Defense and inhibition integrated mesoporous nanoselenium delivery system against tomato gray mold†
Jiawei
Liu‡
,
Xufeng
Zhu‡
,
Xu
Chen
,
Yanan
Liu
,
Youcong
Gong
,
Guanglong
Yuan
,
Jie
Liu
 * and
Lanmei
Chen
* and
Lanmei
Chen
Department of Chemistry, College of Chemistry and Materials Science, Jinan University, Guangzhou 510630, China. E-mail: tliuliu@jnu.edu.cn; Fax: +86 020 85220223; Tel: +86 020 85220223
First published on 18th November 2019
Abstract
Botrytis cinerea (B. cinerea) is a necrotic and nutritive pathogenic fungus that causes gray mold in a variety of economic crops. Due to its multiple infestation patterns and its latent infestation, it is difficult to control. However, traditional chemical antifungal agents are not suitable for long-term treatment of gray mold because of their high toxicity and resistance. In this study, we synthesized mesoporous nano-selenium (Se NPs) with high loading efficiency to combine with the antifungal drug thiophanate-methyl (TM), which enhanced TM's water solubility while reducing its dosage. Based on the synthesis and secretion of oxalic acid by B. cinerea after infection, acid-responsive polyacrylic acid (PAA) was used to plug the mesopores of Se NPs, which led to a highly loaded and responsive nano-drug delivery system (TM@Se@PAA NPs) for effective control of drug release. We demonstrated that TM@Se@PAA NPs at low concentration had a rapid and effective antifungal effect against B. cinerea and did not induce drug resistance. Based on the excellent resistance to B. cinerea in vitro, we designed three in vivo delivery models. The results showed that TM@Se@PAA NPs could effectively prevent and inhibit the growth of B. cinerea, and multiple applications did not produce significant toxic side effects on plants. In particular, TM@Se@PAA NPs could improve the photosynthetic efficiency of plants and promote plant growth to some extent. The results of this study indicated that TM@Se@PAA NPs are promising for the prevention and inhibition of B. cinerea and other destructive fungal diseases.
Environmental significanceIn the field of agriculture, the environmental problems caused by the use of chemical antifungal agents have aroused researchers' concerns. With the increasing supervision of the use of chemical antifungal agents, it is urgent to find antifungal agents with low environmental pollution. Here, highly biocompatible TM@Se@PAA NPs were constructed containing Se that is beneficial to plant growth. At the same time, TM@Se@PAA NPs could enhance TM's hydrophilicity and reduce the possibility of environmental pollution by allowing the dosage of TM to be reduced. In particular, many plant experiments showed that TM@Se@PAA NPs could be used to deliver the antifungal drug TM to enhance plant prevention of and resistance to gray mold and improve photosynthesis efficiency. Therefore, the functionalized nano-selenium developed in this study provides an important reference value for the treatment of phytopathogenic fungi and for environmentally friendly fungicides. |
1. Introduction
As a fungal disease caused by Botrytis cinerea (B. cinerea), gray mold is ubiquitous in cash crops such as tomatoes, grapes, potatoes and strawberries, costing 1.26 billion dollars each year globally for its control.1–3B. cinerea can infect different organs of plants, including petals, fruits, leaves and stems, through wounds or natural openings and cause severe decay and wilting.4,5 In addition, B. cinerea has multiple modes of attack on host plants, including the production of phytotoxic metabolites, synthesis and secretion of cell wall-degrading enzymes and oxalic acid (necrosis-inducing activity), and stimulation of oxidative stress.6,7 At the same time, it also has the features of latent infection and low-temperature pathogenicity, which can be carried into the market by postharvest fruits, resulting in fruit rotting during storage, transportation and marketing, causing serious economic losses.3 Besides, it is difficult to rapidly and effectively control plant gray mold during the period of infection, and it is necessary to prevent the infection of B. cinerea in advance and enhance the defense ability of plants.8,9 At present, the main way to control B. cinerea is chemical treatment. However, long-term use of high-dose chemical antifungal agents will not only lead to high residues and environmental and food contamination, but also to strain resistance.10–13 Secondly, the most direct way to prevent gray mold is to improve plant culture conditions, such as reducing air humidity, reducing planting density, making variable temperature and ventilation, etc., but the cost is high.14 In addition, biocontrol agents based on antagonistic microorganisms have also been used to control plant gray mold, such as Trichoderma harzianum and Bacillus strains, but due to their limited biological control activities, they have not been widely used.15In recent years, nano-antimicrobial materials have become a focus of research due to their excellent antimicrobial properties and non-resistance.16 Among them, Ag NPs and TiO2 NPs have been strongly confirmed, and mesoporous nano-selenium (Se NPs) has also been found to have antimicrobial activity recently.17–22 However, due to the limited research and application of nano-antimicrobial materials in plant disease, and the lack of impact on plant physiology at the molecular level, they are still in the research stage.23 Selenium has been studied in agricultural applications for decades and is a beneficial element for plant growth and development.24 Appropriate amounts of Se can help increase crop yield, improve the quality of agricultural products, enhance the stress resistance of plants and alleviate the stress damage caused by pests, diseases and weeds.25,26 In addition, studies have shown that reasonable Se supplementation plays an active role in human health, such as in Keshan disease and Kashin–Beck disease, and Se has a certain anti-cancer activity.27–29 In order to further explore the broad-spectrum antimicrobial properties of Se NPs, we plan to use them for developing a new B. cinerea inhibitor, which may bring new therapeutic strategies for the prevention and control of plant gray mold. At the same time, such a system can supply plants with Se to promote their growth and avoid the limitations of chemical antimicrobial agents.
In this study, we synthesized a novel nano-selenium-methylthiophanate therapy system for prevention and treatment of multiple gray mold plant models (grape, tomato) caused by B. cinerea. First of all, we selected the low-toxicity broad-spectrum antifungal agent thiophanate-methyl (TM) as the drug, which can affect the cell division and spore germination of pathogens by interfering with mycelial formation, thereby killing the bacteria.30–32 In order to achieve high loading efficiency of TM, we prepared a spherical mesoporous Se NPs carrier by a redox and template method. By combining TM with mesoporous Se NPs, the hydrophobicity of TM is improved, and the sensitivity of B. cinerea to TM is improved, thereby improving the utilization and antifungal activity of TM. At the same time, on the basis of oxalic acid and other acidic substances secreted by B. cinerea after infecting plants, accompanied by degradation of plant cell walls and pH reduction,33 we used pH-responsive polyacrylic acid (PAA)34 to block the pores of Se NPs after drug loading, which can achieve an optimal release speed of the drug according to whether the plant is infected or not. The experimental results showed that the resulting nano-drug delivery system (TM@Se@PAA NPs) had rapid and effective resistance to B. cinerea in vitro, and could effectively prevent and inhibit the growth of B. cinerea. Based on this excellent property, we designed three modes of administration in plants (Scheme 1): continuous administration of TM@Se@PAA NPs nano-formulation, first inoculating with B. cinerea before TM@Se@PAA NPs, and adding TM@Se@PAA NPs before inoculation of B. cinerea. The results showed that long-term use of TM@Se@PAA NPs can promote plant growth, improve tomato quality, and has good biocompatibility; and the other two modes of administration proved able to enhance the ability of plants to defend against and resist gray mold. More importantly, TM@Se@PAA NPs with high efficiency and low toxicity are also suitable for the development of nano-pesticides (high water solubility, sustained-release performance, high efficiency),35 which may become a substitute for traditional pesticides for the treatment of gray mold, and provide a new model for the development of environmentally friendly pesticides. Therefore, the functionalized Se NPs we have developed have great potential in the treatment of plant fungal diseases.
