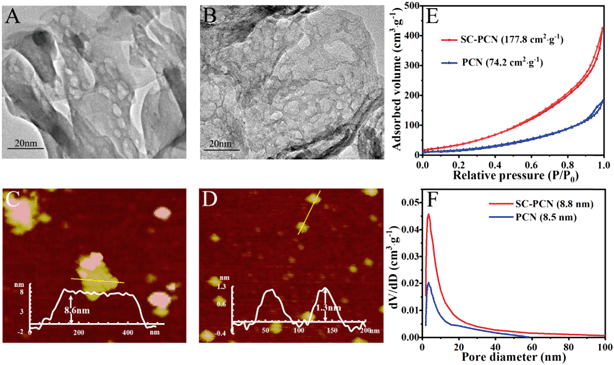
The TEM and AFM images of PCN and SC-PCN are shown in Fig. 1A–D. Compared with PCN, SC-PCN had more pores and a thinner and clearer laminar structure. The average diameter of SC-PCNs was estimated to be 60 nm and the thickness of SC-PCNs was approximately 1.3 nm. Furthermore, N2 sorption–desorption isotherms were investigated (Fig. 1E). The porous ultrathin g-C3N4 nanosheets exhibit a type IV adsorption–desorption isotherm with an H2 hysteresis loop, indicating the presence of a mesoporous structure. The estimated BET surface area of SC-PCNs was about 177.8 m2 g−1, which was 2.5-fold larger than that of PCNs (74.2 m2 g−1). The corresponding pore size distribution (Fig. 1F) confirmed that SC-PCNs possess more mesoporous structures than PCNs. It should be noted that a large BET surface area usually provides more specific active sites, which improves the adsorption ability.To investigate the specific effects of second calcination on the formation of a structure with more porosity, N2 sorption–desorption experiments of different treatment processes of the products were performed, as shown in Table S1.† Compared to the values for primary PCNs, the BET surface areas and pore sizes increased greatly via the second calcination process. Some unstable amorphous portions still existed after first calcination without the HNO3 treating process. During the second calcination process, g-C3N4 could be exfoliated into ultrathin nanosheets, which was attributed to the residual amorphous components disintegrating into gases so as to form a more apparent mesoporous structure. Noticeably, the unstable amorphous residues of primary g-C3N4 could be removed through the HNO3 pre-treatment, leading to no obvious increase in the BET surface area of the resulting g-C3N4. Consequently, the second calcination process without pre-treatment led to a larger BET surface area. Afterwards, due to the acid treatment, the surfaces possessed more hydroxyl groups.
The XRD patterns and FT-IR spectra of PCNs and SC-PCNs are shown in Fig. S1.† PCNs exhibit a typical g-C3N4 layered structure with two pronounced characteristic peaks: (i) the sharp and strong shoulder peak at approximately 27.6° originates from the (002) interlayer stacking of aromatic systems for graphitic materials; (ii) the weak peak at 2θ = 13.1° (100) derives from the in-planar repeated tri-s-triazine units. After the second calcination process, the intensity of the (002) diffraction peak decreased sharply, indicating that the resulting SC-PCNs possessed a thinner layer structure, as confirmed by the TEM images. The characteristic FT-IR spectrum of PCNs is similar to that of SC-PCNs; it exhibits a strong peak at 810 cm−1 (breathing modes of the triazine units), bands at 1123–1630 cm−1 (stretching modes of the CN heterocycles), and broad bands between 2740 and 3600 cm−1 (stretching modes of hydrogen-containing groups). The FT-IR spectrum of SC-PCN exhibits that the intensity of the stretching vibration of OH increases obviously. The elemental analysis results (Table S2†) further confirmed that the contents of the hydrogen and oxygen elements on the surface of the samples increased after second calcination and acid treatment. These results suggest that the surface of SC-PCNs possesses more –OH groups, which is favorable for the adsorption of TNP molecules by forming hydrogen bonds.
