Open Access Article
Open Access ArticleStructure and magnetism of the Rh4+-containing perovskite oxides La0.5Sr0.5Mn0.5Rh0.5O3 and La0.5Sr0.5Fe0.5Rh0.5O3†
Nijat
Hasanli
a,
Alex
Scrimshire
b,
Paul A.
Bingham
b,
Robert G.
Palgrave
 c and
Michael A.
Hayward
c and
Michael A.
Hayward
 *a
*a
aDepartment of Chemistry, University of Oxford, Inorganic Chemistry Laboratory, South Parks Road, Oxford, OX1 3QR, UK. E-mail: michael.hayward@chem.ox.ac.uk
bMaterials and Engineering Research Institute, Sheffield Hallam University, City Campus, Howard Street, Sheffield, S1 1WB, UK
cDepartment of Chemistry, Department of Chemistry, University College London, 20 Gordon Street, London, WC1H 0AJ, UK
First published on 4th August 2020
Abstract
Synchrotron X-ray powder diffraction data indicate that La0.5Sr0.5Mn0.5Rh0.5O3 and La0.5Sr0.5Fe0.5Rh0.5O3 adopt distorted perovskite structures (space group Pnma) with A-site and B-site cation disorder. A combination of XPS and 57Fe Mössbauer data indicate the transition metal cations in the two phases adopt Mn3+/Rh4+ and Fe3+/Rh4+ oxidation state combinations respectively. Transport data indicate both phases are insulating, with ρ vs. T dependences consistent with 3D variable-range hopping. Magnetisation data reveal that La0.5Sr0.5Mn0.5Rh0.5O3 adopts a ferromagnetic state below Tc ∼ 60 K, which is rationalized on the basis of coupling via a dynamic Jahn–Teller distortion mechanism. In contrast, magnetic data reveal La0.5Sr0.5Fe0.5Rh0.5O3 undergoes a transition to a spin-glass state at T ∼ 45 K, attributed to frustration between nearest-neighbour Fe–Rh and next-nearest-neighbour Fe–Fe couplings.
Introduction
Transition-metal oxides which adopt the ABO3 perovskite structure exhibit a wide variety of complex physical properties, including superconductivity, colossal magnetoresistance, ferroelectricity and a wide variety of other coupled electronic and magnetic behaviours.1 The rich diversity of electronic and magnetic phenomena displayed by perovskite oxides can be attributed to the strong coupling between the local electronic states of the octahedrally coordinated transition-metal cations, which is facilitated by strong covalency in the B–O–B links which connect the BO6 units.2Recently there has been much interest in perovskite oxide compounds which combine 3d and 4d or 3d and 5d transition-metal cations, because the contrasting features of the 3d (narrow metal d-bands, large on-site electron–electron repulsion) and 4d/5d (broad d-bands, low on-site repulsion, strong spin–orbit coupling for 5d) cations can result in novel behaviour when they are combined.3,4 For example, Sr2FeMoO6 exhibits half-metallic ferromagnetic behaviour attributed to the interaction between the localized 3d5 electronic configuration of Fe3+ and the delocalized 4d1 electrons of Mo5+,5 with similar behaviour observed in other 3d/4d and 3d/5d systems.6,7
In a broader context the inter-cation magnetic couplings in mixed 3d/4d and 3d/5d perovskite oxides do not generally appear to follow the Goodenough Kanamouri rules8 which dominate 3d systems.9–11 However, to date only a subset of the 3d/4d and 3d/5d perovskite oxide phases which could be envisioned have been reported,12 so it is hard to formulate broad magnetic coupling rules for this class of compound. To help address this situation, we have been studying mixed perovskite phases containing rhodium.
