Toxicity of zinc oxide and iron oxide engineered nanoparticles to Bacillus subtilis in river water systems†
Samuel K.
Leareng
 a,
Eunice
Ubomba-Jaswa
b and
Ndeke
Musee
*a
a,
Eunice
Ubomba-Jaswa
b and
Ndeke
Musee
*a
aEmerging Contaminants Ecological and Risk Assessment (ECERA) Group, Department of Chemical Engineering, University of Pretoria, Private Bag X20, Hatfield 0028, Pretoria, South Africa. E-mail: Ndeke.musee@up.ac.za; museen2012@gmail.com
bWater Research Commission, Pretoria, South Africa
First published on 28th October 2019
Abstract
Zinc oxide (nZnO) and iron oxide (nFeOx) engineered nanoparticles (ENPs) are widely used in consumer products and industrial applications, and consequently, are continuously being released into the environment. Numerous studies have reported on the toxicity of ENPs to bacteria, especially in synthetic aqueous exposure media. However, investigations in natural aqueous exposure media such as river water are limited. Herein, the toxicity of nZnO and maghemite iron oxide (γ-nFe2O3) to Bacillus subtilis was investigated in two natural river water samples: the Elands River (ER) and the Bloubank River (BR). Four endpoints, namely, cell viability, cell membrane integrity, adenosine triphosphate levels (ATP), and reactive oxygen species (ROS) production, were evaluated to determine the effects of the ENPs on the bacteria. nZnO induced significant reduction in cell viability and membrane integrity at higher tested concentrations of 100 and 1000 μg L−1 in ER; but none were observed in BR. In addition, a higher decrease in ATP levels was observed in ER than in BR, and the ROS production was negligible irrespective of the ENP type and exposure media. γ-nFe2O3 induced no effects on B. subtilis on all tested endpoints. These results demonstrated that the observed differences in the effects of nZnO towards B. subtilis were influenced by the physicochemical properties of each river's water. Therefore, the unique physicochemical properties of natural aqueous media were established to be the key determinant attributes in enhancing or inhibiting the effects of ENPs on bacteria.
Environmental significanceThe increasing global production and associated uses of zinc oxide (nZnO) and iron oxide (nFeOx) engineered nanoparticles are likely to increase their presence, for example, in freshwater systems. Thus, assessment of likely environmental impacts of ENPs is critical, although to date very limited studies have investigated their effects on microorganisms under relevant environmental exposure-media and -concentration(s) scenarios. Herein, the effects of nZnO and γ-nFe2O3 on Bacillus subtilis were assessed in natural water media using multiple endpoints with varying degrees of toxicity sensitivities to contaminants. The toxicity of nZnO was shown to be dependent on water physicochemical properties; however, the same properties were observed to mitigate likely γ-nFe2O3–cell interactions. Findings on sub-lethal endpoints like ATP production revealed nanoparticle toxicity unlike traditional endpoints such as cell viability where no bacterial effects were observed. Our study contributes towards the understanding of plausible nanoparticle–organism interaction mechanisms in natural water matrices, necessary for prediction of meaningful environmental outcomes. |
Introduction
Increasingly, numerous consumer products have incorporated engineered nanoparticles (ENPs) in recent years1–5 due to their unique physicochemical properties to enhance functionality and meet consumer needs. Among the most produced and used top five ENPs in the fast-growing nanotechnology market are zinc oxide (nZnO) and iron oxide (nFeOx),6,7 with medium-term production growth expected to increase.8 For example, nZnO is widely incorporated in consumer products, e.g. cosmetics, sunscreens, nanomedicine, paints and coatings, due to its UV radiation blocking properties;9,10 whereas due to its superior properties and functionalities (e.g. magnetic, catalytic, etc.), γ-nFe2O3 is used in biomedical imaging, environmental remediation catalysis, fertilizers, pigments, sensors, and biomedical and lithium ion batteries.11–14 Therefore, as the use and applications of both ENPs become more common, their release into and eventual accumulation in the environment continue to grow exponentially.6,15,16 As a result, this exponential growth will likely surpass current concentrations of iron oxides and nZnO predicted at 12.8 to 44 ng L−1,17 and 2 to 360 ng L−1 (ref. 18–20) in Europe, respectively, for example.The antimicrobial properties of ENPs heighten their likelihood to exert adverse impacts on non-target and useful microbes once released into aquatic systems,10,15,21 for example, Sinorhizobium meliloti22 and Shewanella oneidensis,23 respectively, which are essential for nitrogen fixation and metal reducing capacity. To date, the observed ENP toxicity to microbes has been attributed to oxidative stress through generation of reactive oxygen species (ROS), release of toxic metallic ions, surface contact, cell membrane damage, and internalization of ENPs.10,21,24,25 Both the interaction of ENPs and microorganisms and their concomitant toxicity are dependent on inherent physicochemical properties of ENPs and water chemistry.5,26–28 In particular, numerous studies have demonstrated the influence of water chemistry (e.g. pH, ionic strength (IS), light, and natural organic matter (NOM))29,30 on ENP transformation, and the eventually observed toxicity to bacteria.31,32
Notably, most of these studies investigated the influence of water chemistry on ENP transformation and observed toxicity predominantly in synthetic media although such media are too simplistic to accurately represent the actual complex aquatic ecosystems.33,34 This is because of the complexities and high variability of freshwater systems that can alter the transformation of ENPs,35–37 and in turn, yield different toxicological outcomes, for example, to microbes.31,38 Thus, data and information on the effects of ENPs on microbes in these deterministic freshwater systems remain scarce, therefore, impeding realistic assessment of ENP risks to the environment.
To date, experimental toxicity investigations of ENPs have been conducted at concentrations several orders of magnitude above both modelled and measured ENP concentrations,39–41 and consequently, this has severely limited our ability to realistically assess their likely implications for aquatic systems. Only a few studies, for instance, have demonstrated the effects of ENPs on bacteria at concentrations within an order of magnitude to those predicted or measured in actual environmental matrices. Yi and Cheng28 evaluated the effects of nAg on Bacillus subtilis in natural waters whilst Wilke et al.42 examined the effects of Ag and TiO2 on Escherichia coli. Studies from Gray's group43,44 have also demonstrated the importance of using toxicity measures like adenosine triphosphate (ATP) depletion to determine the effects of ENPs on microorganisms to obtain a complete understanding of the likely risks posed by ENPs in the environment, even at very low concentrations where cell death is not apparent. Even under dark conditions, for example, at concentrations <250 μg L−1, the obtained results demonstrated that bacterial ATP levels can be significantly reduced by nAg and nZnO (ref. 42 and 44) with likely adverse implications for the environment.
The highlighted knowledge gaps raise the need to understand the implications of ENPs in aquatic systems, particularly at environmentally relevant concentrations. Therefore, in this work the toxicological effects of nZnO and γ-nFe2O3 on B. subtilis as influenced by water chemistry characteristics were evaluated. Here, the natural water chemistry matrix differences were due to the distinct sources (two river water systems). B. subtilis, an environmentally ubiquitous organism, was selected as the model organism due to its ability to tolerate extreme environmental conditions and stress.28,45 Secondly, this organism has been used in nanotoxicity studies, and is reported to be more sensitive to ENPs compared to Gram-negative bacteria e.g. E. coli, or other Gram-positive bacteria like Streptococcus aureus.46 Understanding the induced toxicity of ENPs to microorganisms in natural water by investigating four end-points: cell viability, membrane integrity, ATP levels, and oxidative stress from ROS can aid in better assessment of their risk to natural water matrices such as river water used in this study.