3.2 Fluorescence determination of PCNs toward TNP
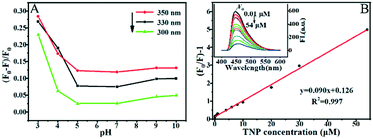
The as-prepared aqueous suspension of PCNs was clear and stable, and it could show obvious fluorescence characteristics and could be quenched after the adsorption of TNP. The spectra of PCN fluorescence and TNP UV-vis absorption in different pH solutions are shown in Fig. S2A and B.† PCNs exhibited a strong emission peak at 447 nm when the excitation wavelength from 300 to 380 nm was used, and the strongest emission signal was located at 330 nm. It was noticed that TNP has a wide absorption band (pH ≥ 3), which nearly overlapped the excitation spectrum of PCNs to a large extent. This overlap strongly favored fluorescence quenching by IFE in the presence of TNP. Notably, a strong absorption peak of HNO3 at 300 nm is seen for the strong acid solution (pH < 3). Thus, it is not suitable to detect TNP in a strong acid HNO3 solution. From Fig. S3C,† it is found that the strong fluorescence signal of PCNs slightly decreases from pH 3 to 10.Based on the above results, the change rate [(F0 − F)/F0] of fluorescence intensity under the conditions of different excitation wavelengths (300, 330 and 350 nm) and pH solutions (pH = 3, 4, 5, 7, 9 and 10) is shown in Fig. 2A. It can be seen clearly that the fluorescence intensity of PCNs with TNP at pH 3 exhibits the largest change ratio and slightly decreases from pH 3 to 5. It is nearly unchanged from pH 6 to 11. Obviously, the effect of quenching is the best under the excitation wavelength of 350 nm and at pH 3 for TNP detection. Thus, the optimized conditions are mainly considered in the following investigation for sensitively detecting TNP.
As shown in Fig. 2B, the fluorescence intensity gradually decreases with the increase in the amount of TNP. The quenching process could be quantitatively described by the widely adopted normal SVE. A distinct linear relationship is shown between [(F0/F) − 1] and the TNP concentration from 0.01 to 54 μM, with LOD of 4 nM. From Fig. S3,† it is further confirmed that it has a larger slope value by the linear equation under the excitation wavelength of 350 nm than that for 330 or 300 nm, and it is much more sensitive at pH 3 compared to that at pH 4. Interestingly, it also exhibits a certain capacity for detecting TNP by utilizing the fluorescence quenching of PCNs at pH 7.
3.3 Fluorescence determination of SC-PCNs toward TNP
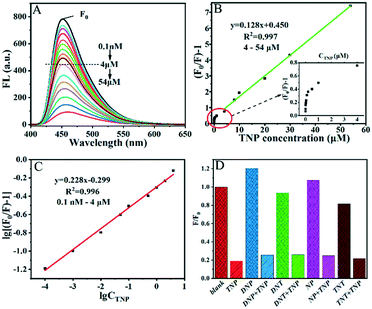
Compared with PCNs, SC-PCNs possess more mesopores and surface hydroxyl groups, which is very favorable for TNP adsorption and to improve the fluorescence determination capability.As shown in Fig. 3A–C, the fluorescence intensity of SC-PCNs gradually decreases on increasing the amount of added TNP under the conditions of 350 nm excitation and pH 3. A distinct linear relationship is shown between [(F0/F) − 1] and the TNP concentration from 4 to 54 μM by the widely adopted normal SVE. Its slope value (KSV = 0.128) is larger than that of PCNs. Significantly, a specific linear relationship of lg(F0/F − 1) vs.lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) CTNP is obtained by using the double logarithmic plot method in the ultralow concentration range of TNP from 0.1 nM to 4 μM, by which LOD of 0.04 nM is confirmed. It should be noted that it is not suitable to detect TNP in the ultralow concentration with PCNs. In addition, the fluorescence determination of TNP by SC-PCNs in neutral conditions was investigated. It was shown that SC-PCNs could also have good capacity for detecting TNP at pH 7 with the lower slope value, as shown in Fig. S4.† These results indicate that the fluorescence determination capability of SC-PCNs toward TNP is much competitive with other fluorescence methods from pH 3 to 7.