A range of Rh-containing perovskite oxides of the form LaM0.5Rh0.5O3 have been reported (M = Cr, Mn, Fe, Co, Ni and Cu) with M/Rh cation disordered structures.13–16 The unit cell volumes of these LaM0.5Rh0.5O3 compounds are broadly consistent with an M3+/Rh3+ oxidation state combination, with the exception of LaCu0.5Rh0.5O3 which appears to adopt a configuration close to a Cu2+/Rh4+ combination.13,15 However, Curie constants obtained by fitting the Curie–Weiss law to temperature-dependent magnetization data deviate from values expected for combinations of M3+ and low-spin, S = 0 Rh3+, casting some doubt on this oxidation state assignment.13 In the case of LaCo0.5Rh0.5O3 the deviations are large and temperature dependent and have been attributed to both a change in the spin-state of Co3+ and the presence of Co2+/Rh4+ oxidation state combinations.17–19
Here we report two further cation-disordered rhodium-containing perovskite oxides, La0.5Sr0.5Mn0.5Rh0.5O3 and La0.5Sr0.5Fe0.5Rh0.5O3. Using a combination of XPS and 57Fe Mössbauer spectroscopy we demonstrate that, even though these compounds were prepared at ambient pressure, the rhodium cations adopt an Rh4+ oxidation state, resulting in ferromagnetic behaviour for La0.5Sr0.5Mn0.5Rh0.5O3 and spin-glass behaviour for La0.5Sr0.5Fe0.5Rh0.5O3.
Experimental
Synthesis
Samples of La0.5Sr0.5Mn0.5Rh0.5O3 and La0.5Sr0.5Fe0.5Rh0.5O3 were prepared via a high-temperature ceramic route. Suitable quantities of La2O3 (99.999%, dried at 900 °C) SrCO3 (99.994%), Rh2O3 (99.99%, dried at 800 °C) and either MnO2 (99.997%) or Fe2O3 (99.997%) were ground together in an agate pestle and mortar and then heated in air to 1000 °C in an alumina crucible. The resulting powders were then reground and pressed into pellets. Samples of La0.5Sr0.5Mn0.5Rh0.5O3 were then heated in air at 1300 °C for 4 periods of 48 h. Samples of La0.5Sr0.5Fe0.5Rh0.5O3 were heated under flowing oxygen at 1300 °C for 3 periods of 48 h. All samples were reground and pressed into pellets between heating periods.Characterization
X-ray powder diffraction data were collected using a PANalytical X′pert diffractometer incorporating an X'celerator position-sensitive detector (monochromatic Cu Kα1 radiation). High-resolution synchrotron X-ray powder diffraction data (SXRD) were collected using the I11 instrument at the Diamond Light Source Ltd. Diffraction patterns were collected using Si-calibrated X-rays with an approximate wavelength of 0.825 Å from samples sealed in 0.3 mm diameter borosilicate glass capillaries. Rietveld refinements were performed using the GSAS suite of programs.20 Magnetization data were collected using a Quantum Design MPMS SQUID magnetometer. Four-probe resistivity measurements were performed using a home-made apparatus on bars cut from sintered pellets. 57Fe Mössbauer spectroscopy measurements utilized acrylic absorber discs with a sample area of 1.767 cm2 which were loaded to present 2.16 × 10−3 g cm−2 of Fe, and achieve a Mössbauer thickness of 1. Samples were homogeneously mixed with graphite to achieve this level of loading. The 14.4 keV γ-rays were supplied by the cascade decay of 25 mCi 57Co in a Rh matrix source, oscillated at constant acceleration by a SeeCo W304 drive unit, and detected using a SeeCo 45431 Kr proportional counter operating with 1.745 kV bias voltage applied to the cathode. All measurements were calibrated relative to α-Fe foil. Spectral data were fitted using the Recoil software package,21 using Lorentzian line shapes.X-ray photoelectron spectroscopy (XPS) analysis was performed using a Kratos Axis SUPRA XPS fitted with a monochromated Al Kα X-ray source (1486.7 eV), a spherical sector analyser and 3 multichannel resistive plate, 128 channel delay line detectors. All data was recorded at 150 W and a spot size of 700 × 300 μm. Survey scans were recorded at a pass energy of 160 eV, and high-resolution scans recorded at a pass energy of 20 eV. Electronic charge neutralization was achieved using a magnetic immersion lens. Filament current = 0.27 A, charge balance = 3.3 V, filament bias = 3.8 V. All sample data was recorded at a pressure below 10−8 Torr and a room temperature of 294 K. Data was analysed using CasaXPS v2.3.18PR1.0. C 1s sp3 peaks were calibrated to 284.8 eV.