Experimental procedure
Freshwater sampling
The freshwater samples were collected from two rivers, namely; the Elands River (ER) (25°32′58.4′′S 28°33′53.4′′E, Gauteng Province, South Africa) and Bloubank River (BR) (26°01′20.3′′S 27°26′31.6′′E, North West Province, South Africa). The collected water samples (from ER and BR) were used as exposure matrices to represent different complex environmental surface freshwater chemistries. The collected river water was filtered using Whatman No. 1 filter paper (pore size: 11 μm) followed by filtration through 0.2 μm pore sized membrane filters to remove microorganisms and larger particles. All water samples were stored at 4 °C until analysis. The physicochemical properties of both river water systems are summarised in Table 1.| Parameter | Unit | Bloubank River (BR) water | Elands River (ER) water |
|---|---|---|---|
| a Dissolved organic matter. b Chemical oxygen demand. c Ionic strength – calculated using Visual MINTEQ (version 3.1). | |||
| pH | 7.9 | 8.1 | |
| DOCa | mg C L−1 | 8.25 | 5.51 |
| Electrical conductivity | ms m−1 25 °C | 39.8 | 19.6 |
| CODb | mg L−1 | 21.3 | 6.67 |
| Alkalinity | mg L−1 | 217 | 75.6 |
| NH4+ | mg L−1 | 3.4 | 4.27 |
| NO3− | mg L−1 | 0.2 | 0.33 |
| Cl− | mg L−1 | 12.9 | 17.1 |
| SO42− | mg L−1 | 6.77 | 9.03 |
| PO4 | mg L−1 | 1.23 | 0.57 |
| Fe3+ | mg L−1 | <0.004 | <0.004 |
| Zn2+ | mg L−1 | 0.01 | 0.008 |
| Ca2+ | mg L−1 | 36 | 14 |
| Mg2+ | mg L−1 | 31 | 9.82 |
| Na+ | mg L−1 | 22.4 | 15.6 |
| K+ | mg L−1 | 3.13 | 4.24 |
| ISc | mM | 5.23 | 2.43 |
Materials
nZnO (<100 nm, 20% dispersion in H2O, CAS 1314-13-2), nFe2O3 (γ-Fe2O3, <50 nm, nanopowder, CAS 1309-37-1), 2′,7′-dichlorofluorescein diacetate (DCF-DA) and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich, South Africa. According to the manufacturer, the particle sizes were <100 nm and <50 nm for nZnO and nFe2O3, respectively. All chemicals were analytical grade reagents and used as received without further purification. The Bacillus subtilis (ATCC 11774) strain was purchased from Anatech (Johannesburg, South Africa).Characterization of ENPs
The ENP size and morphology were characterised by transmission electron microscopy (TEM) (JEM 2010F, JEOL Ltd., Japan), with the ENP diameter measured using ImageJ software (National Institutes of Health, USA) based on particle size analysis from several micrographs. The phase composition was determined using a Bruker D8 Advance powder X-ray diffractometer (PXRD) with monochromatized Cu Kα radiation with a wavelength (λ) of 1.54 Å. The hydrodynamic diameter (HDD) and zeta potential (ζ-potential) for ENP suspensions in river water were characterised using dynamic light scattering (DLS) on a Zetasizer Nano-ZS instrument (Malvern Instruments, UK), and the results are listed in Table S1 (ESI†). Concentrations of 20 mg L−1 ZnO and 5 mg L−1 γ-nFe2O3 were used for hydrodynamic size and ζ-potential measurements.To determine the dissolved metal concentrations of nZnO and γ-nFe2O3, suspensions of ENPs were prepared similarly to experimental conditions (bacterial culture and exposure preparation), without the bacteria. Nanoparticle suspensions were filtered through regenerated cellulose centrifugal filters with a 3 kDa molecular weight cut-off (Merck Millipore, Darmstadt, Germany) by centrifugation for 30 min at 4000g (Eppendorf 5810 R, Eppendorf, Germany), to remove undissolved ENPs, but allow dissolved ions in the aqueous phase to pass through. The dissolved fraction was acidified with 5 μL of concentrated HNO3 and the concentration of dissolved ions in the supernatant was analysed by inductively coupled plasma mass spectrometry (ICP-MS) (ICPE-9820, Shimadzu, Japan).
Visual MINTEQ (version 3.1, https://vminteq.lwr.kth.se) was used to predict speciation of zinc in ER and BR water based on the parameters listed in Table 1. The Stockholm humic model (SHM) was used with default parameters as model inputs.
Bacterial culture and exposure preparation
Bacterial strain B. subtilis was plated on sterilized lysogeny broth (LB) agar plates and stored at 4 °C until use. For cell viability studies, a single colony was inoculated in LB and incubated at 30 °C with shaking at 150 rpm overnight until cells attained the mid-exponential phase (0.4–0.5 at OD600nm). Bacterial cells were harvested by centrifugation at 7500g for 5 min, and washed once with physiological saline (0.85% NaCl), and then once using filtered river water. The cells were finally re-suspended in filtered river water and the final concentration of cells used in the exposure tests was adjusted to 2 × 108 CFU mL−1 (≈0.3 at OD600nm) measured by direct plating counting.Stock solutions of ENPs at a concentration of 100 mg L−1 were prepared using ultrapure water (18 MΩ cm resistivity, Elga PureLab Option System, United Kingdom), and sonicated for 20 min using an ultrasonic bath prior to exposure experiments. The stock suspensions were diluted to target nominal concentrations of 10, 100 and 1000 μg L−1 for nZnO and 10, 100, 1000 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg L−1 for γ-nFe2O3 in 250 mL flasks containing bacterial cells (OD600nm of 0.3) and river water to achieve a final volume of 25 mL. The controls were run without ENP suspensions. The flasks were incubated at room temperature (20–23 °C) on a rotating shaker at 75 rpm for 2 h under visible light (338 lux).
000 μg L−1 for γ-nFe2O3 in 250 mL flasks containing bacterial cells (OD600nm of 0.3) and river water to achieve a final volume of 25 mL. The controls were run without ENP suspensions. The flasks were incubated at room temperature (20–23 °C) on a rotating shaker at 75 rpm for 2 h under visible light (338 lux).
Cell viability and membrane integrity
Both exposed and non-exposed (control) samples were serially diluted in 0.85% NaCl, and thereafter, viable bacteria were determined on the LB agar plate following the drop count method.47 Nine drops of 20 μL for each dilution were transferred onto solid LB agar plates. The plates were incubated overnight at 37 °C. The bacterial viability following exposure to ENP suspensions was measured by counting the number of colony forming units (CFU) from the appropriate dilution on nutrient agar plates. Hence, the percentage of viable cells was determined by comparing the CFU per mL of the culture as a ratio of the number of CFU from ENP exposed samples to that from non-exposed (control) samples. All viability experiments were conducted in triplicate and repeated twice.A Live/Dead BacLight kit (Molecular Probes, US) was used to test the cell membrane integrity of the bacteria following exposure to ENPs. A hundred microliters of ENP exposed and non-exposed (control) samples were transferred to individual wells in a 96-well microplate (Greiner Bio-One, Austria), combined with 100 μL of SYT09/PI mixture (10/60 μM), and then mixed thoroughly. PI and SYTO9 stain nucleic acids were used to differentiate between intact cells (live organisms – stained in green) and damaged cells (dead organisms – stained in red), respectively. The microplate was then incubated with assay reagents for 15 min at room temperature (20–23 °C) in the dark (covered with aluminium foil). Fluorescence was measured with excitation and emission wavelengths for SYTO9 and PI of 485/538 nm (green) and 485/635 (red), respectively, using a Fluoroskan Ascent FL microplate reader (Thermo Fisher, USA). A calibration curve was obtained using cells with known percentages of intact cells. For each test, three replicates were included in each treatment per plate, and two plates were used to ensure reproducibility.
Oxidative stress from ROS
Intracellular ROS production following exposure to ENPs in the river water was determined as a measure of oxidative stress using the membrane permeable non-fluorescent dye 2′,7′-dichlorofluorescein diacetate (DCF-DA, Sigma Aldrich). DCF-DA is converted into fluorescent 2′,7′-dichlorofluorescein (DCF) after reacting with ROS, thus making the cell fluoresce. Following the 2 h exposure, 150 μL of the exposed and non-exposed samples were transferred to 96-well microplates and incubated with DCF-DA (100 μM final concentration) for 30 min at 37 °C under dark conditions (covered using aluminium foil). The DCF fluorescence intensity was measured with a Fluoroskan Ascent FL microplate reader (Thermo Fisher, USA) at excitation and emission wavelengths of 485 and 538 nm, respectively, to quantify ROS activity in both the treated and control groups. ENPs without bacteria were also incubated with DCF-DA as controls. ROS production was expressed as the percentage fluorescence of the control over the exposed samples. For each test, three replicates of each treatment were added per plate, and two plates were used to ensure reproducibility.Bacterial ATP levels
The BacTiter-Glo microbial cell viability assay (Promega, Germany) was used to quantify bacterial ATP levels. This was achieved by measuring luminescence signal intensity from the reaction of luciferin and ATP to test bacterial activity changes in response to ENP treatment. A hundred microliters of ENP exposed and non-exposed samples were transferred to individual wells in a 96-well microplate combined with 100 μL of the BacTiter-Glo reagent and mixed thoroughly. The microplate was then incubated for 5 min at room temperature (20–23 °C) under dark conditions (covered with aluminium foil). The luminescence signal was measured using the Fluoroskan Ascent FL microplate reader, and the results were expressed as the relative percentage of ATP of exposed bacteria to the control. For each test, three replicates were included in each treatment per plate, and two independent microplates were used to ensure reproducibility of the results. Also, the potential interference of the ENPs with the BacTiter-Glo assay reagent was analysed to ascertain whether the effects observed were wholly due to ENP treatment.Statistical analysis
Data herein are expressed as the mean with corresponding standard deviation (SD). Two-way analysis of variance (ANOVA) was used to evaluate statistical differences followed by post hoc Tukey's multiple comparison tests. Differences between samples were considered statistically significant when p < 0.05. All analyses were conducted with GraphPad Prism V7.04 (GraphPad Prism software Inc., San Diego, CA, USA).Results and discussion
Nanoparticle characterization
γ-nFe2O3 had a hexagonal shape and an average particle size of 41 ± 25 nm. nZnO exhibited non-uniform shapes consisting of hepta-, penta- and hexagonal, and rod-like shapes, with diameters ranging from 15 to 57 nm due to the asymmetry of the morphology. The representative TEM images of the ENPs are shown in Fig. S1a, b, e and f.† Results from XRD revealed that the crystalline phase of nZnO was zincite (Fig. S1c†) whereas that of nFe2O3 was maghemite (γ-nFe2O3) (Fig. S1d†). The ζ-potential for both ENPs was negative in all river water samples (Table S1†), and in a narrow range between −12.3 ± 0.6 and −15.1 ± 1.3 mV.Zetasizer results indicated immediate aggregation of nZnO and nFe2O3 in both river water samples post-sonication (Table S1†). nZnO had average sizes of 512 ± 22 and 1069 ± 187 nm in ER and BR, respectively, whereas nFe2O3 had HDD values of 958 ± 188 nm and 1056 ± 120 nm in ER and BR, respectively (Table S1†). High aggregation of ENPs observed in both river water samples was associated with low ζ-potential of between −12.3 ± 0.6 and −15.1 ±1.3 mV; considered to be too low as ζ-potential of ± 30 mV is required to maintain ENPs dispersed by charge stabilization against aggregation.48–51 For both ENPs, increased aggregation was more significant in BR compared to ER after 2 h (Table S1†).