 | ||
| Scheme 1 Schematic illustration for synthetic procedure of TM@Se@PAA NPs and the three modes of administration to tomato plants. | ||
2. Materials and methods
2.1. Materials and reagents
Hexadecyltrimethylammonium bromide (CTAB) and TM were obtained from Shanghai YuanYe Biotechnology Co. Ltd (Shanghai, China); thiol-polyethylene glycol-carboxyl (SH-PEG-COOH, MW400) was purchased from Creative PEGWorks (USA); sodium selenite (Na2SeO3), ascorbic acid (Vc), and PAA powder (MW2000) were purchased from Shanghai Maclean Biochemical Co. Ltd (Shanghai, China); sodium hydroxide (NaOH), anhydrous ethanol (EtOH), hydrochloric acid (HCl), and acetone were purchased from Guangzhou Chemical Reagent Factory. Ultrapure Milli-Q water (18.2 MW) was used in the experiments. Potato dextrose agar (PDA) medium was purchased from AoBoXing Product (Beijing, China); zinc (40 nm) and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 909 Calcofluor White Stain were purchased from Sigma-Aldrich; B. cinerea (GIM 3.47) was obtained from Guangdong Province Microbial Strain Depository center in China. The experimental tomato seeds were obtained from Guangdong Kenong Vegetable Seed Co. Ltd; grape and tomato fruits were purchased from Huiwanjia Supermarket, Shipai Taoyu Road, Tianhe District, Guangzhou City, Guangdong Province, China; the above materials for plant experiments were sent to the laboratory for disinfection.
909 Calcofluor White Stain were purchased from Sigma-Aldrich; B. cinerea (GIM 3.47) was obtained from Guangdong Province Microbial Strain Depository center in China. The experimental tomato seeds were obtained from Guangdong Kenong Vegetable Seed Co. Ltd; grape and tomato fruits were purchased from Huiwanjia Supermarket, Shipai Taoyu Road, Tianhe District, Guangzhou City, Guangdong Province, China; the above materials for plant experiments were sent to the laboratory for disinfection.
2.2. Synthesis and characterization of TM@Se@PAA NPs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCl (19
HCl (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and refluxed at 80 °C for 12 h to remove CTAB and Zn, then washed with acetone several times. Finally, solid Se NPs were obtained after freeze-drying.
1) and refluxed at 80 °C for 12 h to remove CTAB and Zn, then washed with acetone several times. Finally, solid Se NPs were obtained after freeze-drying.
The nano-components corresponding to each synthesis stage were characterized by various analytical techniques. The morphology and structure of Se NPs, TM@Se NPs and TM@Se@PAA NPs were recorded by transmission electron microscopy (TEM; Hitachi, H-7650), and their size distribution and ζ-potential were measured by a Nano-ZS instrument (Malvern Instruments Limited). The pore size distribution of mesoporous Se NPs was determined by nitrogen adsorption–desorption measurements, and the crystal form of each stage was determined by X-ray diffraction (XRD) analysis, and then characterized by Fourier transform infrared (FT-IR) spectroscopy and ultraviolet-visible (UV-vis) spectroscopy. In addition, the loading of TM was measured and calculated by thermogravimetric analysis and UV-vis spectroscopy, and the amount of TM released under different pH conditions was measured.
2.3. Tomato cultivation
Tomato seeds were soaked in a series of concentrations of nano-preparations of TM@Se@PAA NPs for 24 h, then embedded in potting soil, and placed in a 16/8 hour light/dark cycle photoperiod and watered properly once in two/three days; other seeds soaked in sterile water were the blank control group and cultured under the same conditions; all of them were found to germinate. After seed germination, three pots of seedlings were sprayed with three concentrations of TM@Se@PAA NPs (50, 100, 200 mg L−1) once every 7 days, each dose being about 1 mL per plant, and the other groups were not treated. It was preliminarily determined that the nano-preparations were non-toxic to seed germination and seeds could grow healthily. Photographs were taken regularly to record the growth status (Fig. S11†) in order to ensure the subsequent experiments. Similarly, the same test was performed on chrysanthemum seeds with TM@Se@PAA NPs (50, 100, 200 mg L−1). Besides, after chrysanthemum seed germination, three pots of seedlings were sprayed with three concentrations of Se NPs (25, 50, 100 mg L−1) once every 7 days, each dose being about 1 mL per plant, and the other groups were not treated.2.4. Antifungal activity of TM@Se@PAA NPs in vitro
The spore suspension was obtained by washing B. cinerea for 7 days on PDA medium with sterile water. The concentration of the spore suspension was diluted to 2 × 105 spores per mL for use by counting with a hemocytometer.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 to the above medium to make 25 mL drug medium after ultraviolet sterilization, and then 20 μL of B. cinerea suspension was inoculated onto each medium to observe fungal colony growth.
9 to the above medium to make 25 mL drug medium after ultraviolet sterilization, and then 20 μL of B. cinerea suspension was inoculated onto each medium to observe fungal colony growth.
2.5. Morphological observation of B. cinerea
The mature B. cinerea suspension was incubated with four different components (sterile water, TM, TM@Se@PAA NPs and carbendazim) for 12 h at 26 °C. Afterwards, each was centrifuged and washed three times with sterile water, then fixed with 2.5% glutaraldehyde solution at 4 °C overnight. Next, B. cinerea suspensions were dehydrated in sequence by 30%, 50%, 70%, 80%, 90%, 95%, 100% ethanol for 15 min each, and sprayed with gold for scanning electron microscopy (SEM) imaging observation.Sterile water (control), TM, TM@Se@PAA NPs and carbendazim (100 μL; 100 mg L−1) were added separately into Petri dishes containing PDA medium (5 mL). After incubation for 2 days, 500 μL of B. cinerea suspension was separately added to each medium. In the subsequent 12 h, 24 h and other time periods, fungal solutions from each medium were severally added to a microslide, then Calcofluor White Stain agent and 10% potassium hydroxide were added and mixed for 1 min. The changes of B. cinerea morphology at different time periods were observed by fluorescence microscopy to analyze how the drugs inhibited the growth of B. cinerea.
2.6. Plant experiment
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether (1
petroleum ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent was used to stir and extract them at 600 r min−1 for 2 h. After filtration, the acetone phase in the filtrate was discarded and the organic phase was made up to 10 mL with petroleum ether; then the absorbance value was measured by a UV-vis spectrometer to calculate the lycopene content. Petroleum ether was used as the blank control. Besides, 2 mL concentrated HNO3 was added to 0.3 g tomato homogenate to soak overnight. The resulting solution was heated to dryness after it became transparent. Then a few drops of perchloric acid were added to heat, waiting for the white smoke to be exhausted; after that, it was removed for cooling, and 2% HNO3 was used to make up to 5 mL for testing.
1) solvent was used to stir and extract them at 600 r min−1 for 2 h. After filtration, the acetone phase in the filtrate was discarded and the organic phase was made up to 10 mL with petroleum ether; then the absorbance value was measured by a UV-vis spectrometer to calculate the lycopene content. Petroleum ether was used as the blank control. Besides, 2 mL concentrated HNO3 was added to 0.3 g tomato homogenate to soak overnight. The resulting solution was heated to dryness after it became transparent. Then a few drops of perchloric acid were added to heat, waiting for the white smoke to be exhausted; after that, it was removed for cooling, and 2% HNO3 was used to make up to 5 mL for testing.