Results
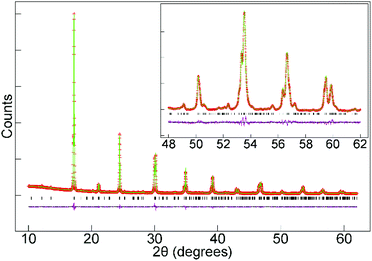
Structural characterisation of La0.5Sr0.5Mn0.5Rh0.5O3
SXRD data collected from La0.5Sr0.5Mn0.5Rh0.5O3 at room temperature could be readily indexed using an orthorhombic unit cell (a = 5.483 Å, b = 7.776 Å, 5.536 Å) with extinction conditions consistent with the Pnma (# 62) space group. A structural model was constructed based on the reported structure of LaMn0.5Rh0.5O3,15 but with the La3+ replaced by a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of La3+
1 ratio of La3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sr2+. This model was refined against the SXRD data to achieve a good fit as shown in Fig. 1 and detailed in Table 1, with selected bond lengths listed in Table 2. SXRD data provide no evidence for Mn/Rh cation-order, with a model based on the reported cation-ordered structure of LaSrNiRuO6 (space group P21/n)11 reverting to a disordered structure when the Mn and Rh occupancies were refined.
Sr2+. This model was refined against the SXRD data to achieve a good fit as shown in Fig. 1 and detailed in Table 1, with selected bond lengths listed in Table 2. SXRD data provide no evidence for Mn/Rh cation-order, with a model based on the reported cation-ordered structure of LaSrNiRuO6 (space group P21/n)11 reverting to a disordered structure when the Mn and Rh occupancies were refined.
| Atom | x | y | z | Occupancy | U iso (Å2) |
|---|---|---|---|---|---|
| La0.5Sr0.5Mn0.5Rh0.5O3 space group Pnma (#62). a = 5.4830(1) Å, b = 7.7769(1) Å, 5.5364(1) Å, volume = 236.08(1). Formula mass: 240.18 g mol−1, Z = 4. Radiation source: Synchrotron X-ray, λ = 0.82622 Å. χ2 = 19.75; wRp = 2.92%; Rp = 1.88%. | |||||
| La/Sr | 0.5181(1) | ¼ | 0.0023(1) | 0.5/0.5 | 0.009(1) |
| Mn/Rh | 0 | 0 | 0 | 0.5/0.5 | 0.004(1) |
| O(1) | 0.0000(6) | ¼ | 0.9434(6) | 1 | 0.009(1) |
| O(2) | 0.2701(8) | 0.0305(4) | 0.2189(6) | 1 | 0.020(1) |
| Cation | Anion | Bond length (Å) |
|---|---|---|
| Mn/Rh | O(1) × 2 | 1.969(1) |
| O(2) × 2 | 1.928(4) | |
| O(2) × 2 | 2.017(4) | |
| La/Sr | O(1) | 2.470(3) |
| O(1) | 2.662(3) | |
| O(1) | 2.859(3) | |
| O(1) | 3.070(3) | |
| O(2) × 2 | 2.490(4) | |
| O(2) × 2 | 2.684(4) | |
| O(2) × 2 | 2.758(3) | |
| O(2) × 2 | 3.117(4) | |
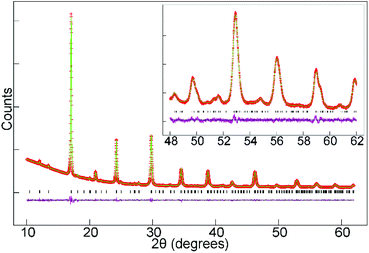
Structural and chemical characterisation of La0.5Sr0.5Fe0.5Rh0.5O3