Fig. S2† summarizes the dissolution results of nZnO in both river water samples. Fe ions could not be detected as their concentration was below the analytical detection limit. Indeed, nFe2O3 is known to exhibit very low or no dissolution in aqueous matrices.15,52 Dissolution of nZnO was observed to be concentration-dependent in a similar fashion to earlier studies.31,53–55 At a nominal exposure concentration of 100 μg L−1, 14 μg L−1 dissolved zinc was measured in ER compared to less than 2 μg L−1 in BR. At a higher nominal exposure concentration of 1000 μg L−1, higher dissolution of nZnO was observed in ER and BR water at values of 366 and 183 μg L−1, respectively.
The observed differences in aggregation and dissolution in ER and BR were attributed to the differences in the water physicochemical properties (Table 1), which are known to influence the transformation processes of ENPs in aqueous matrices.30,35,36 Natural organic matter (NOM) coating on ENPs in aquatic systems can either enhance or inhibit their aggregation and stability through mechanisms like electrostatic interaction and ligand exchange, among others.56–59 Moreover, it has been reported that under high IS conditions and in the presence of NOM, cation binding likely enhances the aggregation of ENPs.30,57,60
In this study, aggregation of both ENPs was observed, with larger aggregate sizes in BR. This may be due to the higher NOM content in BR (Table 1) that could have resulted in adsorption onto ENP surfaces, likely through ligand exchange since both ENPs were negatively charged in both water samples. This is because NOM is known to strongly adsorb due to ligand exchange between carboxyl and hydroxyl groups of NOM and the hydroxyl groups on ENPs.30 Both river water samples a had high NOM content (>5 mg L−1) which is within the reported range of 0.1 to 30 mg L−1 in surface water,59,61,62 and therefore, rendered NOM dependent aggregation highly likely.30,59
Secondly, differences in IS, with both monovalent and divalent ions being higher in BR compared to ER (Table 1), could also explain the enhanced aggregation in the former, as such conditions are known to increase NOM and ENP complexes.59,63–65 The increased aggregation in BR was also linked to the higher concentration of electrolytes (mainly divalent ions e.g. Ca2+, Mg2+, etc.) via compression of the electric double layer (EDL) through the reduction of electrostatic repulsion between particles and/or formation of aggregates by cation bridging.30,55,63,64,66,67 Consequently, nZnO dissolution in BR was significantly lower due to the reduced surface area as larger aggregates were formed compared to those in ER water samples. Dissolved zinc from nZnO could have also been reduced due to complexation with NOM and PO4, resulting in lower concentration of ions measured in BR water. Li et al.31 and Lv et al.68 showed that the release of Zn2+ decreased with increasing concentrations of PO4 due to strong metal-complexation between the phosphates and metal ions. In this study, BR water had a higher concentration of PO4 than ER water, pointing to reduced bioavailability of the ions in the former.
The observed differences in the dissolution of nZnO were therefore linked to the differences in aggregation and water chemistry observed between ER and BR water samples. Similarly, results of Odzak et al.36 indicated enhanced aggregation and low dissolution of nZnO in freshwaters sourced from river and lake waters with high IS ranging between 3.4 and 6.4 mM compared with those characterized by low IS. These researchers' results are consistent with the findings reported herein where BR water samples with a high IS of 5.23 mM had higher aggregation and low dissolution of nZnO when compared to low aggregation and high dissolution observed in ER with a low IS of 2.43 mM.
Overall, results therefore point to NOM coating-controlled release of ions from ENPs in freshwater due to the blockage of active sites, which in turn, inhibits the diffusion of ions from ENP surfaces, thus accounting for the high aggregation and low dissolution observed in BR water samples characterised by both high NOM and IS. In addition, complexation of metal ions with NOM and phosphates could also account for reduced dissolution of nZnO. These outlined transformations have direct or indirect influence on the bioavailability and toxicity of nZnO and γ-nFe2O3 in the two freshwater systems as discussed in the following sections.
Cell viability and membrane integrity
Results of B. subtilis exposure to nZnO and γ-nFe2O3 in ER and BR revealed distinctive cytotoxic effects (Fig. 1A and B). At higher concentrations of 100 and 1000 μg L−1, nZnO reduced the B. subtilis viability in ER with markedly significant effects observed at 1000 μg L−1 (p ≤ 0.001); whereas at 10 μg L−1 nZnO, no viability inhibition was apparent. Conversely, exposure to nZnO in BR had no effect on cell viability at all tested concentrations (Fig. 1A) which was linked to larger aggregates formed in BR water samples as discussed in the Nanoparticle characterization section. This is because larger aggregates could not compromise cell integrity due to the limited or lack of contact with cells. For γ-nFe2O3, irrespective of the exposure concentration (10–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg L−1) no effect on cell viability was observed in both water samples (Fig. 1B).
000 μg L−1) no effect on cell viability was observed in both water samples (Fig. 1B).
Bacterial cell membrane integrity was also evaluated for both ENPs, and the results are summarized in Fig. 2. Effects of nZnO on cell membrane integrity were found to be concentration dependent in both river waters. For instance, results indicated that nZnO induced significantly higher effects on cell membrane integrity disruption at all concentrations in ER with a maximum reduction of 46% (Fig. 2A), and the trend was similar to the reduction in cell viability (61%) observed at the same concentration (Fig. 1A). A 26% cell membrane integrity disruption was observed in BR at 1000 μg L−1, but insignificant minimal effects at lower concentrations (Fig. 2A). Moreover, the cell membrane integrity significantly decreased (p ≤ 0.01) in BR (1000 μg L−1) for nZnO, and the results were similar to those of 100 μg L−1 in ER (Fig. 2A). The observed differences in cell membrane integrity were attributed to two factors: (i) water physicochemical properties (Table S1†) where in ER a marked reduction was observed, likely due to the low aggregation and high dissolution of ENPs (specifically nZnO), and (ii) the type of ENP with nZnO inducing a higher disruption compared to γ-nFe2O3 (Fig. 2).