2.7. Determination of fluorescence parameters for photosynthesis
Six-week-old tomato plants treated with TM@Se@PAA NPs (50, 100, 200 mg L−1) and plants after the live tomato infection test were used to measure the basic parameters with an FMS-2 portable modulation fluorometer, such as: maximum fluorescence , minimum fluorescence
, minimum fluorescence  , steady-state fluorescence emission (Fs), etc. In addition, other fluorescence parameters were calculated by the following formulas: maximum efficiency of PSII
, steady-state fluorescence emission (Fs), etc. In addition, other fluorescence parameters were calculated by the following formulas: maximum efficiency of PSII  , photochemical quenching
, photochemical quenching  , non-photochemical quenching
, non-photochemical quenching  and photosynthetic electron transport efficiency (ETR = ΦPSII × PAR × 0.84 × 0.5). The photosynthetically active radiation (PAR) was measured to be 500 μmol m−2 s−1. The plants were placed in the dark for adaptation for 30 minutes before the measurement, and the experiment was repeated three times.
and photosynthetic electron transport efficiency (ETR = ΦPSII × PAR × 0.84 × 0.5). The photosynthetically active radiation (PAR) was measured to be 500 μmol m−2 s−1. The plants were placed in the dark for adaptation for 30 minutes before the measurement, and the experiment was repeated three times.
3. Results and discussion
3.1. Synthesis and characterization of TM@Se@PAA NPs
The Se NPs were prepared by a Zn and CTAB template-assisted method, using PEG on Se NPs to load TM and covering PAA on the surface by hydrogen bonding and electrostatic interaction. TEM imaging (Fig. 1A) showed the spherical mesoporous structure of Se NPs with good dispersibility and a particle size of about 80 nm. The pore size distribution was measured at about 12 nm according to N2 adsorption and desorption, and the surface area and pore volume of Se NPs were calculated as 447.5 m2 g−1 and 1.12 cm3 g−1 (Fig. 1D). As shown in Fig. 1B, the morphology of Se NPs changed significantly due to the surface binding of a large amount of TM and the size increased to 95 nm; finally, it increased to about 110 nm after encapsulating with PAA (Fig. 1C). The size of the nanoparticles was similar to that measured by dynamic light scattering (Fig. S1†). The corresponding zeta potential value of Se NPs was −22.4 mV in aqueous solution, which changed to +6.7 mV after loading with TM. However, due to the successful loading of PAA, the zeta potential of TM@Se@PAA NPs was finally −31.4 mV (Fig. 1E), and there was no significantly change in the potential within one week, indicating good stability (Fig. S2†). In addition, results of XRD of Se NPs showed diffraction peaks at 31°, 43°, 47° and 56°, consistent with diffraction peaks of triangular selenium (JCPDS 06-0362). The XRD patterns showed that TM@Se NPs and TM@Se@PAA NPs had a strong absorption peak at 30°, and their crystal forms did not change, which proved that Se NPs had good stability as a carrier (Fig. 1I). Moreover, successful preparation of Se NPs, TM@Se NPs and TM@Se@PAA NPs was also confirmed from analysis of FT-IR and UV-vis spectra. By analyzing the FT-IR spectra, compared with Se NPs, the carbon–sulfur double bond (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S), N–H and C
S), N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations may be assigned to characteristic absorption peaks at 1250 cm−1, 1600 cm−1 and 1750 cm−1 for TM@Se NPs, respectively, which indicated that TM was successfully loaded into Se NPs (Fig. 1F). In addition, in the UV-vis spectrum, it could be observed that Se NPs had an absorption peak at 266 nm, and there was a new absorption peak at 208 nm which indicated that TM was successfully loaded. TM@Se NPs showed absorption peaks at 266 nm and 208 nm in the UV-vis spectrum, one of them coinciding with the absorption peak of Se NPs at 266 nm. Therefore, the absorption peak at 208 nm was selected as a qualitative reference for TM and its corresponding absorbance for quantitative reference (Fig. 1G). By measuring the UV-vis spectra for different concentrations of TM, the standard curve was drawn, and the drug loading rate of TM@Se@PAA NPs was calculated to be 19.0% (Fig. S3†). To further verify the TM loading, we performed thermogravimetric analysis. The results showed that Se NPs had good stability which began to decompose at 278 °C, being completely decomposed at 500 °C. The decomposition temperature of TM was 172 °C, which decomposed into carbendazim at higher temperature; this corresponded to the weight in TM@Se@PAA NPs being reduced by 18.5%, which conformed to the TM loading efficiency measured from the UV-vis spectrum (Fig. 1H). Next, in order to study the release efficiency in vitro, we analyzed the release process of TM in vitro under acidic conditions (pH = 5.6) and neutral conditions (pH = 7.4). It was found that TM release was accelerated with a decrease of pH, and released slowly within 7 days under neutral conditions, which could prolong the effective time of TM (Fig. S4†). Combined with the normal growth state of tomato plants, the pH value of soil was 7.2, which was conducive to the stable existence of TM, and oxalic acid and other acidic substances released from plants that were infested could accelerate its release. By the way, the dendritic structure of TM@Se@PAA NPs could effectively deliver the antifungal drug TM in a pH-controlled mechanism. Therefore, the hydrophobic effect of TM was effectively improved through the formation of a stable nano-treatment system with mesoporous selenium being loaded with antifungal drug TM, and it is expected to further enhance its antifungal activity cycle by pH-responsive slow release.
O stretching vibrations may be assigned to characteristic absorption peaks at 1250 cm−1, 1600 cm−1 and 1750 cm−1 for TM@Se NPs, respectively, which indicated that TM was successfully loaded into Se NPs (Fig. 1F). In addition, in the UV-vis spectrum, it could be observed that Se NPs had an absorption peak at 266 nm, and there was a new absorption peak at 208 nm which indicated that TM was successfully loaded. TM@Se NPs showed absorption peaks at 266 nm and 208 nm in the UV-vis spectrum, one of them coinciding with the absorption peak of Se NPs at 266 nm. Therefore, the absorption peak at 208 nm was selected as a qualitative reference for TM and its corresponding absorbance for quantitative reference (Fig. 1G). By measuring the UV-vis spectra for different concentrations of TM, the standard curve was drawn, and the drug loading rate of TM@Se@PAA NPs was calculated to be 19.0% (Fig. S3†). To further verify the TM loading, we performed thermogravimetric analysis. The results showed that Se NPs had good stability which began to decompose at 278 °C, being completely decomposed at 500 °C. The decomposition temperature of TM was 172 °C, which decomposed into carbendazim at higher temperature; this corresponded to the weight in TM@Se@PAA NPs being reduced by 18.5%, which conformed to the TM loading efficiency measured from the UV-vis spectrum (Fig. 1H). Next, in order to study the release efficiency in vitro, we analyzed the release process of TM in vitro under acidic conditions (pH = 5.6) and neutral conditions (pH = 7.4). It was found that TM release was accelerated with a decrease of pH, and released slowly within 7 days under neutral conditions, which could prolong the effective time of TM (Fig. S4†). Combined with the normal growth state of tomato plants, the pH value of soil was 7.2, which was conducive to the stable existence of TM, and oxalic acid and other acidic substances released from plants that were infested could accelerate its release. By the way, the dendritic structure of TM@Se@PAA NPs could effectively deliver the antifungal drug TM in a pH-controlled mechanism. Therefore, the hydrophobic effect of TM was effectively improved through the formation of a stable nano-treatment system with mesoporous selenium being loaded with antifungal drug TM, and it is expected to further enhance its antifungal activity cycle by pH-responsive slow release.