SXRD data collected from La0.5Sr0.5Fe0.5Rh0.5O3 at room temperature could be readily indexed using an orthorhombic unit cell (a = 5.525 Å, b = 7.860 Å, 5.563 Å,) with extinction conditions consistent with the Pnma (# 62) space group. A structural model was constructed, based that used for La0.5Sr0.5Mn0.5Rh0.5O3, and refined against the SXRD data to achieve a good fit as shown in Fig. 2 and detailed in Table 3, with selected bond lengths listed in Table 4. SXRD data provide no evidence for Fe/Rh cation-order, with a model based on the reported cation-ordered structure of LaSrNiRuO6 (space group P21/n)11 reverting to a disordered structure when the Fe and Rh occupancies were refined.| Atom | x | y | z | Occupancy | U iso (Å2) |
|---|---|---|---|---|---|
| La0.5Sr0.5Fe0.5Rh0.5O3 space group Pnma (#62). a = 5.5254(3) Å, b = 7.8602(5) Å, 5.5635(4) Å, volume = 241.63(3). Formula mass: 240.63 g mol−1, Z = 4. Radiation source: Synchrotron X-ray, λ = 0.8259 Å. χ2 = 15.85; wRp = 2.41%; Rp = 1.54%. | |||||
| La/Sr | 0.5138(3) | ¼ | 0.0012(6) | 0.5/0.5 | 0.007(1) |
| Fe/Rh | 0 | 0 | 0 | 0.5/0.5 | 0.003(1) |
| O(1) | 0.9982(5) | ¼ | 0.0369(4) | 1 | 0.009(1) |
| O(2) | 0.2294(3) | 0.9594(1) | 0.2800(2) | 1 | 0.016(1) |
| Cation | Anion | Bond (Å) |
|---|---|---|
| Fe/Rh | O(1) × 2 | 1.976(1) |
| O(2) × 2 | 1.958(1) | |
| O(2) × 2 | 2.033(1) | |
| La/Sr | O(1) | 2.571(4) |
| O(1) | 2.684(3) | |
| O(1) | 2.856(3) | |
| O(1) | 2.995(4) | |
| O(2) × 2 | 2.456(2) | |
| O(2) × 2 | 2.678(2) | |
| O(2) × 2 | 2.849(2) | |
| O(2) × 2 | 3.176(2) | |
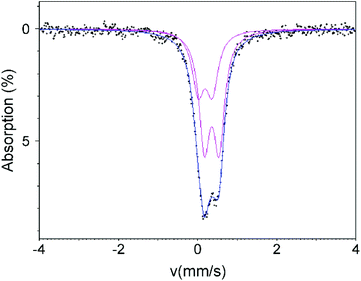
57Fe Mössbauer analysis of La0.5Sr0.5Fe0.5Rh0.5O3
A 57Fe Mössbauer spectrum collected from La0.5Sr0.5Fe0.5Rh0.5O3 at room temperature can be satisfactorily fitted by two doublets as shown in Fig. 3 and detailed in Table 5.| Doublet | C S (mm s−1) | Δ (mm s−1) | HWHM (mm s−1) | Site population (%) |
|---|---|---|---|---|
| 1 | 0.361(18) | 0.371(6) | 0.166(16) | 63(19) |
| 2 | 0.195(42) | 0.355(14) | 0.184(24) | 37(19) |
We attribute the need to use two doublets to obtain a satisfactory fit to the Fe/Rh cation-disordered structure of La0.5Sr0.5Fe0.5Rh0.5O3. Each octahedral transition-metal coordination site in the perovskite framework is surrounded by 6 others. In a disordered Fe/Rh array the majority of the iron cations with have 3 iron neighbours and 3 rhodium neighbours. However there will be a significant number which have Fe2Rh4 or Fe4Rh2 surrounding them and a smaller number with Fe1Rh5 or Fe5Rh1. When we also consider that the Fe3Rh3 environments can be arranged as either fac or mer, and the Fe2Rh4 and Fe4Rh2 as cis or trans, it can