 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Further correction: Understanding cation effects in electrochemical CO2 reduction
Stefan
Ringe
*ab,
Ezra L.
Clark
cd,
Joaquin
Resasco
e,
Amber
Walton
c,
Brian
Seger
d,
Alexis T.
Bell
c and
Karen
Chan
*f
aSUNCAT Center for Interface Science and Catalysis, Department of Chemical Engineering, Stanford University, Stanford, California 94305, USA. E-mail: sringe@stanford.edu
bSUNCAT Center for Interface Science and Catalysis, SLAC National Accelerator Laboratory, Menlo Park, California 94025, USA
cJoint Center for Artificial Photosynthesis, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA
dSurface Physics & Catalysis (SurfCat), Department of Physics Technical University of Denmark, Denmark
eDepartment of Chemical Engineering, University of California, Santa Barbara, California 93117, USA
fCatTheory Center, Department of Physics, Technical University of Denmark, Kongens Lyngby 2800, Denmark. E-mail: kchan@fysik.dtu.dk
First published on 28th January 2020
Abstract
Further correction for ‘Understanding cation effects in electrochemical CO2 reduction’ by Stefan Ringe et al., Energy Environ. Sci., 2019, 12, 3001–3014.
In Fig. 6 of the original correction to this article, the captions (a) and (b) were switched. The Figure should appear with the following text:
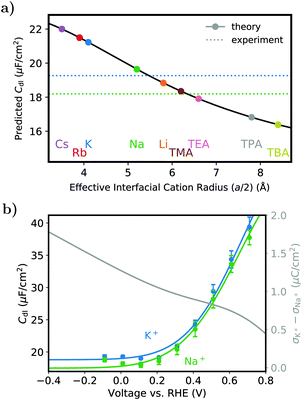 | ||
| Fig. 1 Dependence of the double layer capacitance on the electrolyte-containing cation. (a) Predicted double layer capacitance from the 1D-continuum model. Solid black line: MPB model predicted double layer capacitance of the Au(111) facet as a function of effective interfacial cation radius at 0 V vs. RHE. Values at the here determined interfacial radii are depicted by filled circles, the dashed lines represent experimental results for K+ and Na+ that are shown explicitly in b. (b) Potential-dependence of the double layer capacitance obtained from fitting a RC circuit to the impedance data of a Au(111) single crystal electrode using a 0.05 M KHCO3 or NaHCO3 electrolyte. Filled circles denote the data points (left y-axis), the solid gray line (right y-axis) the difference in surface charge density between both experiments under the assumption of the same PZC of 0.97 V vs. RHE.1 | ||
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
References
- S. Trasatti and E. Lust, in Modern aspects of electrochemistry, ed. J. O'M. Bockris, B. E. Conway and R. E. White, Kluwer Academic/Plenum Publishers, New York, vol. 33 Search PubMed.
| This journal is © The Royal Society of Chemistry 2020 |