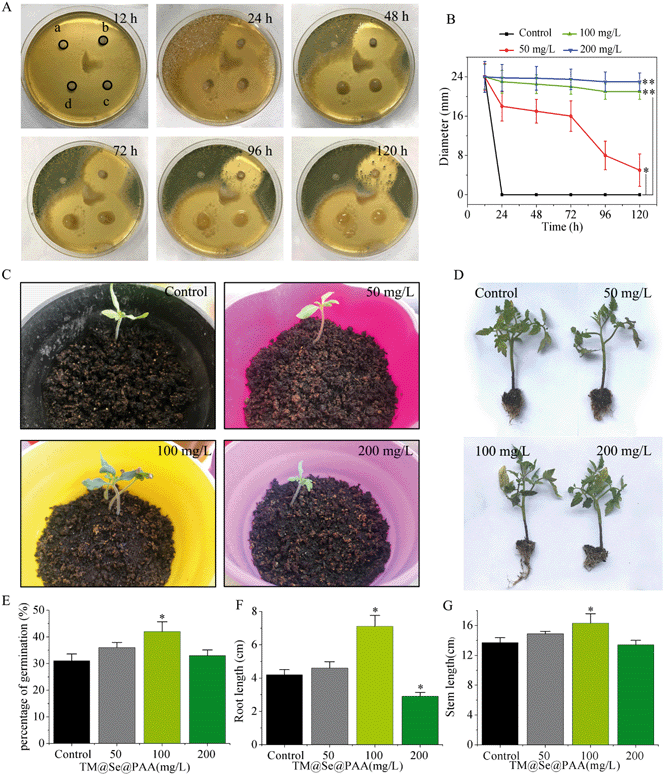
3.2. Determination of antifungal concentration of TM@Se@PAA NPs
To verify the antifungal activity of TM@Se@PAA NPs, we first carried out a paper-based antimicrobial test at different concentrations of TM@Se@PAA NPs (50, 100, 200 mg L−1). As a result, fungal colonies were formed on the filter paper and the boundary of the Petri dish for the control group at 24 h. As for the other concentrations of nano-preparation groups within 72 h, the surrounding of each filter paper was aseptically formed, indicating that TM@Se@PAA NPs at various concentrations could effectively inhibit the formation of colonies within 72 h. With the passage of time, fungal colonies began to form near the filter paper containing 50 mg L−1 TM@Se@PAA NPs at 96 h with a 8.1 ± 2.9 mm bacteriostatic diameter, and finally shrank to 1.4 ± 1.4 mm at 120 h. However, TM@Se@PAA NPs at concentrations of 100 and 200 mg L−1 had no colonies found within 120 h, and the bacteriostatic diameter reached 21 ± 1.3 mm and 23 ± 1.8 mm respectively (Fig. 2A and B), proving that TM@Se@PAA NPs (100 and 200 mg L−1) had good antifungal effect and could effectively inhibit the growth of B. cinerea for a long time. In addition, in order to exclude the material interference during the synthesis process and to compare the fungistatic effect with the commercial fungicide carbendazim, corresponding paper-based antifungal tests were performed. The results showed that separate PAA, Se NPs, and PEG-SH had no fungistatic effect. Besides, carbendazim and TM@Se@PAA NPs had good effects of inhibiting the growth of B. cinerea, while TM@Se NPs showed similar bacteriostasis to both, which may be due to the synergistic antifungal effect of TM and Se NPs (Fig. S5†). The results indicated that TM@Se@PAA NPs showed excellent inhibition effect against B. cinerea in the paper-based antifungal test and could be used for further experiments.In order to further determine the effective antifungal concentration and reduce the toxicity to plants caused by the dosage, we evaluated the germination rate of tomato seeds, the growth of 4-week-old tomato plants and the growth of 2-week-old chrysanthemums. From the germination and growth conditions, tomato seeds treated with three concentrations of TM@Se@PAA NPs all germinated, and the seed germination rate increased by 5%, 11%, 2% (Fig. 2C and E), respectively. After 30 days, they all grew into thicker seedlings (Fig. 2D), which showed good biosafety, and the roots and stems of the seedlings increased to different degrees (Fig. 2F and G). Similarly, the leaf length and root length of 2-week-old chrysanthemum treated with TM@Se@PAA NPs increased significantly, while the sapling stem length did not change distinctly (Fig. S6†). It may be said that TM@Se@PAA NPs at low concentration could increase seed germination rate and promote elongation of stems and leaves of plants, while high concentration (200 mg L−1) was not conducive to plant root growth, but it did not affect the development of other organs of plants. This could be due to long-term exposure to high concentrations of nano-preparations that gradually accumulated in plant roots.36 In addition, to explore the effect of Se NPs on chrysanthemums, we sprayed different concentrations of Se NPs (25, 50 and 100 mg L−1) onto chrysanthemums, and then recorded the stem length, leaf length and roots of one-month-old chrysanthemums. As shown in Fig. S7,† we found that none of the concentrations of Se NPs caused significant toxicity to chrysanthemums, and 25 and 50 mg L−1 Se NPs could promote root growth. In summary, in order to ensure good antifungal effect and plant growth requirements, we recommend using TM@Se@PAA NPs at 100 mg L−1 as the concentration for subsequent experiments.
3.3. Evaluation of TM@Se@PAA NPs for the prevention and inhibition of B. cinerea
To further evaluate the prevention and inhibition ability of TM@Se@PAA NPs against B. cinerea, TM@Se@PAA NPs were previously added to the medium, then TM@Se@PAA NPs were added to the medium containing the mature fungal colonies. The prevention test results of drug medium showed that the control group had many fungal colonies on the medium at 48 h, but no fungal colony was observed on the medium within 96 h after TM@Se@PAA NPs treatment; while a small number of colonies were observed on the medium at 48 h after carbendazim treatment, and the fungal colony zone increased with time (Fig. 3A). These results indicated that TM@Se@PAA NPs had excellent ability to prevent the growth of B. cinerea. Since the drug medium already contained substances that were not conducive to the growth of B. cinerea, its inhibition on the existing strain was not observed. Therefore, we used the fungal colonies that had been cultured for a certain period of time, and then added TM@Se@PAA NPs. The fungistatic effect is shown in Fig. 3B. The medium at 72 h after treatment with TM@Se@PAA NPs was consistent with that at 48 h, indicating that the first dosing could effectively inhibit the expansion of fungal colonies, showing a similar rapid fungistatic effect to carbendazim. Compared with the medium 72 h after TM treatment, the fungal colonies could still spread around, and the antifungal activity was weaker than that of the other two groups. This may be due to the TM@Se@PAA NPs improving the poor water solubility of TM and being better absorbed by the fungus, thus improving the fungistatic effect. Inhibition tests showed that TM@Se@PAA NPs could effectively inhibit the growth and spread of B. cinerea. Therefore, TM@Se@PAA NPs with integrated prevention and inhibition not only significantly prevented the growth of B. cinerea, but also directly killed B. cinerea and restrained its expansion.3.4. Mycelium destruction
The normal morphology of B. cinerea can reflect whether it has the ability to infect a host. In order to analyze whether the nano-preparations can affect the morphology of B. cinerea, we observed the effect of TM@Se@PAA NPs on the morphology of B. cinerea by SEM. The results showed that the mycelium of B. cinerea in the control group was intact, and its spores were numerous and full, and the surface of spores was smooth. After treatment with TM@Se@PAA NPs and carbendazim for 12 h, the mycelium was severely damaged or even broken (indicated by the red arrows in Fig. 4A). At the same time, spores had varying degrees of atrophy, and some of them were accompanied by leakage of contents (indicated by the blue arrows). This confirmed that TM@Se@PAA NPs could damage mycelium integrity and rupture spore membranes to kill B. cinerea (Fig. 4A). In addition, in order to quantify fungal growth and explore whether TM@Se@PAA NPs have long-term resistance to B. cinerea, we stained B. cinerea with Calcofluor White Stain and analyzed morphological changes of B. cinerea in several time periods. Calcofluor White Stain is a non-specific fluorescent dye that binds to