be seen that there are a large number of local environments for the iron cations within the disordered Rh/Fe array, each with a different Mössbauer chemical shift and doublet splitting. It is not possible to resolve this large number of components from the data in a meaningful way, so we have utilized a 2-doublet fit to extract the range of Cs and Δ values spanned by the different Fe local coordination environments. Comparison with literature standards indicates these signals correspond to octahedrally coordinated Fe3+.22,23
To investigate the possibility that some Fe4+ cations are present in La0.5Sr0.5Fe0.5Rh0.5O3 we have performed a further fit in which one doublet was fixed at CS = 0, corresponding to Fe4+. This approach also gives a reasonable fit to the data and indicates a maximum concentration of 12(2)% Fe4+ as described in detail in the ESI.†
XPS analysis of La0.5Sr0.5Mn0.5Rh0.5O3 and La0.5Sr0.5Fe0.5Rh0.5O3
A Rh 3d spectrum collected from La0.5Sr0.5Fe0.5Rh0.5O3 is shown in Fig. 4a and exhibits a well resolved Rh 3d5/2 component centred at BE = 308.9 eV (FWHM = 1.86 eV) and a Rh 3d3/2 component centred at BE = 313.7 eV (FWHM = 2.63 eV). When combined with an O 1s–Rh 3d5/2 separation of 219.6 eV, as shown in Fig. 4a, these values indicate an oxidation state of Rh4+.24,25 Fe 2p spectra (Fig. S2, ESI†) exhibit a well resolved Fe 2p3/2 component centred at BE = 710.1 eV (FWHM = 3.44 eV) and a Fe 2p1/2 component centred at BE = 723.3 eV (FWHM = 4.65 eV), consistent with Fe3+.26,27 These data, in combination with the 57Fe Mössbauer data indicate an Fe3+/Rh4+ oxidation state combination for La0.5Sr0.5Fe0.5Rh0.5O3.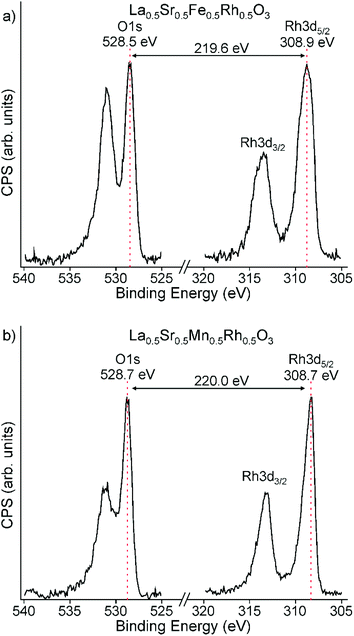 | ||
| Fig. 4 Binding energy separation between O 1s and Rh 3d peaks of (a) LaSrFe0.5Rh0.5O3 and (b) LaSrFe0.5Rh0.5O3 which is consistent with the presence of Rh4+ in both cases. | ||
Similarly, a Rh 3d spectrum collected from La0.5Sr0.5Mn0.5Rh0.5O3, shown in Fig. 4b, exhibits a well resolved Rh 3d5/2 component centred at BE = 308.7 eV (FWHM = 1.45 eV), a Rh 3d3/2 component centred at BE = 313.4 eV (FWHM = 2.16 eV) and a O 1s–Rh 3d5/2 separation of 220.0 eV which also indicate an oxidation state of Rh4+.24,25 Mn 2p spectra (Fig. S3, ESI†) exhibit a well resolved Mn 2p3/2 component centred at BE = 641.6 eV (FWHM = 3.79 eV) and a Mn 2p1/2 component centred at BE = 652.9 eV (FWHM = 4.35 eV), consistent with Mn3+.26,28 These data indicate an Mn3+/Rh4+ oxidation state combination for La0.5Sr0.5Mn0.5Rh0.5O3.
