Using reverse osmosis membranes to control ion transport during water electrolysis†
Abstract
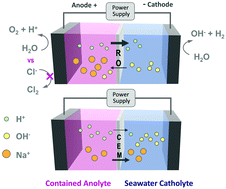
The decreasing cost of electricity produced using solar and wind and the need to avoid CO2 emissions from fossil fuels has heightened interest in hydrogen gas production by water electrolysis. Offshore and coastal hydrogen gas production using seawater and renewable electricity is of particular interest, but it is currently economically infeasible due to the high costs of ion exchange membranes and the need to desalinate seawater in existing electrolyzer designs. A new approach is described here that uses relatively inexpensive commercially available membranes developed for reverse osmosis (RO) to selectively transport favorable ions. In an applied electric field, RO membranes have a substantial capacity for proton and hydroxide transport through the active layer while excluding salt anions and cations. A perchlorate salt was used to provide an inert and contained anolyte, with charge balanced by proton and hydroxide ion flow across the RO membrane. Synthetic seawater (NaCl) was used as the catholyte, where it provided continuous hydrogen gas evolution. The RO membrane resistance was 21.7 ± 3.5 Ω cm2 in 1 M NaCl and the voltages needed to split water in a model electrolysis cell at current densities of 10–40 mA cm−2 were comparable to those found when using two commonly used, more expensive ion exchange membranes.


 Please wait while we load your content...
Please wait while we load your content...