CTNP is obtained by using the double logarithmic plot method in the ultralow concentration range of TNP from 0.1 nM to 4 μM, by which LOD of 0.04 nM is confirmed. It should be noted that it is not suitable to detect TNP in the ultralow concentration with PCNs. In addition, the fluorescence determination of TNP by SC-PCNs in neutral conditions was investigated. It was shown that SC-PCNs could also have good capacity for detecting TNP at pH 7 with the lower slope value, as shown in Fig. S4.† These results indicate that the fluorescence determination capability of SC-PCNs toward TNP is much competitive with other fluorescence methods from pH 3 to 7.
To explore the selectivity of SC-PCNs toward TNP, the fluorescence detection was investigated in the presence of several potentially interfering substances, including various nitroaromatic explosive compounds and metal ions. Fig. 3D and S5† show the percentages of the fluorescence quenching of SC-PCNs in the presence of DNP, DNT, NP, TNT, Pb2+, Zn2+, Fe2+ and Fe3+ with and without TNP. This reveals that these organic interferences and metal ions have a weak effect on the fluorescence intensity change of SC-PCNs, indicating that SC-PCNs exhibit high selectivity for TNP. We also made a comparison of our method to those reported by other researchers, as seen in Table S6.† Noticeably, this work is a novel and important approach in the determination of TNP.
3.4 Mechanism of adsorption
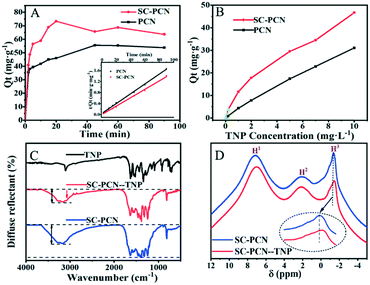
To further elucidate the detection mechanism of SC-PCNs for TNP, adsorption experiments of TNP on PCNs and SC-PCNs at room temperature were performed.As shown in Fig. 4A and Table S3,† the high coefficient (R2) values indicate that the adsorption kinetics process of porous ultrathin g-C3N4 can be described by the pseudo-second-order model equation very well. Thus, it can be represented by eqn (1):
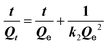 | (1) |
Here, Qt and Qe (mg g−1) present the equilibrium and maximum adsorption capacities of the adsorbent, respectively, t (min) is the contact time, and k2 (g mg−1 min−1) is the adsorption rate constant. This adsorption process mostly involves electron sharing or electron transfer between the adsorbent and the adsorbate. The adsorption of SC-PCNs toward TNP can easily reach saturation within a shorter time (about 20 min) than that of PCNs (about 45 min). The value of k2 for SC-PCNs (0.15 g mg−1 min−1) is larger than that of PCNs (0.0077 g mg−1 min−1), indicating that the adsorption efficiency of SC-PCNs toward TNP is better than that of PCNs. Meanwhile, the adsorption isotherms of SC-PCNs and PCNs to TNP are shown in Fig. 4B. The adsorption isotherm of PCN fits the Freundlich adsorption model, which is expressed by eqn (2):
| Qt = KFCe1/N | (2) |
Here, KF (L g−1) corresponds to the Freundlich constant, N presents the Freundlich parameter associated with the degree of heterogeneity in the system, and Ce (mg L−1) is the equilibrium concentration in the liquid phase.
In comparison, the amounts of TNP adsorbed on SC-PCNs could not be expressed by a single adsorption model. The adsorption isotherm of SC-PCNs fits the Freundlich adsorption model at low concentrations and the Henry adsorption model at ultralow concentrations. The Henry adsorption model is expressed by eqn (3):
| Qt = KhCe | (3) |
Here, Kh (L g−1) is the Henry constant. According to the observed fitting data in Table S3,† the value of KF for SC-PCNs is 3 times that for PCNs. This further proves the better adsorption capacity of SC-PCNs toward TNP. Based on the above comparative investigations, it was confirmed that SC-PCNs could more easily adsorb TNP and display more and stronger monolayer adsorption at ultralow concentrations. Therefore, the ultrasensitive determination of SC-PCN for TNP is attributed to its stronger adsorption ability.