To date, the toxic effects of nZnO on bacteria have been widely reported, with the effects being linked to mechanisms such as the nanoparticle surface contact and uptake, release of ions (Zn2+), and ROS production.31,46,69–74 In this study, the three mechanisms were evaluated in an effort to account for the observed cytotoxic effects of both ENPs on B. subtilis, and the results are discussed in the following paragraphs and sections. Concentration-dependent effects on cell membrane integrity were observed in both water samples (Fig. 2A) but cell viability effects were only observed in ER water samples (Fig. 1A). Our findings are consistent with other studies53,71 where bacterial viability was found to be dependent on nZnO exposure dosage (2 μg L−1 to 5000 mg L−1). Herein, the observed effects were linked to the increased dissolved zinc ion concentrations as the exposure concentration increased (Fig. S2†), which was dependent on the river water chemistry (Table 1). This is consistent with earlier findings where dissolved zinc from nZnO was established to be responsible for the observed toxicity to bacteria as influenced by exposure media chemistry.31,53 Herein, the effects of nZnO in ER may be linked to measured dissolved zinc of 366 μg L−1 compared to 183 μg L−1 in BR at a nominal exposure concentration of 1 mg L−1. Modelled speciation results of dissolved zinc species in both river water systems using Visual MINTEQ showed that about 50% of dissolved zinc formed complexes with DOM in BR water compared to about 39% in ER water (Table S2†); whereas the rest formed labile complexes that could also account for the observed toxicity as similarly reported elsewhere.75
γ-nFe2O3 showed no cell membrane integrity effects on B. subtilis irrespective of the water sample source across all exposure concentrations (Fig. 2B), and the results are in agreement with the literature where no effects were observed on bacteria at concentrations of <70 mg L−1 (<70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg L−1).52,76,77 In this study, no cytotoxic effects of γ-nFe2O3 were observed due to the high aggregation (Table S1†) that, in turn, reduced the bacterial cell–nanoparticle contact. High aggregation was evident at higher exposure concentrations where γ-nFe2O3 sedimented to the bottom of the exposure vessels. And the low solubility of γ-nFe2O3 implied very low amounts or non-release of ions especially in the short 2 h exposure period used in this study, further accounting why no cytotoxic effects were evident.
000 μg L−1).52,76,77 In this study, no cytotoxic effects of γ-nFe2O3 were observed due to the high aggregation (Table S1†) that, in turn, reduced the bacterial cell–nanoparticle contact. High aggregation was evident at higher exposure concentrations where γ-nFe2O3 sedimented to the bottom of the exposure vessels. And the low solubility of γ-nFe2O3 implied very low amounts or non-release of ions especially in the short 2 h exposure period used in this study, further accounting why no cytotoxic effects were evident.
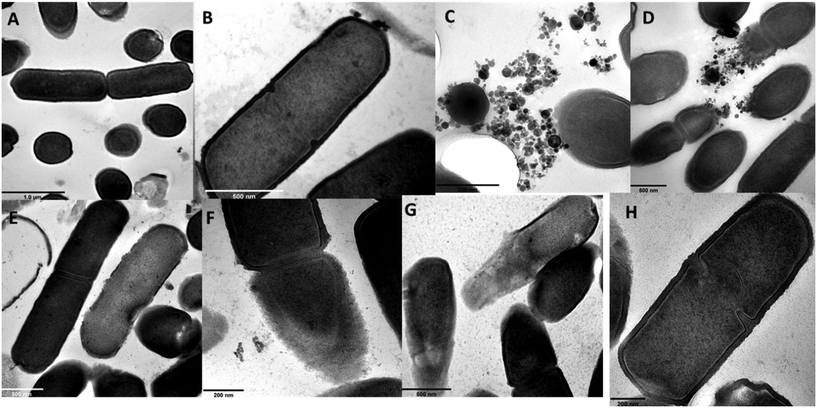
Herein, TEM was used to observe bacteria–ENP interactions and the likely resultant effects on the bacterial cells, and the micrographs are shown in Fig. 3. For γ-nFe2O3, intact bacteria cells at all nominal exposure concentrations (10–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg L−1) were observed with only limited contact between the ENPs and cells as the exposure concentration increased (Fig. 3(A–D)). Raptured cells were observed at higher concentrations of 100 and 1000 μg L−1 for nZnO (Fig. 3F and G), but the cells remained apparently intact at the lower concentration of 10 μg L−1.
000 μg L−1) were observed with only limited contact between the ENPs and cells as the exposure concentration increased (Fig. 3(A–D)). Raptured cells were observed at higher concentrations of 100 and 1000 μg L−1 for nZnO (Fig. 3F and G), but the cells remained apparently intact at the lower concentration of 10 μg L−1.
The cross-sections of B. subtilis following exposure to ENPs (Fig. 4) depict a qualitative assessment on the integrity of membrane structures. At lower concentrations of 10 and 100 μg L−1, γ-nFe2O3 intact cells were evident (Fig. 4A and B); however, at higher ones (1000 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg L−1) as shown in Fig. 4C and D, impairment of the cell walls and membrane was observed likely due to the close proximity of ENPs to the cells enhanced by increased aggregation as the concentration increased as well as likely entrapment of cells in the formed aggregates. For nZnO, raptured cells were observed at 1000 μg L−1 (Fig. 4G), but at 10 and 100 μg L−1 qualitatively higher proportions of unimpaired cells were observed (Fig. 4E and F).
000 μg L−1) as shown in Fig. 4C and D, impairment of the cell walls and membrane was observed likely due to the close proximity of ENPs to the cells enhanced by increased aggregation as the concentration increased as well as likely entrapment of cells in the formed aggregates. For nZnO, raptured cells were observed at 1000 μg L−1 (Fig. 4G), but at 10 and 100 μg L−1 qualitatively higher proportions of unimpaired cells were observed (Fig. 4E and F).
The bacterial cell wall serves as a barrier that controls and/or prevents the entry of certain compounds from the surrounding environment into the cell interior.78,79 Engineered nanoparticles have been reported to show anti-microbial activity through ENP-induced disruption of the membrane.10,71,80 Contact between ENPs and bacteria, for example, where nZnO has been observed to cause cell wall permeability on B. subtilis was reported by Rago54 and on other organisms.22,71 Herein, TEM results point to the restricted contact between ENPs (nZnO and γ-nFe2O3) and bacteria cells with no evidence of uptake, yet cell membrane damage was observed in the case of nZnO. From our findings, negatively charged ENPs (as reported herein for both river water samples, Table S2†) suggest minimal ENP–cell interactions (if any) due to repulsion; however, the ENP–cell surface contact could not be ruled-out especially at higher concentrations where membrane damage occurred for nZnO. For instance, a HDD of 558 ± 28 nm for nZnO as measured by DLS represents the average size of larger-sized particles; however, smaller-sized particles can still interact with the bacteria cells due to the concentration effect as suggested by other researchers.81 Such nZnO–bacteria interactions could also occur aided by mechanisms such as hydrogen bonding, van der Waals forces, and receptor–ligand interactions through bacterial cell moieties where there's weak electrostatic repulsion.74,82,83 However, no such similar effects were observed for γ-nFe2O3 due to the formation of larger aggregates (μm sized) that tended to sediment and settle at the bottom of the vessels, thus leading to no plausible ENP–cell interactions. Zn2+ has also been suggested to attach to the cell membrane and rupture the cell wall leading to loss of membrane integrity.84,85 In addition, in circumstances where disruption of zinc homeostasis occurs due to internalised Zn2+ cell death may occur through the denaturation of protein.85–87 Tong et al.,44 for example, reported marked inhibition of between 10 and 20% on Aeromonas hydrophila and E. coli by nZnO (1000 μg L−1) under dark and light conditions without increased ROS production and with minimal contact between nZnO and bacterial cells. Therefore, results herein show that Zn2+ effects may be via reduction of cellular functioning due to the pure chemical effect on the bacterial cells.
ATP production
In this study, the ability of ENPs to disrupt ATP production was also evaluated. Results of measured ATP abundance following exposure of B. subtilis to nZnO and γ-nFe2O3 in both water samples for 1 h are summarised in Fig. 5. ATP levels were observed to decrease as a function of time, nominal exposure concentration, and water chemistry (Fig. 5). For example, nZnO exhibited significant concentration-dependent effects on ATP levels which were more pronounced in ER compared to BR water samples (Fig. 5A). To date, only limited studies have investigated the likely effects of ENPs on bacterial ATP levels.42–44 Tong et al.,44 for example, reported significant concentration-dependent reduction in ATP levels exerted on E. coli and A. hydrophila by nZnO in lake water at concentrations of 250 and 1000 μg L−1 following 1 h incubation under dark conditions.Herein, our results revealed significant reduction in ATP levels even at the lower concentration of 10 μg L−1 nZnO (p < 0.05) following 1 h incubation in river water under visible light. To account for these findings, we propose two plausible mechanisms. First, the depletion of cellular ATP may have been due to the disruption of the cellular membrane leading to the loss of homeostasis in cells.54,87 Secondly, the release of ions following the dissolution of ENPs (in this case nZnO into dissolved zinc species) may have deactivated energy-dependent reactions in the cells as previously observed in toxicity studies of silver and zinc.88,89 In most organisms, Zn is an essential micronutrient required for biochemical processes; however, when present at elevated quantities Zn may also interfere with biological pathways.89 Therefore, the dissolved zinc from nZnO may have been taken up via the transport chains without causing damage to the cell membrane – but rather induced denaturation of ribosomes and suppression of enzymes and proteins involved in ATP production – leading to the disruption of the cell functionality.90,91 This is consistent with the findings of nZnO toxicity in this study as evidenced by reduction in cell viability (Fig. 1A) and cell membrane integrity (Fig. 2A). This correlation is highly plausible due to two reasons. First, because minimal ENP–cell contact was established, thus unlikely cell membrane perforations were observed (Fig. 3E–G). And secondly, results herein indicate that the observed reduction in ATP levels followed the dissolution patterns responsible for the release of dissolved zinc as also suggested earlier by Tong et al.44 Therefore, the observed varying nZnO effects between water matrices from different sources were linked to differences in dissolution as influenced by water chemistry characteristics.