cellulose and chitin in the cell walls of fungi and other organisms. It can be used to quickly detect fungi and exhibits purple-blue fluorescence.37,38 After incubation with different components (control, TM, TM@Se@PAA NPs and carbendazim) for 2 days, the mycelial morphology of B. cinerea was analyzed by fluorescence microscopy after staining with Calcofluor White Stain. The experiment showed that the hyphae of B. cinerea in the control group enlarged obviously, and the number of spores increased significantly with time. However, after treatment with TM@Se@PAA NPs and carbendazim at 12 h, the mycelium had different degrees of damage and fracture, and the fluorescence intensity was 0.23 and 0.3 times that of the control group, respectively. Besides, the complete mycelium could not be observed after 24 h, and the number of spores decreased significantly. It may be that TM@Se@PAA NPs could delay the release of TM, which not only inhibited the growth of B. cinerea, but also effectively restrained the number of spores with a long-term mechanism. In contrast, although the growth of B. cinerea treated with TM was inhibited to some extent within 24 h, the fungus still had relatively integral morphology (Fig. 4B). From the fluorescence quantification intensity, TM@Se@PAA NPs showed the best inhibition effect on the growth of B. cinerea. Its fluorescence intensity (arbitrary units) was 11.3, which was 0.3 times that of the TM group and 56 times lower than that of the control group (Fig. 4C). The results showed that the mesoporous Se NPs delivery system could directly destroy the fungal membrane, even break the hyphae, and eliminate the spores, which could effectively kill B. cinerea.3.5. Prevention and treatment using TM@Se@PAA NPs on tomato leaves infected with B. cinerea
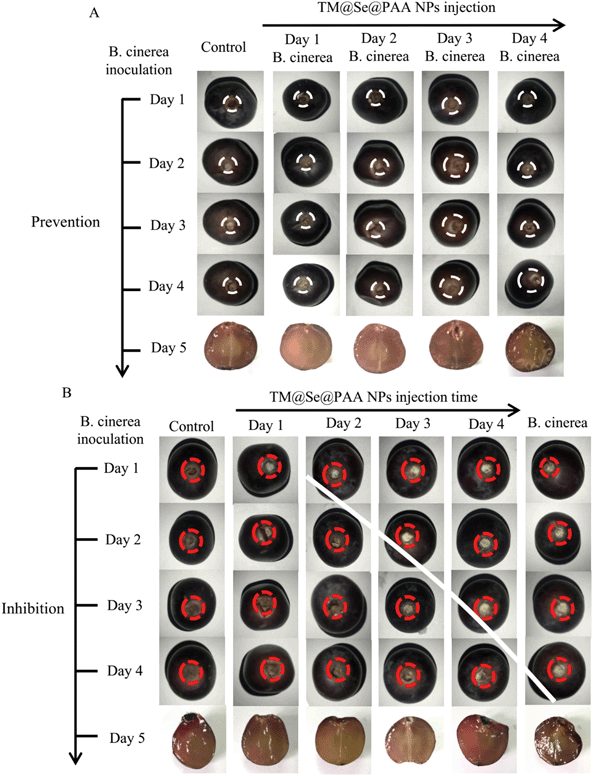
Based on the good antifungal properties and low toxicity of TM@Se@PAA NPs, the preventive and inhibitory effects of the nanoparticles on plant leaves infected by B. cinerea were investigated. We designed two modes of administration: first, TM@Se@PAA NPs were added to the leaves in advance, then B. cinerea was inoculated at certain intervals; second, we inoculated with B. cinerea and then added TM@Se@PAA NPs. This enabled us to explore both prevention and inhibition of tomato gray mold. Initially, the feasibility of TM@Se@PAA NPs for resistance to B. cinerea in tomato was analyzed by in vitro leaf inoculation method. It could be clearly seen for the B. cinerea group (Fig. 5): its lesion location was not limited to the inoculation site (black circle), which spread over many parts of the leaves, and had eroded into the interior of the blade, showing irreversible damage. For the TM@Se@PAA NPs + B. cinerea (prevention) group (Fig. 5): within two days after treatment with TM@Se@PAA NPs, the state of the two groups of leaves (day 2 and day 3) was similar to that of the control group, and no significant pathological phenomenon occurred; besides, there was minimal damage degree of leaf tissue in the experimental group eventually, showing a good preventive effect against B. cinerea. For the B. cinerea + TM@Se@PAA NPs (inhibition) group (Fig. 5): although there were different degrees of damage, it was observed from the enlarged view of the wound on the fifth day that the damage only remained on the surface of the leaves, which was not enough to destroy the veins. To some extent, TM@Se@PAA NPs may effectively reduce the infection of B. cinerea on tomato plants and improve the ability of TM to control tomato gray mold. The results showed that the prevention group with TM@Se@PAA NPs had the best effect on controlling leaf gray mold, and the damaged area of the leaves was the smallest. At the same time, the inhibition group proved that TM@Se@PAA NPs could slow down the infection of leaves by B. cinerea. Therefore, the above results showed that TM@Se@PAA NPs could prevent and control the infection of tomato leaves by B. cinerea within a period of time and restrain further decay and damage.3.6. Preventive and inhibitory tests of B. cinerea infecting tomato plants
As B. cinerea may cause multiple organ damage and decay after infecting plants, and to further prove the preventive and inhibitory effects of TM@Se@PAA NPs, we then carried out two similar administration modes for live plants to evaluate the efficacy in controlling gray mold in tomato. In the control group, the leaves remained in their original state; in the B. cinerea group, the inoculation site had been eroded, and there were many disease spots around it, indicating that B. cinerea had successfully invaded the host plant. Comparing with the TM@Se@PAA NPs + B. cinerea (prevention) and B. cinerea + TM@Se@PAA NPs (inhibition) groups: there was no lesion formation and gray-brown damage at the inoculum; TM@Se@PAA NPs not only effectively prevented B. cinerea from infecting tomato plants, but also promoted the healing of wounds to some extent (Fig. 6A). Moreover, combined with the in vitro leaf inoculation test, the results of disease resistance evaluation were similar, but the incidence, speed and extent of infecting leaves of living plants were lower than that of in vitro leaves.Chlorophyll is an essential component of photosynthesis in plants, and its content changes can reflect the physiological condition of plants. In order to further explore the damage caused by B. cinerea after infecting tomato, we measured the changes of chloroplast pigment content in leaves at day 5. Among them, chlorophyll a is the main component of photosynthesis, which reflects the absorption of long-wavelength light by plants, and the absorption and conversion of light energy; while chlorophyll b mainly absorbs short-wavelength light and participates in absorption and transmission of light energy.39,40 The results (Fig. 6B and C) showed that the content of chlorophyll a decreased by 32.6% after infection by B. cinerea, but the content of chlorophyll b increased to 0.181 mg g−1, which was similar to that for the nano-treated group. This indicated that a plant infected by B. cinerea may retain the ability to absorb and transfer light energy, but the efficiency of converting light energy into heat and chemical energy is decreased significantly. In the prevention and inhibition groups, the chloroplast pigment contents were increased to different extents, which effectively reduced the decrease of chlorophyll a caused by B. cinerea, and the carotenoid content in the inhibition group was significantly increased compared with the other groups (Fig. 6D). It was preliminarily indicated that the nano-treatment of plants might effectively inhibit fungal infection, increase the content of chloroplast pigments, and possibly improve the photosynthesis efficiency. From the results of in vitro and in vivo leaf inoculation tests, TM@Se@PAA NPs might be used as a new antifungal agent for preventing and curing gray mold of tomato.