To obtain more insights into the molecular interaction between SC-PCNs and TNP, FT-IR and 1H-NMR spectroscopies were utilized. As shown in Fig. 4C, the broad band at 2800–3500 cm−1 for SC-PCNs is slightly weaker after the adsorption of TNP, but a new small peak at 3057 cm−1 appears differently from that of pure SC-PCNs, indicating that TNP is adsorbed on SC-PCNs. From the 1H-NMR spectra in Fig. 4D and 5, distinct peaks at 7.17 and 2.16 ppm are observed, which are assigned to –NH and –NH2 in the melem units. The peak at −1.14 ppm is assigned to –COH in the electron-rich cavity of SC-PCNs. A slight chemical shift in the –COH peak on SC-PCNs after TNP adsorption is found, which means that TNP is successfully grafted onto SC-PCNs through the hydrogen bonds between –COH (SC-PCN) and O2N– (TNP).31
Moreover, the zeta potential measurements of PCNs and SC-PCNs have the same variation trend, as shown in Fig. S6.† The zeta potential reduces with the increase in pH, and the maximum positive value is obtained at pH 3. SC-PCNs possess a positive charge and display a zeta potential of 37.5 mV at pH 3. It should be noted that the –COH group of SC-PCNs is beneficial for the formation COH2+ in an acidic environment, which promotes the formation of more hydrogen bonds. In addition, SC-PCNs should have certain molecular chemical interactions with TNP by π–π interactions so as to be favorable for TNP adsorption.31
3.5 Mechanism of fluorescence quenching
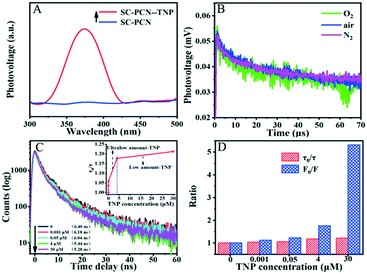
The fluorescence of g-C3N4 could be effectively quenched by adsorbed TNP through strong IFE, as shown in previous literature, which was also confirmed at low concentrations of TNP in this work. The PET effect could be neglected since the adsorption model of TNP on PCNs was mainly multilayer adsorption in the detection process. However, the adsorption amount of TNP on PCNs at ultralow concentrations was very low, due to which it was not feasible to detect TNP. As for SC-PCNs, they could exhibit strong adsorption ability toward TNP at ultralow concentrations with a monolayer adsorption model. In this case, both IFE and PET contributed to the quenching of fluorescence of g-C3N4. Unfortunately, the PET effect is frequently neglected. Based on the combination effects of IFE and PET, DL SVE is developed successfully to describe this fluorescence quenching process.To elucidate the PET mechanism of SC-PCNs for TNP, the SPS responses of SC-PCNs without and with TNP adsorption in N2 were investigated. As shown in Fig. 6A, it is clear that SC-PCNs have no SPS signal, while the SPS response of SC-PCNs is significantly enhanced after adsorbing TNP. This result indicates that the photogenerated charge transfer and separation occur between SC-PCNs and TNP. Notably, the formed hydrogen bonds are favorable for charge transport so as to offer an effective “fluorescer-bridge-quencher” channel.