Results summarized in Fig. 5 also show the influence of the ENP type on ATP production. The results in Fig. 5B show that ATP levels following exposure to γ-nFe2O3 (compared to nZnO in Fig. 5A) were insignificant irrespective of the nominal exposure concentration and water chemistry in both water samples. In certain cases, however, like in BR γ-nFe2O3 was observed to induce increased ATP production levels above the control after 30 min incubation (Fig. 5B). To the authors' knowledge, this is the first time that ATP levels on bacteria exposed to γ-nFe2O3 in natural water samples were observed (10–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg L−1). The lack of significant effects observed by ATP measurements, even at 10
000 μg L−1). The lack of significant effects observed by ATP measurements, even at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg L−1, suggests that γ-nFe2O3 may not pose any undesirable effect on microorganisms in aquatic systems, particularly at current predicted environmental concentrations of 28 ng L−1.92
000 μg L−1, suggests that γ-nFe2O3 may not pose any undesirable effect on microorganisms in aquatic systems, particularly at current predicted environmental concentrations of 28 ng L−1.92
ROS production
To determine whether oxidative stress contributed to the observed effects of ENPs, ROS production was evaluated. Results showed that nZnO and nFe2O3 induced no significant change in intracellular ROS levels on B. subtilis, compared to the control, in both water samples under visible light conditions (Fig. 6). Hence, these findings indicate that ROS production and oxidative stress were not linked to the observed nZnO toxicity.To date, numerous studies have reported cell damage due to oxidative stress to be among the key toxicity causing mechanisms for metal-based ENPs.25,74,93–95 For example, nZnO has been observed to induce significant ROS generation from E. coli relative to the control even in the absence of UV illumination71 – although at higher concentration of 8 mg L−1 (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg L−1) unlikely to be found in actual freshwater systems. Dasari and Hwang32 observed cytotoxic effects in natural river water following exposure of nZnO to bacterial assemblages at 100 and 1000 μg L−1 under both dark and light (sunlight) conditions; however, ROS generation was similar to the controls. Similarly, findings of Rago et al.54 revealed no induction of oxidative stress (ROS production) to B. subtilis following exposure to nZnO (10–250
000 μg L−1) unlikely to be found in actual freshwater systems. Dasari and Hwang32 observed cytotoxic effects in natural river water following exposure of nZnO to bacterial assemblages at 100 and 1000 μg L−1 under both dark and light (sunlight) conditions; however, ROS generation was similar to the controls. Similarly, findings of Rago et al.54 revealed no induction of oxidative stress (ROS production) to B. subtilis following exposure to nZnO (10–250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg L−1), yet cytotoxic effects were observed.
000 μg L−1), yet cytotoxic effects were observed.
Herein, results show that nZnO induced cytotoxicity and cell membrane damage to B. subtilis in both river water samples, but the observed effects could not be accounted for by ROS production (as it was absent). In addition, our results are consistent with observations of Dasari et al.32 where cytotoxicity was evident but could not be linked to oxidative stress. As argued by Kadiyala et al.83 and the evidence of the results from this study, cytotoxicity and cell membrane damage to B. subtilis indicate that ROS generation cannot be the predominant mechanism to account for the nZnO antimicrobial activity.87 Therefore, more analysis is essential to elucidate the likely oxidative stress implications on the toxicity of ENPs, and particularly generate knowledge that can offer valuable insights that can account for the current contradictory data.
Environmental implications
The need to generate ENP toxicity data from an environmental point of view, especially to build an understanding on their impacts on bacteria – organisms known to play a key role in efficient and effective functioning of ecological systems – remains of high importance. This is because adverse effects on bacteria due to ENPs can trigger far reaching yet undesirable implications. To contribute towards achieving this objective, in this study, multiple lines of evidence on effects (lethal and sub-lethal) of ENPs to bacteria were considered – particularly at likely relevant exposure concentrations and in complex dynamic natural water matrices (sourced from two rivers). Evidence shows that differences in water chemistry properties play a significant role in the observed (or lack thereof) effects of ENPs on microorganisms as they control the extent of ENP bioavailability and transformation(s) in a given medium. This means that, based on the results generated from this study, it is not possible to generalize the effects of ENPs in freshwater, (whether at organism or sub-lethal levels); rather the role of the water chemistry should be carefully considered.To date, very limited studies have investigated the implications of ENPs to microorganisms in actual environmental matrices at relevant exposure concentrations such as freshwater used in this study. To contribute and improve the risk assessment of ENPs in the environment, data presented herein particularly at sub-lethal levels (cell membrane integrity and ATP production) should be considered even when there is no evidence of effects on whole body organisms, or even in the absence of widely known mechanisms of toxicity such as ROS production. This is because, exclusion of non-standard toxicity endpoints can lead to flawed conclusions if only absence of adverse effects of ENPs to microorganisms (based on effect or lethal concentration endpoints) are exclusively considered, and especially given the increasing concentrations of these emerging contaminants in different environmental compartments. Moreover, current divergent and fragmented data especially on the mechanisms of toxicity and levels of toxicity to bacteria mostly generated in synthetic exposure media, has hindered our ability to draw meaningful conclusions concerning the extent of ENP implications to the environment. Thus, this points to two priority areas that need urgent consideration, if risk assessment of ENPs in the environment particularly to microorganisms is to achieve a certain degree of standardization among experts in different fields (technical, regulatory and policy). The two areas entails consideration of non-standard methods to assess effects and mechanisms of toxicity (e.g. molecular approaches) especially at low ENPs concentrations, and using actual environmental matrices (e.g. sediments, freshwater, etc.).
Overall, the study findings suggest that γ-nFe2O3 at current environmental concentrations may not pose a risk to environmental organisms such as bacteria based on both physiological and sub-lethal effects. In contrast, the effects of nZnO on bacteria were dependent on water chemistry where dissolution was a key precursor parameter to the observed toxicity. These results suggest that sub-cellular effects need to be incorporated jointly with traditional endpoints to establish the toxicity of ENPs, as the case with nZnO in this study vividly illustrates. However, findings herein cannot be deemed representative of all possible permutations of environmental conditions which could not be considered in a single study. This raises the need to broaden testing conditions (represented by different sources of freshwater) including aspects such as irradiation and use of sensitive trophic levels e.g. crustaceans in natural water matrices. More data generated using natural waters as exposure media and in consideration of multiple toxicity endpoints will improve the possibility of undertaking meaningful ecological risk assessments of ENPs. Such an approach will aid in drawing firm conclusions necessary to map out proactive approaches aiming to manage nanoscale contaminants in a responsible and sustainable manner.
Conclusions
In this study, the toxicity of nZnO and γ-nFe2O3 in natural water samples with varying physicochemical parameters was assessed. Results indicated that cell viability, cell membrane integrity, and ATP production were more diminished in ER (lower NOM, low IS, etc.) compared to those in BR (high NOM, high IS, etc.) water samples for nZnO exposure. However, γ-nFe2O3 induced very low or no cytotoxicity to microorganisms at exposure concentrations investigated in this work. In addition, we demonstrated that the toxicity of ENPs to B. subtilis was dependent chiefly on the differences in IS and NOM in the studied water samples. ROS production was observed to be negligible for both ENPs. Moreover, there were no observed interactions of nZnO and bacteria indicating that the effects of nZnO were likely driven by dissolved zinc, and water chemistry played a key role as evidenced by the differences in its dissolution between the two water samples.This study also illustrates the benefit of using multiple endpoints to assess the toxicity of ENPs as valuable insights into discrete effects were gained as certain endpoints showed no apparent responses but others revealed likely deleterious effects. For instance, in certain cases cell viability could not reveal cytotoxic effects of nZnO whereas cell membrane damage and ATP production demonstrated the effects of ENPs on bacteria. These findings suggest a plausible correlation between dissolved zinc and observed effects at different concentrations in the two water sources. This implies the induced adverse interference on the metabolic pathways and cell membrane structures, which in turn, leads to the observed outcomes on bacteria; however, the nanoparticulate effects cannot be ruled out. Overall, the study highlighted the complexity and variations in natural water sample physicochemical properties that should be considered when establishing the toxicity of ENPs to bacteria.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the University of Pretoria (UP) (Grant No.: A0Y229) and the Water Research Commission (WRC) (K5/2509/1), South Africa. The contents are the sole responsibility of the authors and do not necessarily reflect the views of UP or the WRC. The authors would like to thank the Centre for Microbial Ecology and Genomics (CMEG) for use of their facilities and equipment, the Laboratory for Microscopy and Microanalysis at the University of Pretoria for assistance with microscopy sample preparation and analysis, and Botswana International University of Science and Technology (BIUST) for use of their equipment. And, the comments and suggestions of three anonymous reviewers are highly acknowledged since they aided in improving the quality of the manuscript.References
- F. Gottschalk, T. Sun and B. Nowack, Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies, Environ. Pollut., 2013, 181, 287–300 CrossRef CAS PubMed.