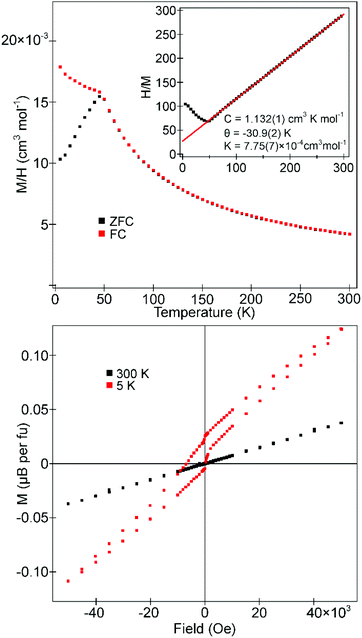
Magnetic characterisation of La0.5Sr0.5Mn0.5Rh0.5O3
Zero-field cooled (ZFC) and field cooled (FC) magnetization data collected from La0.5Sr0.5Mn0.5Rh0.5O3 in an applied field of 100 Oe (Fig. 5) diverge weekly below 100 K, before diverging more strongly below 60 K, consistent with the onset of magnetic order. Data in the range 100 < T/K < 300 do not obey the Curie–Weiss law. Magnetization-field data collected at 5 K are consistent with ferromagnetic behaviour with a coercive field of 190 Oe (as shown more clearly in Fig. S3 in the ESI†) and a saturated moment of 1.43μB per formula unit (2.86μB per Mn).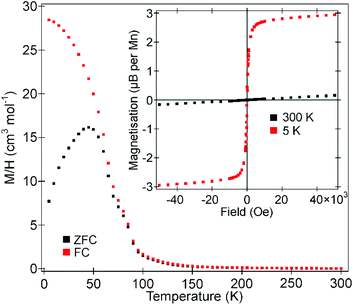 | ||
| Fig. 5 ZFC and FC magnetisation data collected from La0.5Sr0.5Mn0.5Rh0.5O3 in an applied field of 100 Oe. Inset shows magnetisation-field isotherms collected at 300 K and 5 K. | ||
Temperature-dependent 4-probe transport measurements indicate that La0.5Sr0.5Mn0.5Rh0.5O3 is highly resistive, with a semiconducting/insulating temperature dependence (δρ/δT < 0) as shown in Fig. 6. A plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ρ against T−1/4 is linear in the range 90 < T/K < 300 consistent with 3D variable range hopping.
ρ against T−1/4 is linear in the range 90 < T/K < 300 consistent with 3D variable range hopping.
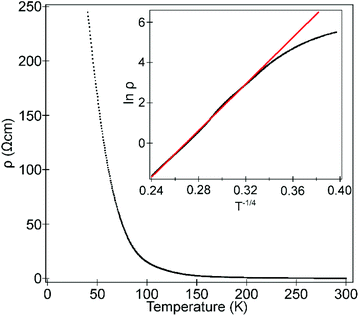 | ||
| Fig. 6 Temperature-dependent resistivity of La0.5Sr0.5Mn0.5Rh0.5O3. Inset shows fit to 3D variable-range hopping model. | ||
Magnetic characterisation of La0.5Sr0.5Fe0.5Rh0.5O3
ZFC and FC data collected from La0.5Sr0.5Fe0.5Rh0.5O3 (Fig. 7) can be fit by the Curie–Weiss law (χ = C/(T − θ) + K) in the temperature range 60 < T/K < 300 to yield value of C = 1.132(1) cm3 K mol−1, θ = −30.9 K, K = 7.75(7) × 10−4 cm3 mol−1, as shown in Fig. 6. For T < 45 K the ZFC and FC data diverge, indicative of a magnetic phase transition. Magnetization data collected at 300 K exhibit a linear field dependence. In contrast, magnetization data collected at 5 K after cooling from 300 K in an applied field of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe exhibit hysteresis and are displaced from the origin, indicative of spin-glass behaviour.