Normally, g-C3N4 exhibits a gradually increasing positive TPV response as the amount of oxygen increases due to the electron trap effect of adsorbing O2.32 It was noticed that the TPV responses of SC-PCNs after adsorbing TNP also exhibited a positive signal but no significant change with increasing amounts of adsorbed O2, as shown in Fig. 6B. This illustrated that the adsorption of TNP could effectively trap electrons so as to make the corresponding holes preferentially diffuse to the surfaces of the tested electrode and weaken the influence of the oxygen's capture effect. This is understandable since TNP as an electron-deficient compound with nitro groups can accept electrons easily and the LUMO energy level of the electron-rich g-C3N4 is rather high.33,34
To better understand the quenching process, the time-resolved fluorescence decay processes of SC-PCNs at different TNP concentrations were recorded. As is well known, the fluorescence lifetime is mostly unaffected by IFE but would be substantially changed by an efficient PET process. Fig. 6C shows that the average lifetime change of SC-PCNs after adsorbing TNP is obvious at an ultralow concentration and then, it decreases with the continuous increase in the TNP concentration. This further proves that the PET effect is great at ultralow concentrations of TNP. The ratios of fluorescence intensity (F0/F) and average lifetime (τ0/τ) of SC-PCNs after adsorbing different concentrations of TNP in pH 3 are shown in Fig. 6D. The subscript 0 denotes the fluorescence intensity or lifetime of SC-PCNs in the absence of TNP; (τ0/τ) represents the change in fluorescence lifetime, which implies the change in fluorescence intensity based on PET,35 whereas (F0/F) represents the change in the fluorescence intensity among the combined fluorescence quenching processes, including PET and IFE. It can be seen clearly that (τ0/τ) accounts for a major proportion at ultralow concentrations of TNP compared to (F0/F), indicating that PET plays a vital role during the whole fluorescence quenching process, while its role is very small at low concentrations of TNP. Therefore, it is further confirmed that IFE and PET influence the fluorescence quenching at ultralow concentrations of TNP, while IFE is mainly dominant at low concentrations of TNP.
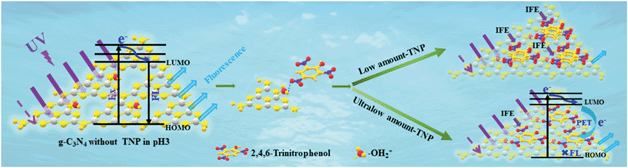
Based on the above-mentioned discussion, a possible mechanism of the fluorescence detection for TNP is illustrated in Scheme 2. SC-PCNs exhibit a strong and blue fluorescence signal in a pH 3 solution. After adsorbing TNP at low concentrations, the fluorescence of SC-PCNs remarkably decreases due to the IFE process, which is attributed to the multilayer adsorption. Meanwhile, after adsorbing TNP at ultralow concentrations, the fluorescence of SC-PCNs is influenced by both IFE and PET, which is attributed to monolayer adsorption through the formed hydrogen bonds between COH2+ (SC-PCN) and O2N– (TNP) in an acidic environment.
3.6 Application of the TNP detection in water samples
The adaptability of the proposed method for the detection of TNP was also attempted. Because TNP was undetectable in the selected water sample by SC-PCNs, a recovery study was carried out with the water sample by spiking with standard TNP solutions (0.02, 1 and 10 μM). The average results obtained after six repetitions are given in Table S4.† The obtained recoveries of the samples varied from 94.6% to 118.0%, and the relative standard deviation (RSD) of each sample was in the range from 2.39% to 11.18%. These results indicate that this SC-PCN-based fluorescence method exhibits a great potential for practical TNP detection in water samples.Conclusions
In this study, ultrathin porous g-C3N4 for the fluorescence determination of TNP in environmental water was easily synthesized via combining second calcination with HNO3-treatment processes. SC-PCNs could play important roles in promoting the detection of TNP at ultralow concentrations in an acidic environment due to the following two aspects: (i) they possessed larger surface areas and more –OH2+ adsorption sites, which resulted in strong monolayer adsorption ability of TNP; (ii) their fluorescence could be decayed by dual factors (IFE and PET) and thus, the process could be well expressed by DL SVE. The proposed fluorescence detection method could solve the problem of insufficient interactions between g-C3N4 and TNP and a selected suitable description model. The LOD value was as low as 0.04 nM with excellent competition with current methods. This new strategy is flexible to explore the fluorescence quenching process related to multiple quenching factors and provides new insights into the fluorescence detection methods for other analytes of interest.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for financial support from the NSFC project (U1805255 and 11704105), Special Funding for Postdoctoral of Heilongjiang Province (LBH-Z17188) and the Fundamental Research Funds of University in Heilongjiang Province KJCXZD201707.Notes and references
- X. C. Sun, Y. Wang and Y. Lei, Fluorescence based explosive detection: from mechanisms to sensory materials, Chem. Soc. Rev., 2015, 44, 8019–8061 RSC.