3.7. Evaluation of the prevention and treatment effect of TM@Se@PAA NPs by grape inoculation in vitro
Due to its high latency, B. cinerea can infect plants in low-temperature environments, and be carried into the market by postharvest fruits, causing fruit to rot during storage, transportation and marketing.41,42 At the same time, since the fruit is the main invasive part for B. cinerea, combined with the results of the in vitro and in vivo leaf inoculation tests, we carried out the inoculation test on grapes to further confirm the antifungal properties of TM@Se@PAA NPs. The results of prevention experiment showed that there was no significant change in the appearance of grapes in day 1 and day 2 groups, and no signs of fungal invasion were observed on the fifth day. Besides, the flesh of grapes remained white and translucent, even better than the control group. In addition, the grapes in day 3 and day 4 groups showed varying signs of bruising degrees in their appearance, while the internal flesh of the grapes was dark and slightly rotten (Fig. 7A). Compared with the results of in vitro leaf test, the grape contains more nutrients for B. cinerea to ingest, which is more conducive to its infestation and reproduction. Also, the results of inhibition experiment could directly confirm the strong antifungal ability of TM@Se@PAA NPs. By adding TM@Se@PAA NPs to the grapes on which had formed colonies, it was clear that the white colonies on the surface of grapes faded away rapidly (within the white curve). Among them, the grapes after injection of TM@Se@PAA NPs within three days maintained a certain appearance, and the flesh was in good condition; while the grapes in day 4 group showed large areas of white colonies, the peel of grape was obviously collapsed, and the interior was rotten (Fig. 7B). The above experiments indicated that tomato plants and their organs could be protected from early infection by B. cinerea by timely dosing. In addition, similar experiments were performed on tomatoes in vitro, and the results were similar to those of grapes in vitro, but the therapeutic effect was relatively insignificant due to the low degree of fungal infection (Fig. S8 and S9†). Therefore, TM@Se@PAA NPs have a time-based effect for the prevention and inhibition of B. cinerea in vitro with grapes and tomatoes, and should be prevented and treated at reasonable times.3.8. Effects of TM@Se@PAA NPs on postharvest tomato fruit
From a consumer's point of view, the appearance and color of fruit are the most direct factors to judge its quality and flavor. We photographed the appearance of the ripe fruit of different tomato plants after harvesting, and then cut the internal pulp to observe the quality of each tomato. As shown in Fig. 8A, the skin of the tomato was fruity and the flesh was red and bright, showing good appearance and pulp quality. Besides, we weighed each tomato and found that the group treated with TM@Se@PAA NPs had a slight increase in weight compared with the control group (Fig. 8B). To further analyze the effects of TM@Se@PAA NPs on tomato fruits, we measured various components in tomato fruits treated with different concentrations of TM@Se@PAA NPs. Tomato fruit contains a variety of pigments, of which lycopene is the main one of ripe tomato and has strong antioxidant activity.43,44 The content of lycopene is the most intuitive reflection of fruit color, and also affects the quality of the final product. The results of UV-vis spectroscopy showed that the lycopene content of the fruits treated by TM@Se@PAA NPs was significantly improved. Among them, the lycopene content of fruit treated with 100 mg L−1 TM@Se@PAA NPs was 0.0857 μg g−1, which was 1.9 times that of the control group (Fig. 8C). In addition, the shelf life of fruit is an important quality characteristic. Some researches have shown that anthocyanin enrichment could prolong the storage time of tomato, and enhance the inhibition of B. cinerea on postharvest fruits.45,46 The results showed that high concentration of TM@Se@PAA NPs (100 and 200 mg L−1) could effectively increase the anthocyanin content of the fruit by 21.6% and 15.5%, while there was no significant difference between the control group and the 50 mg L−1 plant group (Fig. 8D). Since the dosing site of the nano-preparations was the leaf, we used ICP-MS to measure whether the content of Se in tomato fruit had changed, to explore the absorption and utilization of TM@Se@PAA NPs by the plant. The results showed that selenium content in tomatoes depended on the concentration of TM@Se@PAA NPs, and we calculated that each nano-treated fruit contains Se in the range of 10.8–16.8 μg (Fig. 8E). Combined with the intake of Se which is limited to 400–450 μg per day,47 even consumption of multiple selenium-enriched tomatoes will not cause selenium poisoning, and will satisfy reasonable selenium supplementation. In general, long-term application of TM@Se@PAA NPs did not cause obvious toxic side effects to plants, but significantly increased the content of lycopene and selenium in fruits.3.9. Effects of TM@Se@PAA NPs on plant photosynthesis
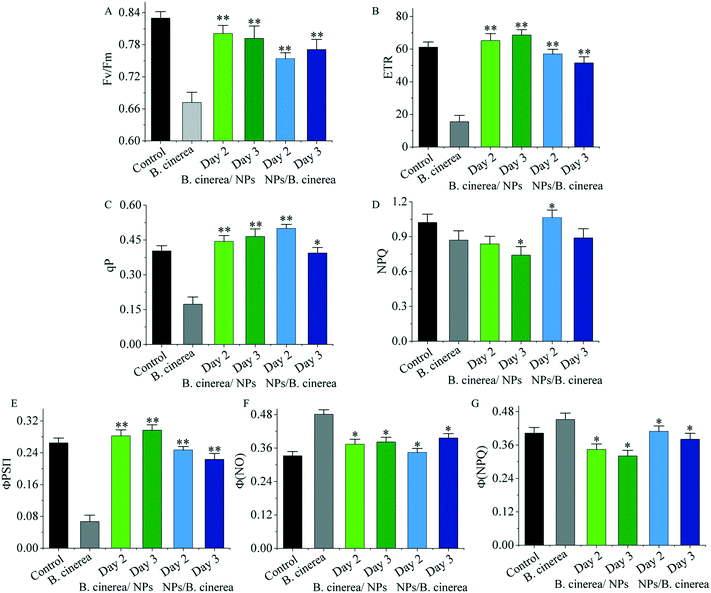
As is well known, a common method for assessing plant photosynthetic activity is to measure the chlorophyll fluorescence parameters of plants under normal growth and stress conditions.48,49 Firstly, we measured a series of photosynthesis parameters of tomato plants treated with different concentrations of TM@Se@PAA NPs after dark adaptation for 30 min to further explore the effects on plant photosynthesis. The maximum photochemical efficiency (Fv/Fm) in normal plant leaves ranged from 0.8 to 0.84, and the measured Fv/Fm of control and nano-treated groups were both above 0.82, indicating that the plants were in a good physiological state (Fig. S10A†). At the same time, the Fv/Fm of the B. cinerea group was significantly decreased to 0.67, while the degree of decrease was reduced after treatment with TM@Se@PAA NPs (B. cinerea/NPs) that stopped Fv/Fm from reducing (Fig. 9A). Compared with the control group, the qP of the nano-treated group increased to some extent, indicating that TM@Se@PAA NPs may promote the absorption of light energy by plants for photochemical electron transfer; at the same time, we measured that the relative ETR was increased by 9.24%, 27.18% and 8.31%, respectively, compared with the control group (65.62); and the up-regulation of the NPQ indicated that TM@Se@PAA NPs may promote plants to efficiently convert excess light energy into heat, so as to dissipate and weaken the damage of excess light to plants (Fig. S9B–D†). Similarly, both ETR (15.52) and qP (0.173) in the B. cinerea group were significantly reduced, indicating that their photosystem II (PSII) had been obviously impaired. Interestingly, the NPQ of the B. cinerea/NPs group decreased to different degrees, indicating that the heat dissipation capacity of plants declined (Fig. 9B–D). Since the Fv/Fm for different concentrations of TM@Se@PAA NPs did not show a significant difference, the actual quantum yield (ΦPSII) was calculated to find that the values for