000 Oe exhibit hysteresis and are displaced from the origin, indicative of spin-glass behaviour.
Temperature-dependent 4-probe transport measurements indicate that La0.5Sr0.5Fe0.5Rh0.5O3 is highly resistive, with a semiconducting/insulating temperature dependence (δρ/δT < 0) as shown in Fig. 8. A plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ρ against T−1/4 is linear in the range 50 < T/K < 300 consistent with 3D variable range hopping.
ρ against T−1/4 is linear in the range 50 < T/K < 300 consistent with 3D variable range hopping.
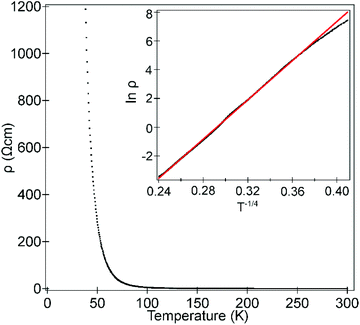 | ||
| Fig. 8 Temperature-dependent resistivity of La0.5Sr0.5Fe0.5Rh0.5O3. Inset shows fit to 3D variable-range hopping model. | ||
Discussion
SXRD data indicate that in common with other reported La1−xSrxMn0.5Rh0.5O3 phases,15,29 La0.5Sr0.5Mn0.5Rh0.5O3 adopts a cation-disordered, a−a−c+ distorted, GdFeO3-type perovskite structure. Comparison with the reported structures of LaMn0.5Rh0.5O3 and La0.75Sr0.25Mn0.5Rh0.5O3 reveals that both the unit cell volumes (V = 243.92 Å3, 240.01 Å3 and 236.08 Å3 for x = 0, 0.25 and 0.5 respectively) and average (Mn/Rh)–O bond lengths ((Mn/Rh)–O = 2.019 Å, 1.995 Å and 1.971 Å for x = 0, 0.25 and 0.5 respectively) decrease on substitution of La3+ by Sr2+, consistent with oxidation of the transition metal cations. In addition it should be noted that there is no evidence of an ordered Jahn–Teller distortion (long-range orbital order) of the (Mn/Rh)O6 octahedra in La0.5Sr0.5Mn0.5Rh0.5O3 (Table 2) or any other La1−xSrxMn0.5Rh0.5O3 phases (despite the unambiguous presence of Mn3+ in LaMn0.5Rh0.5O3)15 which is attributed to suppression by the disordered arrangement of the Mn and Rh cations.La0.5Sr0.5Fe0.5Rh0.5O3 also adopts a cation-disordered, a−a−c+ distorted, GdFeO3-type perovskite structure. Comparison with the reported structure of LaFe0.5Rh0.5O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 again reveals that substitution of La3+ with Sr2+ leads to a contraction in unit cell volume, consistent with oxidation of the transition metals.
13 again reveals that substitution of La3+ with Sr2+ leads to a contraction in unit cell volume, consistent with oxidation of the transition metals.
XPS and 57Fe Mössbauer data indicate the predominant oxidation state combinations of La0.5Sr0.5Mn0.5Rh0.5O3 and La0.5Sr0.5Fe0.5Rh0.5O3 are Mn3+/Rh4+ and Fe3+/Rh4+ respectively. This is unexpected considering the oxide chemistry of these transition metals. For example, Mn3+ is not particularly stable in perovskite oxides, as illustrated by the observation that LaMnO3 must be prepared under low oxygen partial pressures to avoid the formation of mixed-valent Mn3+/4+ ‘LaMnO3+δ’ phases.30 In contrast the preparation of Mn4+ containing perovskite oxides, such as SrMnO3, can be readily achieved by heating in air.31 Rhodium shows the opposite trend with Rh3+ perovskites such as LaRhO3 being readily prepared in air,32 while Rh4+ perovskite oxides such as SrRhO3 require high-pressure synthesis conditions.33 It is therefore surprising that combining the two ‘more stable’ perovskite phases LaRhO3 and SrMnO3 to form La0.5Sr0.5Mn0.5Rh0.5O3 leads to a change in oxidation states from a Mn4+/Rh3+ combination in the ternary phases to a Mn3+/Rh4+ in the quaternary product. Rh4+ has been observed in perovskite oxides prepared at ambient pressure when it is combined with either electronegative elements such as Cu (LaCu0.52+Rh0.54+O3)15 or elements which only exhibit a single divalent cation oxidation state (LaZn0.52+Rh0.54+O3, LaMg0.52+Rh0.54+O3).34 However, the observation of Rh4+ in the presence of cations such as Fe3+ and Mn3+ which can be oxidized relatively easily is unexpected and suggests the 3d and 4d levels of Mn/Fe and Rh respectively are of similar energy.