- L. H. Cao, F. Shi, W. M. Zhang, S. Q. Zang and T. C. W. Mak, Selective sensing of Fe3+ and Al3+ ions and detection of 2,4,6-trinitrophenol by a water-stable terbium-based metal-organic framework, Chem. – Eur. J., 2015, 21(44), 15705–15721 CrossRef CAS PubMed.
- L. Havlíková, R. Matyáš, L. Ihnát, L. Nováková and D. Šatínský, Degradation study of nitroaromatic explosives 2-diazo-4,6-dinitrophenol and picramic acid using HPLC and UHPLC-ESI-MS/MS, Anal. Methods, 2014, 6(13), 4761–4768 RSC.
- J. R. Huang, L. Y. Wang, C. C. Shi, Y. J. Dai, C. P. Gu and J. H. Liu, Selective detection of picric acid using functionalized reduced graphene oxide sensor device, Sens. Actuators, B, 2014, 196, 567–573 CrossRef CAS.
- S. Hamad, G. K. Podagatlapalli, M. A. Mohiddon and V. R. Soma, Cost effective nanostructured copper substrates prepared with ultrafast laser pulses for explosives detection using surface enhanced Raman scattering, Appl. Phys. Lett., 2014, 104, 263104 CrossRef.
- Y. Wang and Y. N. Ni, Molybdenum Disulfide Quantum Dots as a Photoluminescence Sensing Platform for 2,4,6-Trinitrophenol Detection, Anal. Chem., 2014, 86, 7463–7470 CrossRef CAS PubMed.
- Y. X. Han, Y. L. Chen, J. Feng, J. J. Liu, S. D. Ma and X. G. Chen, One-pot synthesis of fluorescent silicon nanoparticles for sensitive and selective determination of 2,4,6-trinitrophenol in aqueous solution, Anal. Chem., 2017, 89, 3001–3008 CrossRef CAS PubMed.
- Z. Y. Yao, Y. D. Qiao, H. Q. Liang, W. Q. Ge, L. Zhang, Z. Cao and H. C. Wu, Approach Based on Polyelectrolyte-Induced Nanoassemblies for Enhancing Sensitivity of Pyrenyl Probes, Anal. Chem., 2016, 88, 10605–10610 CrossRef CAS PubMed.
- X. J. Hong, Q. Wei, Y. P. Cai, S. R. Zheng, Y. Yu, Y. Z. Fan, X. Y. Xu and L. P. Si, 2-Fold interpenetrating bifunctional Cd-metal−organic frameworks: highly selective adsorption for CO2 and sensitive luminescent sensing of nitro aromatic 2,4,6-trinitrophenol, ACS Appl. Mater. Interfaces, 2017, 9, 4701–4708 CrossRef CAS PubMed.
- B. B. Chen, Z. X. Liu, H. Y. Zou and C. Z. Huang, Highly selective detection of 2,4,6-trinitrophenol by using newly developed terbium-doped blue carbon dots, Analyst, 2016, 141, 2676–2681 RSC.
- T. P. Huynh, A. Wojnarowicz, A. Kelm, P. Woznicki, P. Borowicz, A. Majka, F. D'Souza and W. Kutner, Chemosensor for selective determination of 2,4,6-trinitrophenol using a custom designed imprinted polymer recognition unit cross-linked to a fluorophore transducer, ACS Sens., 2016, 1, 636–639 CrossRef CAS.
- A. Hakonen, F. C. Wang, P. O. Andersson, H. Wingfors, T. Rindzevicius, M. S. Schmidt, V. R. Soma, S. C. Xu, Y. Q. Li, A. Boisen and H. A. Wu, Hand-held femtogram detection of hazardous picric acid with hydrophobic Ag nanopillar SERS substrates and mechanism of elasto-capillarity, ACS Sens., 2017, 2, 198–202 CrossRef CAS PubMed.