the nano-treatment group were 0.311, 0.361 and 0.309, respectively, which were higher than that for the control group of 0.284. This proved that the effective photosynthesis efficiency of plants was improved to some extent (Fig. S10E†). In addition, the decrease of the non-regulated quantum yield (ΦNO) of the nano-treated groups indicated that the degree of photo-damage was low. While the regulatory quantum yield (ΦNPQ) of each group was around 0.41, which indicated that plants could effectively absorb light energy and have good light protection (Fig. S10F and G†). Similarly, the ΦPSII of the B. cinerea group was 0.067, which was much lower than that the other groups. The up-regulation of ΦNO showed that the plants in the B. cinerea group could not effectively utilize light energy under the same illumination due to the excess photo-damage; besides, the ΦNPQ results showed that the plants still had a certain degree of light protection (Fig. 9E–G). Therefore, the experimental results indicated that the application of TM@Se@PAA NPs nano-preparations did not cause damage to the plant photosynthesis system, but reduced the low photosynthesis efficiency caused by B. cinerea infection, and played the role of protective agent.4. Conclusion
We have successfully prepared an integrated prevention and inhibition mesoporous nanoselenium delivery system (TM@Se@PAA NPs) which has the following advantages: (1) based on the synthesis and secretion of oxalic acid and other acid substances after B. cinerea infects plants, TM@Se@PAA NPs can effectively release the antifungal drug TM; (2) compared with TM, TM@Se@PAA NPs had good water solubility, and low-dose and high-efficiency antifungal effect, and even long-term application to plants did not affect growth and development notably; and (3) in vitro fruit tests and plant in vivo and in vitro experiments showed that B. cinerea could be prevented and inhibited effectively by TM@Se@PAA NPs within a certain period of time, and enhanced the resistance of plants to gray mold; besides, the photosynthetic efficiency of plants was improved. The experimental results of this study indicated that TM@Se@PAA NPs might be used as a new antifungal agent to prevent and resist plant gray mold, and bring an important reference value for the treatment of other fungal diseases of plants.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21877051, 81803027, 21701034), the Natural Science Foundation of Guangdong Province (2018A030310628), the Planned Item of Science and Technology of Guangdong Province (2016A020217011), Projects of Special Innovative of Department of Education of Guangdong Province (2017KTSCX078) and Project of Young Innovative Talents in Universities and Colleges of Department of Education of Guangdong Province (2018KQNCX100).References
- E. P. Benito, A. T. Have, J. W. V. T. Klooster and J. A. L. V. Kan, Fungal and plant gene expression during synchronized infection of tomato leaves by Botrytis cinerea, Eur. J. Plant Pathol., 1998, 104, 207–220 CrossRef CAS.
- C. He, Z. Zhang, B. Li, Y. Xu and S. Tian, Effect of natamycin on Botrytis cinerea and Penicillium expansum—Postharvest pathogens of grape berries and jujube fruit, Postharvest Biol. Technol., 2019, 151, 134–141 CrossRef CAS.
- R. Dean, J. A. Van Kan, Z. A. Pretorius, K. E. Hammond-Kosack, A. Di Pietro, P. D. Spanu, J. J. Rudd, M. Dickman, R. Kahmann and J. Ellis, The Top 10 fungal pathogens in molecular plant pathology, Mol, Plant Pathol., 2012, 13, 414–430 Search PubMed.
- D. Steiger, Global economic importance of Botrytis protection, 2007 Search PubMed.
- B. Williamson, B. Tudzynski, P. Tudzynski and J. A. van Kan, Botrytis cinerea: the cause of grey mould disease, Mol. Plant Pathol., 2007, 8, 561–580 CrossRef CAS PubMed.
- M. Choquer, E. Fournier, C. Kunz, C. Levis, J.-M. Pradier, A. Simon and M. Viaud, Botrytis cinerea virulence factors: new insights into a necrotrophic and polyphageous pathogen, FEMS Microbiol. Lett., 2007, 277, 1–10 CrossRef CAS.
- J. A. van Kan, Licensed to kill: the lifestyle of a necrotrophic plant pathogen, Trends Plant Sci., 2006, 11, 247–253 CrossRef CAS PubMed.
- S. M. Sanzani, L. Schena, V. De Cicco and A. Ippolito, Early detection of Botrytis cinerea latent infections as a tool to improve postharvest quality of table grapes, Postharvest Biol. Technol., 2012, 68, 64–71 CrossRef.
- A. Wszelaki and E. Mitcham, Effect of combinations of hot water dips, biological control and controlled atmospheres for control of gray mold on harvested strawberries, Postharvest Biol. Technol., 2003, 27, 255–264 CrossRef CAS.
- S. Wilkinson, V. Pastor, S. Paplauskas, P. Pétriacq and E. Luna, Long-lasting β-aminobutyric acid-induced resistance protects tomato fruit against Botrytis cinerea, Plant Pathol., 2018, 67, 30–41 CrossRef CAS.
- P. Leroux, R. Fritz, D. Debieu, C. Albertini, C. Lanen, J. Bach, M. Gredt and F. Chapeland, Mechanisms of resistance to fungicides in field strains of Botrytis cinerea, Pest Manage. Sci., 2002, 58, 876–888 CrossRef CAS.
- M. Komárek, E. Čadková, V. Chrastný, F. Bordas and J.-C. Bollinger, Contamination of vineyard soils with fungicides: a review of environmental and toxicological aspects, Environ. Int., 2010, 36, 138–151 CrossRef.
- M. Leroch, M. Kretschmer and M. Hahn, Fungicide resistance phenotypes of Botrytis cinerea isolates from commercial vineyards in South West German, J. Phytopathol., 2011, 159, 63–65 CrossRef CAS.
- A. Schmid, C. Daniel and F. Weibel, Effect of cultural methods on leaf spot (Mycosphaerella fragariae) and gray mold (Botrytis cinerea) damage in strawberries, BioControl, 2005, 50, 179–194 CrossRef.
- J. P. Lee, S.-W. Lee, C. S. Kim, J. H. Son, J. H. Song, K. Y. Lee, H. J. Kim, S. J. Jung and B. J. Moon, Evaluation of formulations of Bacillus licheniformis for the biological control of tomato gray mold caused by Botrytis cinerea, Biol. Control, 2006, 37, 329–337 CrossRef.
- I. Ocsoy, M. L. Paret, M. A. Ocsoy, S. Kunwar, T. Chen, M. You and W. Tan, Nanotechnology in plant disease management: DNA-directed silver nanoparticles on graphene oxide as an antibacterial against Xanthomonas perforans, ACS Nano, 2013, 7, 8972–8980 CrossRef CAS PubMed.
- A. Panáček, M. Kolář, R. Večeřová, R. Prucek, J. Soukupova, V. Kryštof, P. Hamal, R. Zbořil and L. Kvítek, Antifungal activity of silver nanoparticles against Candida spp, Biomaterials, 2009, 30, 6333–6340 CrossRef PubMed.
- Y. Li, W. Zhang, J. Niu and Y. Chen, Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles, ACS Nano, 2012, 6, 5164–5173 CrossRef CAS PubMed.
- P. A. Tran and T. J. Webster, Selenium nanoparticles inhibit Staphylococcus aureus growth, Int. J. Nanomed., 2011, 6, 1553 CAS.
- Y. Xia, T. Xu, C. Wang, Y. Li, Z. Lin, M. Zhao and B. Zhu, Novel functionalized nanoparticles for tumor-targeting co-delivery of doxorubicin and siRNA to enhance cancer therapy, Int. J. Nanomed., 2018, 13, 143 CrossRef CAS PubMed.
- X. Huang, X. Chen, Q. Chen, Q. Yu, D. Sun and J. Liu, Investigation of functional selenium nanoparticles as potent antimicrobial agents against superbugs, Acta Biomater., 2016, 30, 397–407 CrossRef CAS PubMed.