- S. F. Hansen, L. R. Heggelund, P. R. Besora, A. Mackevica, A. Boldrin and A. Baun, Nanoproducts–what is actually available to European consumers?, Environ. Sci.: Nano, 2016, 3, 169–180 RSC.
- Project on Emerging Nanotechnologies (PEN), Consumer Products Inventory: An inventory of nanotechnology-based consumer products introduced on the market, Available at http://www.nanotechproject.org, 2018.
- The Nanodatabase, Jointly Published by: DTU Environment, the Danish Ecological Council and Danish Consumer Council., www.nanodb.dk, 2016.
- K. Hegde, S. K. Brar, M. Verma and R. Y. Surampalli, Current understandings of toxicity, risks and regulations of engineered nanoparticles with respect to environmental microorganisms, Nanotechnol. Environ. Eng., 2016, 1, 5 CrossRef.
- F. Gottschalk, C. Lassen, J. Kjoelholt, F. Christensen and B. Nowack, Modeling flows and concentrations of nine engineered nanomaterials in the Danish environment, Int. J. Environ. Res. Public Health, 2015, 12, 5581–5602 CrossRef CAS PubMed.
- R. W. Lai, K. W. Yeung, M. M. Yung, A. B. Djurišić, J. P. Giesy and K. M. Leung, Regulation of engineered nanomaterials: current challenges, insights and future directions, Environ. Sci. Pollut. Res., 2018, 25, 3060–3077 CrossRef CAS.
- Ricardo Energy and Environment, Support for 3rd regulatory review on nanomaterials- environmental legislation: Interim/Background Report, 2016.
- J. W. Wiechers and N. Musee, Engineered inorganic nanoparticles and cosmetics: facts, issues, knowledge gaps and challenges, J. Biomed. Nanotechnol., 2010, 6, 408–431 CrossRef CAS.
- J. Hou, Y. Wu, X. Li, B. Wei, S. Li and X. Wang, Toxic effects of different types of zinc oxide nanoparticles on algae, plants, invertebrates, vertebrates and microorganisms, Chemosphere, 2018, 193, 852–860 CrossRef CAS.
- D. L. Huber, Synthesis, properties, and applications of iron nanoparticles, Small, 2005, 1, 482–501 CrossRef CAS.
- E. Demangeat, M. Pedrot, A. Dia, M. Bouhnik-Le-Coz, F. Grasset, K. Hanna, M. Kamagate and F. Cabello-Hurtado, Colloidal and chemical stabilities of iron oxide nanoparticles in aqueous solutions: the interplay of structural, chemical and environmental drivers, Environ. Sci.: Nano, 2018, 5, 992–1001 RSC.
- Y. Zhang, B. Wu, H. Xu, H. Liu, M. Wang, Y. He and B. Pan, Nanomaterials-enabled water and wastewater treatment, NanoImpact, 2016, 3, 22–39 CrossRef.
- S. Rajput, L. P. Singh, C. U. Pittman Jr and D. Mohan, Lead (Pb2+) and copper (Cu2+) remediation from water using superparamagnetic maghemite (γ-Fe2O3) nanoparticles synthesized by Flame Spray Pyrolysis (FSP), J. Colloid Interface Sci., 2017, 492, 176–190 CrossRef CAS.
- C. Lei, L. Zhang, K. Yang, L. Zhu and D. Lin, Toxicity of iron-based nanoparticles to green algae: Effects of particle size, crystal phase, oxidation state and environmental aging, Environ. Pollut., 2016, 218, 505–512 CrossRef CAS.
- N. Musee, A model for screening and prioritizing consumer nanoproduct risks: A case study from South Africa, Environ. Int., 2017, 100, 121–131 CrossRef CAS PubMed.
- Y. Wang and B. Nowack, Environmental Risk Assessment of Engineered Nano-SiO2, Nano Iron Oxides, Nano-CeO2, Nano-Al2O3, and Quantum Dots, Environ. Toxicol. Chem., 2018, 37, 1387–1395 CrossRef CAS.
- F. Gottschalk, C. Ort, R. W. Scholz and B. Nowack, Engineered nanomaterials in rivers–exposure scenarios for Switzerland at high spatial and temporal resolution, Environ. Pollut., 2011, 159, 3439–3445 CrossRef CAS.
- T. Y. Sun, F. Gottschalk, K. Hungerbühler and B. Nowack, Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials, Environ. Pollut., 2014, 185, 69–76 CrossRef CAS.
- E. Dumont, A. C. Johnson, V. D. Keller and R. J. Williams, Nano silver and nano zinc-oxide in surface waters–Exposure estimation for Europe at high spatial and temporal resolution, Environ. Pollut., 2015, 196, 341–349 CrossRef CAS.
- N. Musee, M. Thwala and N. Nota, The antibacterial effects of engineered nanomaterials: implications for wastewater treatment plants, J. Environ. Monit., 2011, 13, 1164–1183 RSC.
- S. Bandyopadhyay, G. Plascencia-Villa, A. Mukherjee, C. M. Rico, M. José-Yacamán, J. R. Peralta-Videa and J. L. Gardea-Torresdey, Comparative phytotoxicity of ZnO NPs, bulk ZnO, and ionic zinc onto the alfalfa plants symbiotically associated with Sinorhizobium meliloti in soil, Sci. Total Environ., 2015, 515, 60–69 CrossRef.
- T. Qiu, J. Bozich, S. Lohse, A. Vartanian, L. Jacob, B. Meyer, I. Gunsolus, N. Niemuth, C. Murphy and C. Haynes, Gene expression as an indicator of the molecular response and toxicity in the bacterium Shewanella oneidensis and the water flea Daphnia magna exposed to functionalized gold nanoparticles, Environ. Sci.: Nano, 2015, 2, 615–629 RSC.
- A. Ivask, K. Juganson, O. Bondarenko, M. Mortimer, V. Aruoja, K. Kasemets, I. Blinova, M. Heinlaan, V. Slaveykova and A. Kahru, Mechanisms of toxic action of Ag, ZnO and CuO nanoparticles to selected ecotoxicological test organisms and mammalian cells in vitro: a comparative review, Nanotoxicology, 2014, 8, 57–71 CrossRef CAS.
- C. Peng, W. Zhang, H. Gao, Y. Li, X. Tong, K. Li, X. Zhu, Y. Wang and Y. Chen, Behavior and potential impacts of metal-based engineered nanoparticles in aquatic environments, Nanomaterials, 2017, 7, 21 CrossRef.
- M. Zhu, H. Wang, A. A. Keller, T. Wang and F. Li, The effect of humic acid on the aggregation of titanium dioxide nanoparticles under different pH and ionic strengths, Sci. Total Environ., 2014, 487, 375–380 CrossRef CAS.
- C. Ren, X. Hu and Q. Zhou, Influence of environmental factors on nanotoxicity and knowledge gaps thereof, NanoImpact, 2016, 2, 82–92 CrossRef.
- J. Yi and J. Cheng, Effects of water chemistry and surface contact on the toxicity of silver nanoparticles to Bacillus subtilis, Ecotoxicology, 2017, 26, 639–647 CrossRef CAS.
- S. M. Majedi, B. C. Kelly and H. K. Lee, Role of combinatorial environmental factors in the behavior and fate of ZnO nanoparticles in aqueous systems: a multiparametric analysis, J. Hazard. Mater., 2014, 264, 370–379 CrossRef CAS.
- Z. Wang, L. Zhang, J. Zhao and B. Xing, Environmental processes and toxicity of metallic nanoparticles in aquatic systems as affected by natural organic matter, Environ. Sci.: Nano, 2016, 3, 240–255 RSC.