- C. L. Zhang, S. M. Zhang, Y. H. Yan, F. Xia, A. N. Huang and Y. Z. Xian, Highly fluorescent polyimide covalent organic nanosheets as sensing probes for the detection of 2,4,6-trinitrophenol, ACS Appl. Mater. Interfaces, 2017, 9, 13415–13421 CrossRef CAS PubMed.
- Y. R. Tang, Y. Y. Su, N. Yang, L. C. Zhang and Y. Lv, Carbon nitride quantum dots: A novel chemiluminescence system for selective detection of free chlorine in water, Anal. Chem., 2014, 86, 4528–4535 CrossRef CAS PubMed.
- S. H. Cao, H. Chen, F. Jiang, Z. X. Hu and X. Wang, Construction of acetaldehyde-modified g-C3N4 ultrathin nanosheets via ethylene glycol-assisted liquid exfoliation for selective fluorescence sensing of Ag+, ACS Appl. Mater. Interfaces, 2018, 10, 44624–44633 CrossRef CAS PubMed.
- W. Chen, S. Kong, J. Wang, L. P. Du, W. Cai and C. S. Wu, Enhanced fluorescent effect of graphitic C3N4@ZIF-8 nanocomposite contribute to its improved sensing capabilities, RSC Adv., 2019, 9, 3734–3739 RSC.
- M. C. Rong, L. P. Lin, X. H. Song, T. T. Zhao, Y. X. Zhong, J. W. Yan, Y. R. Wang and X. Chen, A Label-free fluorescence sensing approach for selective and sensitive detection of 2,4,6-trinitrophenol (TNP) in aqueous solution using graphitic carbon nitride nanosheets, Anal. Chem., 2015, 87, 1288–1296 CrossRef CAS PubMed.
- H. Z. Xie, B. Feng, J. Hou and S. Y. Ai, A highly sensitive dual-signaling assay via inner filter effect between g-C3N4 and gold nanoparticles for organophosphorus pesticides, Sens. Actuators, B, 2018, 255, 2232–2239 CrossRef CAS.
- J. Hassanzadeh, B. R. Moghadam, A. Sobhani-Nasab, F. Ahmadi and M. Rahimi-Nasrabadi, Specific fluorometric assay for direct determination of amikacin by molecularly imprinting polymer on high fluorescent g-C3N4 quantum dots, Spectrochim. Acta, Part A, 2019, 214, 451–458 CrossRef CAS PubMed.
- H. Z. Xie, J. Dong, J. L. Duan, J. Y. Hou, S. Y. Ai and X. Y. Li, Magnetic nanoparticles-based immunoassay for aflatoxin B1 using porous g-C3N4 nanosheets as fluorescence probes, Sens. Actuators, B, 2019, 278, 147–152 CrossRef CAS.
- H. F. Yang, X. B. Li, X. X. Wang, W. F. Chen, W. Bian and M. F. Choi, Silver-doped graphite carbon nitride nanosheets as fluorescent probe for the detection of curcumin, Luminescence, 2018, 33, 1062–1069 CrossRef CAS PubMed.
- Y. R. Guo, Q. Liu, Z. H. Li, Z. G. Zheng and X. M. Fang, Enhanced photocatalytic hydrogen evolution performance of mesoporous graphitic carbon nitride co-doped with potassium and iodine, Appl. Catal., B, 2017, 221, 362–370 CrossRef.
- S. N. Talapaneni, G. P. Mane, D. H. Park, K. S. Lakhi, K. Ramadass, S. Joseph, W. M. Skinner, U. Ravon, K. Al-Bahilyb and A. Vinu, Diaminotetrazine based mesoporous C3N6 with a well-ordered 3D cubic structure and its excellent photocatalytic performance for hydrogen evolution, J. Mater. Chem. A, 2017, 5(34), 18183–18192 RSC.