- V. Bartůněk, B. Vokatá, K. Kolářová and P. Ulbrich, Preparation of amorphous nano-selenium-PEG composite network with selective antimicrobial activity, Mater. Lett., 2019, 238, 51–53 CrossRef.
- S. J. Soenen, W. J. Parak, J. Rejman and B. Manshian, (Intra) cellular stability of inorganic nanoparticles: effects on cytotoxicity, particle functionality, and biomedical applications, Chem. Rev., 2015, 115, 2109–2135 CrossRef CAS PubMed.
- Z. Zhu, Y. Zhang, J. Liu, Y. Chen and X. Zhang, Exploring the effects of selenium treatment on the nutritional quality of tomato fruit, Food Chem., 2018, 252, 9–15 CrossRef CAS PubMed.
- E. A. Pilon-Smits, C. F. Quinn, W. Tapken, M. Malagoli and M. Schiavon, Physiological functions of beneficial elements, Curr. Opin. Plant Biol., 2009, 12, 267–274 CrossRef CAS PubMed.
- M. Hasanuzzaman, M. A. Hossain and M. Fujita, Selenium in higher plants: physiological role, antioxidant metabolism and abiotic stress tolerance, J. Plant Sci., 2010, 5, 354–375 CrossRef CAS.
- X. Wang, Z. Miao, Y. Ma, H. Chen, H. Qian and Z. Zha, One-pot solution synthesis of shape-controlled copper selenide nanostructures and their potential applications in photocatalysis and photothermal therapy, Nanoscale, 2017, 9, 14512–14519 RSC.
- D. R. Ellis and D. E. Salt, Plants, selenium and human health, Curr. Opin. Plant Biol., 2003, 6, 273–279 CrossRef CAS PubMed.
- X. Wang, F. Li, X. Yan, Y. Ma, Z.-H. Miao, L. Dong, H. Chen, Y. Lu and Z. Zha, Ambient aqueous synthesis of ultrasmall Ni0. 85Se nanoparticles for noninvasive photoacoustic imaging and combined photothermal-chemotherapy of cancer, ACS Appl. Mater. Interfaces, 2017, 9, 41782–41793 CrossRef CAS PubMed.
- C. de Oliveira Datovo and D. J. Soares, Sensitivity of field isolates of Botryotinia ricini to fluazinam and thiophanate-methyl, Trop. Plant Pathol., 2019, 44, 205–208 CrossRef.
- T. R. Roberts, T. R. Roberts, D. H. Hutson and P. J. Jewess, Metabolic pathways of agrochemicals: insecticides and fungicides, Metabolic pathways of agrochemicals: insecticides and fungicides, R. S. C., 1998 Search PubMed.
- M. Cycoń, M. Wójcik and Z. Piotrowska-Seget, Biodegradation kinetics of the benzimidazole fungicide thiophanate-methyl by bacteria isolated from loamy sand soil, Biodegradation, 2011, 22, 573–583 CrossRef PubMed.
- A. Walz, I. Zingen-Sell, M. Loeffler and M. Sauer, Expression of an oxalate oxidase gene in tomato and severity of disease caused by Botrytis cinerea and Sclerotinia sclerotiorum, Plant Pathol., 2008, 57, 453–458 CrossRef CAS.
- L. Yuan, Q. Tang, D. Yang, J. Z. Zhang, F. Zhang and J. Hu, Preparation of pH-responsive mesoporous silica nanoparticles and their application in controlled drug delivery, J. Phys. Chem. C, 2011, 115, 9926–9932 CrossRef CAS.
- M. H. Siddiqui, M. H. Al-Whaibi and F. Mohammad, Nanotechnology and plant sciences, Springer International Publishing, Cham., 2015, vol. 10, p. 303, 978–973 Search PubMed.
- M. H. Lahiani, Z. A. Nima, H. Villagarcia, A. S. Biris and M. V. Khodakovskaya, Assessment of effects of the long-term exposure of agricultural crops to carbon nanotubes, J. Agric. Food Chem., 2017, 66, 6654–6662 CrossRef PubMed.
- F. R. Rossi, A. R. Krapp, F. Bisaro, S. J. Maiale, F. L. Pieckenstain and N. Carrillo, Reactive oxygen species generated in chloroplasts contribute to tobacco leaf infection by the necrotrophic fungus Botrytis cinerea, Plant J., 2017, 92, 761–773 CrossRef CAS PubMed.
- L. Fritz and R. E. Triemer, A rapid simple technique utilizing calcofluor white M2R for the visualization of dinoflagellate thecal plates1, J. Phycol., 1985, 21, 662–664 CrossRef.
- J. P. Connelly, M. G. Müller, R. Bassi, R. Croce and A. R. Holzwarth, Femtosecond transient absorption study of carotenoid to chlorophyll energy transfer in the light-harvesting complex II of photosystem II, Biochemistry, 1997, 36, 281–287 CrossRef CAS PubMed.
- J. Liu, J. Wang, X. Yao, X. Dong and W. Chen, Fine mapping and photosynthetic characteristics of the lower chlorophyll b 1 mutant in rice (Oryza sativa L.), Plant Breed., 2015, 134, 661–667 CrossRef CAS.
- E. Silva-Moreno, J. Brito-Echeverría, M. López, J. Ríos, I. Balic, R. Campos-Vargas and R. Polanco, Effect of cuticular waxes compounds from table grapes on growth, germination and gene expression in Botrytis cinerea, World J. Microbiol. Biotechnol., 2016, 32, 74 CrossRef PubMed.
- W.-T. Xu, K.-L. Huang, F. Guo, W. Qu, J.-J. Yang, Z.-H. Liang and Y.-B. Luo, Postharvest grapefruit seed extract and chitosan treatments of table grapes to control Botrytis cinerea, Postharvest Biol. Technol., 2007, 46, 86–94 CrossRef CAS.
- H. Sies and W. Stahl, Lycopene: antioxidant and biological effects and its bioavailability in the human, Proc. Soc. Exp. Biol. Med., 1998, 218, 121–124 CrossRef CAS PubMed.
- J. Javanmardi and C. Kubota, Variation of lycopene, antioxidant activity, total soluble solids and weight loss of tomato during postharvest storage, Postharvest Biol. Technol., 2006, 41, 151–155 CrossRef CAS.
- Y. Zhang, E. Butelli, R. De Stefano, H.-J. Schoonbeek, A. Magusin, C. Pagliarani, N. Wellner, L. Hill, D. Orzaez and A. Granell, Anthocyanins double the shelf life of tomatoes by delaying overripening and reducing susceptibility to gray mold, Curr. Biol., 2013, 23, 1094–1100 CrossRef CAS PubMed.
- A. Sass-Kiss, J. Kiss, P. Milotay, M. Kerek and M. Toth-Markus, Differences in anthocyanin and carotenoid content of fruits and vegetables, Food Res. Int., 2005, 38, 1023–1029 CrossRef CAS.
- M. P. Rayman, The importance of selenium to human health, Lancet, 2000, 356, 233–241 CrossRef CAS.
- K. Sitko, S. Rusinowski, H. M. Kalaji, M. Szopiński and E. Małkowski, Photosynthetic efficiency as bioindicator of environmental pressure in A. halleri, Plant Physiol., 2017, 175, 290–302 CrossRef CAS PubMed.
- H. M. Kalaji, A. Jajoo, A. Oukarroum, M. Brestic, M. Zivcak, I. A. Samborska, M. D. Cetner, I. Łukasik, V. Goltsev and R. J. Ladle, Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions, Acta Physiol. Plant., 2016, 38, 102 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9en00859d |
| ‡ Jiawei Liu and Xufeng Zhu contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2020 |