- M. Li, D. Lin and L. Zhu, Effects of water chemistry on the dissolution of ZnO nanoparticles and their toxicity to Escherichia coli, Environ. Pollut., 2012, 173, 97–102 CrossRef PubMed.
- T. P. Dasari and H.-M. Hwang, Effect of humic acids and sunlight on the cytotoxicity of engineered zinc oxide and titanium dioxide nanoparticles to a river bacterial assemblage, J. Environ. Sci., 2013, 25, 1925–1935 CrossRef CAS.
- M. Heinlaan, M. Muna, M. Knöbel, D. Kistler, N. Odzak, D. Kühnel, J. Müller, G. S. Gupta, A. Kumar and R. Shanker, Natural water as the test medium for Ag and CuO nanoparticle hazard evaluation: an interlaboratory case study, Environ. Pollut., 2016, 216, 689–699 CrossRef CAS.
- M. Thwala, S. J. Klaine and N. Musee, Interactions of metal-based engineered nanoparticles with aquatic higher plants: A review of the state of current knowledge, Environ. Toxicol. Chem., 2016, 35, 1677–1694 CrossRef CAS.
- Y.-H. Peng, Y.-C. Tsai, C.-E. Hsiung, Y.-H. Lin and Y.-H. Shih, Influence of water chemistry on the environmental behaviors of commercial ZnO nanoparticles in various water and wastewater samples, J. Hazard. Mater., 2017, 322, 348–356 CrossRef CAS.
- N. Odzak, D. Kistler and L. Sigg, Influence of daylight on the fate of silver and zinc oxide nanoparticles in natural aquatic environments, Environ. Pollut., 2017, 226, 1–11 CrossRef CAS.
- J. R. Conway, A. S. Adeleye, J. Gardea-Torresdey and A. A. Keller, Aggregation, dissolution, and transformation of copper nanoparticles in natural waters, Environ. Sci. Technol., 2015, 49, 2749–2756 CrossRef CAS PubMed.
- C. T. T. Binh, T. Tong, J.-F. O. Gaillard, K. A. Gray and J. J. Kelly, Common freshwater bacteria vary in their responses to short-term exposure to nano-TiO2, Environ. Toxicol. Chem., 2014, 33, 317–332 CrossRef CAS PubMed.
- P. A. Holden, J. L. Gardea-Torresdey, F. Klaessig, R. F. Turco, M. Mortimer, K. Hund-Rinke, E. A. Cohen Hubal, D. Avery, D. Barceló and R. Behra, Considerations of environmentally relevant test conditions for improved evaluation of ecological hazards of engineered nanomaterials, Environ. Sci. Technol., 2016, 50, 6124–6145 CrossRef CAS PubMed.
- C.-W. Huang, S.-W. Li and V. H.-C. Liao, Chronic ZnO-NPs exposure at environmentally relevant concentrations results in metabolic and locomotive toxicities in Caenorhabditis elegans, Environ. Pollut., 2017, 220, 1456–1464 CrossRef CAS.
- Z. Sheng and Y. Liu, Potential impacts of silver nanoparticles on bacteria in the aquatic environment, J. Environ. Manage., 2017, 191, 290–296 CrossRef CAS.
- C. M. Wilke, T. Tong, J.-F. O. Gaillard and K. A. Gray, Attenuation of Microbial Stress Due to Nano-Ag and Nano-TiO2 Interactions under Dark Conditions, Environ. Sci. Technol., 2016, 50, 11302–11310 CrossRef CAS.
- C. M. Wilke, J.-F. Gaillard and K. A. Gray, The critical role of light in moderating microbial stress due to mixtures of engineered nanomaterials, Environ. Sci.: Nano, 2018, 5, 96–102 RSC.
- T. Tong, C. M. Wilke, J. Wu, C. T. T. Binh, J. J. Kelly, J.-F. O. Gaillard and K. A. Gray, Combined Toxicity of Nano-ZnO and Nano-TiO2: From Single- to Multinanomaterial Systems, Environ. Sci. Technol., 2015, 49, 8113–8123 CrossRef CAS PubMed.
- A. M. Earl, R. Losick and R. Kolter, Ecology and genomics of Bacillus subtilis, Trends Microbiol., 2008, 16, 269–275 CrossRef CAS.
- Y.-W. Baek and Y.-J. An, Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus, Sci. Total Environ., 2011, 409, 1603–1608 CrossRef CAS.
- C. Pagnout, S. Jomini, M. Dadhwal, C. Caillet, F. Thomas and P. Bauda, Role of electrostatic interactions in the toxicity of titanium dioxide nanoparticles toward Escherichia coli, Colloids Surf., B, 2012, 92, 315–321 CrossRef CAS PubMed.
- A. Hitchman, G. H. S. Smith, Y. Ju-Nam, M. Sterling and J. R. Lead, The effect of environmentally relevant conditions on PVP stabilised gold nanoparticles, Chemosphere, 2013, 90, 410–416 CrossRef CAS.
- T. M. Riddick, Control of Colloid Stability through Zeta Potential and its Relationship to Cardiovascular Disease, Livingston Publishing, Wynnewood, PA, 1968 Search PubMed.
- R. W. O'Brien and L. R. White, Electrophoretic mobility of a spherical colloidal particle, J. Chem. Soc., Faraday Trans. 2, 1978, 74, 1607–1626 RSC.
- G. V. Lowry, R. J. Hill, S. Harper, A. F. Rawle, C. O. Hendren, F. Klaessig, U. Nobbmann, P. Sayre and J. Rumble, Guidance to improve the scientific value of zeta-potential measurements in nanoEHS, Environ. Sci.: Nano, 2016, 3, 953–965 RSC.
- D. Wang, Z. Lin, T. Wang, Z. Yao, M. Qin, S. Zheng and W. Lu, Where does the toxicity of metal oxide nanoparticles come from: the nanoparticles, the ions, or a combination of both?, J. Hazard. Mater., 2016, 308, 328–334 CrossRef CAS PubMed.
- M. Li, S. Pokhrel, X. Jin, L. Mädler, R. Damoiseaux and E. M. Hoek, Stability, bioavailability, and bacterial toxicity of ZnO and iron-doped ZnO nanoparticles in aquatic media, Environ. Sci. Technol., 2010, 45, 755–761 CrossRef.
- I. Rago, C. R. Chandraiahgari, M. P. Bracciale, G. De Bellis, E. Zanni, M. C. Guidi, D. Sali, A. Broggi, C. Palleschi and M. S. Sarto, Zinc oxide microrods and nanorods: different antibacterial activity and their mode of action against Gram-positive bacteria, RSC Adv., 2014, 4, 56031–56040 RSC.
- N. Musee, J. N. Zvimba, L. M. Schaefer, N. Nota, L. M. Sikhwivhilu and M. Thwala, Fate and behavior of ZnO-and Ag-engineered nanoparticles and a bacterial viability assessment in a simulated wastewater treatment plant, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2014, 49, 59–66 CrossRef CAS.
- M. Baalousha, A. Manciulea, S. Cumberland, K. Kendall and J. R. Lead, Aggregation and surface properties of iron oxide nanoparticles: influence of pH and natural organic matter, Environ. Toxicol. Chem., 2008, 27, 1875–1882 CrossRef CAS.
- R. Khan, M. Inam, S. Zam, D. Park and I. Yeom, Assessment of key environmental factors influencing the sedimentation and aggregation behavior of zinc oxide nanoparticles in aquatic environment, Water, 2018, 10, 660 CrossRef.
- S. Yu, J. Liu, Y. Yin and M. Shen, Interactions between engineered nanoparticles and dissolved organic matter: A review on mechanisms and environmental effects, J. Environ. Sci., 2018, 63, 198–217 CrossRef.
- A. Philippe and G. E. Schaumann, Interactions of dissolved organic matter with natural and engineered inorganic colloids: a review, Environ. Sci. Technol., 2014, 48, 8946–8962 CrossRef CAS.
- B. Collin, M. Auffan, A. C. Johnson, I. Kaur, A. A. Keller, A. Lazareva, J. R. Lead, X. Ma, R. C. Merrifield and C. Svendsen, Environmental release, fate and ecotoxicological effects of manufactured ceria nanomaterials, Environ. Sci.: Nano, 2014, 1, 533–548 RSC.
- A. Nebbioso and A. Piccolo, Molecular characterization of dissolved organic matter (DOM): a critical review, Anal. Bioanal. Chem., 2013, 405, 109–124 CrossRef CAS.
- S. M. Louie, R. D. Tilton and G. V. Lowry, Critical review: impacts of macromolecular coatings on critical physicochemical processes controlling environmental fate of nanomaterials, Environ. Sci.: Nano, 2016, 3, 283–310 RSC.