- G. P. Mane, S. N. Talapaneni, K. S. Lakhi, H. Ilbeygi, U. Ravon, K. Al-Bahily, T. Mori, D. H. Park and A. Vinu, Highly ordered nitrogen-rich mesoporous carbon nitrides and their superior performance for sensing and photocatalytic hydrogen generation, Angew. Chem., 2017, 129, 8601–8605 CrossRef.
- Q. Gu, Y. S. Li, L. S. Yin, J. L. Long, X. X. Wang and C. Xue, Template-free synthesis of porous graphitic carbon nitride microspheres for enhanced photocatalytic hydrogen generation with high stability, Appl. Catal., B, 2015, 165, 503–510 CrossRef CAS.
- Z. Z. Lin and X. C. Wang, Nanostructure engineering and doping of conjugated carbon nitride semiconductors for hydrogen photosynthesis, Angew. Chem., Int. Ed., 2013, 52, 1735–1738 CrossRef CAS PubMed.
- Y. T. Xiao, G. H. Tian, W. Li, Y. Xie, B. J. Jiang, C. G. Tian, D. Y. Zhao and H. G. Fu, Molecule self-assembly synthesis of porous few-layer carbon nitride for highly efficient photoredox catalysis, J. Am. Chem. Soc., 2019, 141, 2508–2515 CrossRef CAS PubMed.
- T. H. Wang, Y. X. Sun, T. Y. Chen and Y. Hu, Research article spectral properties of the interaction between hesperidin of tangerine peel's active ingredient with protein, Int. J. Pharmacol., 2018, 14, 106–1065 Search PubMed.
- T. N. Tikhonova, E. A. Shirshin, G. S. Budylin, V. V. Fadeev and G. P. Petrova, Assessment of the europium(III) binding sites on albumin using fluorescence spectroscopy, J. Phys. Chem. B, 2014, 118, 6626–6633 CrossRef CAS PubMed.
- R. S. Mani, F. Karimi-Busheri, M. Fanta, C. E. Cass and M. Weinfeld, Spectroscopic studies of DNA and ATP binding to human polynucleotide kinase: evidence for a ternary complex, Biochemistry, 2003, 42, 12077–12084 CrossRef CAS PubMed.
- E. L. Guan, M. Xie, Y. Shi, X. L. Zhang and J. H. Zhan, Dimolecular interaction between graphitic carbon nitride nanosheets and phenols: a mechanism study, Carbon, 2016, 102, 462–469 CrossRef CAS.
- F. Raziq, L. Q. Sun, Y. Y. Wang, X. L. Zhang, M. Humayun, S. Ali, L. L. Bai, Y. Qu, H. T. Yu and L. Q. Jing, Synthesis of large surface-area g-C3N4 comodified with MnOx and Au-TiO2 as efficient visible-light photocatalysts for fuel production, Adv. Energy Mater., 2017, 1701580 Search PubMed.
- S. Mukherjee, A. V. Desai, B. Manna, A. I. Inamdar and S. K. Ghosh, Exploitation of Guest Accessible Aliphatic Amine Functionality of a Metal−Organic Framework for Selective Detection of 2,4,6-trinitrophenol (TNP) in Water, Cryst. Growth Des., 2015, 15, 4627–4634 CrossRef CAS.
- X. L. Zhang, X. X. Zhang, J. D. Li, J. H. Sun, J. Bian, S. Y. Wang, Y. Qu, C. L. Qin and L. Q. Jing, Exceptional visible-light activities of g-C3N4 nanosheets dependent on the unexpected synergistic effects of prolonging charge lifetime and catalyzing H2 evolution with H2O, Appl. Catal., B, 2018, 237, 50–58 CrossRef CAS.
- A. Shi, J. H. Sun, Q. H. Zeng, C. Shao, Z. C. Sun, H. B. Li, X. G. Kong and J. L. Zhao, Photoluminescence quenching of CdTe/CdS core-shell quantum dots in aqueous solution by ZnO nanocrystals, J. Lumin., 2011, 131, 1536–1540 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9en01165j |
| This journal is © The Royal Society of Chemistry 2020 |