- S.-W. Bian, I. A. Mudunkotuwa, T. Rupasinghe and V. H. Grassian, Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: influence of pH, ionic strength, size, and adsorption of humic acid, Langmuir, 2011, 27, 6059–6068 CrossRef CAS PubMed.
- A. J. Miao, X. Y. Zhang, Z. Luo, C. S. Chen, W. C. Chin, P. H. Santschi and A. Quigg, Zinc oxide–engineered nanoparticles: dissolution and toxicity to marine phytoplankton, Environ. Toxicol. Chem., 2010, 29, 2814–2822 CrossRef CAS PubMed.
- X. He, W. G. Aker, P. P. Fu and H.-M. Hwang, Toxicity of engineered metal oxide nanomaterials mediated by nano–bio–eco–interactions: a review and perspective, Environ. Sci.: Nano, 2015, 2, 564–582 RSC.
- D. Zhou and A. A. Keller, Role of morphology in the aggregation kinetics of ZnO nanoparticles, Water Res., 2010, 44, 2948–2956 CrossRef CAS.
- J. Fang, A. Shijirbaatar, D.-H. Lin, D.-J. Wang, B. Shen and Z.-Q. Zhou, Stability of co-existing ZnO and TiO2 nanomaterials in natural water: Aggregation and sedimentation mechanisms, Chemosphere, 2017, 184, 1125–1133 CrossRef CAS PubMed.
- J. Lv, S. Zhang, L. Luo, W. Han, J. Zhang, K. Yang and P. Christie, Dissolution and microstructural transformation of ZnO nanoparticles under the influence of phosphate, Environ. Sci. Technol., 2012, 46, 7215–7221 CrossRef CAS PubMed.
- M. Heinlaan, A. Ivask, I. Blinova, H.-C. Dubourguier and A. Kahru, Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus, Chemosphere, 2008, 71, 1308–1316 CrossRef CAS PubMed.
- V. Aruoja, H. C. Dubourguier, K. Kasemets and A. Kahru, Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata, Sci. Total Environ., 2009, 407, 1461–1468 CrossRef CAS.
- A. Kumar, A. K. Pandey, S. S. Singh, R. Shanker and A. Dhawan, Engineered ZnO and TiO2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli, Free Radical Biol. Med., 2011, 51, 1872–1881 CrossRef CAS PubMed.
- A. Jain, R. Bhargava and P. Poddar, Probing interaction of Gram-positive and Gram-negative bacterial cells with ZnO nanorods, Mater. Sci. Eng., C, 2013, 33, 1247–1253 CrossRef CAS PubMed.
- Y. C. Huang, R. Fan, M. A. Grusak, J. D. Sherrier and C. Huang, Effects of nano-ZnO on the agronomically relevant Rhizobium–legume symbiosis, Sci. Total Environ., 2014, 497, 78–90 CrossRef PubMed.
- Y. H. Leung, X. Xu, A. P. Ma, F. Liu, A. M. Ng, Z. Shen, L. A. Gethings, M. Y. Guo, A. B. Djurišić and P. K. Lee, Toxicity of ZnO and TiO2 to Escherichia coli cells, Sci. Rep., 2016, 6, 35243 CrossRef CAS PubMed.
- M. Li, L. Zhu and D. Lin, Toxicity of ZnO nanoparticles to Escherichia coli: mechanism and the influence of medium components, Environ. Sci. Technol., 2011, 45, 1977–1983 CrossRef CAS PubMed.
- M. Auffan, W. Achouak, J. Rose, M.-A. Roncato, C. Chanéac, D. T. Waite, A. Masion, J. C. Woicik, M. R. Wiesner and J.-Y. Bottero, Relation between the redox state of iron-based nanoparticles and their cytotoxicity toward Escherichia coli, Environ. Sci. Technol., 2008, 42, 6730–6735 CrossRef CAS PubMed.
- A. Azam, A. S. Ahmed, M. Oves, M. S. Khan, S. S. Habib and A. Memic, Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study, Int. J. Nanomed., 2012, 7, 6003–6009 CrossRef CAS PubMed.
- W. Dowhan and M. Bogdanov, in New comprehensive biochemistry, Elsevier, 2002, vol. 36, pp. 1–35 Search PubMed.
- S. Murínová and K. Dercová, Response mechanisms of bacterial degraders to environmental contaminants on the level of cell walls and cytoplasmic membrane, Int. J. Microbiol., 2014, 2014, 873081 Search PubMed.
- A. Sirelkhatim, S. Mahmud, A. Seeni, N. H. M. Kaus, L. C. Ann, S. K. M. Bakhori, H. Hasan and D. Mohamad, Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism, Nano-Micro Lett., 2015, 7, 219–242 CrossRef CAS PubMed.
- D. Wang, Y. Gao, Z. Lin, Z. Yao and W. Zhang, The joint effects on Photobacterium phosphoreum of metal oxide nanoparticles and their most likely coexisting chemicals in the environment, Aquat. Toxicol., 2014, 154, 200–206 CrossRef CAS.
- W. Jiang, H. Mashayekhi and B. Xing, Bacterial toxicity comparison between nano-and micro-scaled oxide particles, Environ. Pollut., 2009, 157, 1619–1625 CrossRef CAS.
- U. Kadiyala, E. S. Turali-Emre, J. H. Bahng, N. A. Kotov and J. S. VanEpps, Unexpected insights into antibacterial activity of zinc oxide nanoparticles against methicillin resistant Staphylococcus aureus (MRSA), Nanoscale, 2018, 10, 4927–4939 RSC.
- J. A. Lemire, J. J. Harrison and R. J. Turner, Antimicrobial activity of metals: mechanisms, molecular targets and applications, Nat. Rev. Microbiol., 2013, 11, 371 CrossRef CAS PubMed.
- E. L. Yu-sen, R. D. Vidic, J. E. Stout, C. A. McCartney and L. Y. Victor, Inactivation of Mycobacterium avium by copper and silver ions, Water Res., 1998, 32, 1997–2000 CrossRef.
- C. B. Anders, J. E. Eixenberger, N. A. Franco, R. J. Hermann, K. D. Rainey, J. J. Chess, A. Punnoose and D. G. Wingett, ZnO nanoparticle preparation route influences surface reactivity, dissolution and cytotoxicity, Environ. Sci.: Nano, 2018, 5, 572–588 RSC.
- K. Matuła, Ł. Richter, W. Adamkiewicz, B. Åkerström, J. Paczesny and R. Hołyst, Influence of nanomechanical stress induced by ZnO nanoparticles of different shapes on the viability of cells, Soft Matter, 2016, 12, 4162–4169 RSC.
- A. Lapresta-Fernández, A. Fernández and J. Blasco, Nanoecotoxicity effects of engineered silver and gold nanoparticles in aquatic organisms, TrAC, Trends Anal. Chem., 2012, 32, 40–59 CrossRef.
- A. Alhasawi, C. Auger, V. Appanna, M. Chahma and V. Appanna, Zinc toxicity and ATP production in Pseudomonas fluorescens, J. Appl. Microbiol., 2014, 117, 65–73 CrossRef CAS PubMed.
- C. Krishnaraj, E. Jagan, S. Rajasekar, P. Selvakumar, P. Kalaichelvan and N. Mohan, Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens, Colloids Surf., B, 2010, 76, 50–56 CrossRef CAS PubMed.
- M. Yamanaka, K. Hara and J. Kudo, Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis, Appl. Environ. Microbiol., 2005, 71, 7589–7593 CrossRef CAS PubMed.
- Y. Wang, L. Deng, A. Caballero-Guzman and B. Nowack, Are engineered nano iron oxide particles safe? An environmental risk assessment by probabilistic exposure, effects and risk modeling, Nanotoxicology, 2016, 10, 1545–1554 CrossRef CAS PubMed.
- G. Vale, K. Mehennaoui, S. Cambier, G. Libralato, S. Jomini and R. F. Domingos, Manufactured nanoparticles in the aquatic environment-biochemical responses on freshwater organisms: a critical overview, Aquat. Toxicol., 2016, 170, 162–174 CrossRef CAS PubMed.
- N. von Moos and V. I. Slaveykova, Oxidative stress induced by inorganic nanoparticles in bacteria and aquatic microalgae–state of the art and knowledge gaps, Nanotoxicology, 2014, 8, 605–630 CrossRef CAS PubMed.
- T. P. Dasari, K. Pathakoti and H.-M. Hwang, Determination of the mechanism of photoinduced toxicity of selected metal oxide nanoparticles (ZnO, CuO, Co3O4 and TiO2) to E. coli bacteria, J. Appl. Microbiol., 2013, 25, 882–888 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9en00585d |
| This journal is © The Royal Society of Chemistry 2020 |