Magnetic behaviour
Transport and magnetization data indicate that La0.5Sr0.5Mn0.5Rh0.5O3 is an electrical insulator which undergoes a transition to a ferromagnetic state at Tc ∼ 60 K. This behaviour is very similar to that reported for other perovskite oxides containing disordered arrays of Mn3+ cations such as LaMn0.5Rh0.5O3 (Tc = 65 K)15,16 and LaMn0.5Ga0.5O3 (Tc = 70 K), with the observed saturated ferromagnetic moment of La0.5Sr0.5Mn0.5Rh0.5O3 (2.86μB per Mn) also being similar to that observed for LaMn0.5Ga0.5O3.35,36The ferromagnetic behaviour of LaMn0.5Ga0.5O3 has been rationalized by observing that partial substitution of the Mn3+ cations in LaMnO3 by a non-Jahn–Teller ion (e.g. Ga3+) suppresses the static Jahn–Teller distortion (orbital ordering) of the MnIIIO6 units present in the unsubstituted phase.35,37 In the absence of a static Jahn–Teller distortion (long-range orbital order), local dynamic Jahn–Teller distortions predominate which make all the Mn–O–Mn exchange couplings ferromagnetic, thus explaining the ferromagnetic order observed for LaMn1−xGaxO3 phases with x > 0.5.35,37 This mechanism can also be invoked to explain the ferromagnetic behaviour of LaMn0.5Rh0.5O3 as it is observed that the substitution of diamagnetic, low-spin d6, Rh3+ into LaMnO3 suppresses the static Jahn–Teller distortion of the MnIIIO6 units in a manner directly analogous to Ga3+ substitution.15,38
We propose that this dynamic Jahn–Teller mechanism is also the origin of the ferromagnetic behaviour of La0.5Sr0.5Mn0.5Rh0.5O3, as the substitution of Rh4+ for Mn3+ suppresses the static Jahn–Teller distortion of the manganese centres, leading to ferromagnetic couplings between Mn3+ cations in this cation-disordered material. It may be expected that the presence of paramagnetic Rh4+ centres would perturb the ferromagnetic Mn–O–Mn couplings, however the observation that the Curie temperature of La0.5Sr0.5Mn0.5Rh0.5O3 (Tc ∼ 60 K) is very similar to those of LaMn0.5Rh0.5O3 (Tc = 65 K)15,16 and LaMn0.5Ga0.5O3 (Tc = 70 K)35,36 indicates any Mn–O–Rh couplings present are weak. It is not clear if the Rh spins contribute to the magnetically ordered state of La0.5Sr0.5Mn0.5Rh0.5O3.
Magnetization data collected from La0.5Sr0.5Fe0.5Rh0.5O3 in the temperature range 60 < T/K < 300 exhibit the temperature-dependence of the Curie–Weiss Law (Fig. 6). However, the Curie constant extracted from these data (C = 1.132(1) cm3 K mol−1) is much smaller than expected for simple paramagnetic behaviour for either an Fe3+/Rh4+ (Cexpected = 2.375 cm3 K mol−1) or an Fe4+/Rh3+ (Cexpected = 1.50 cm3 K mol−1) oxidation state combination. This indicates that strong spin–spin interactions are present in this temperature range, which is consistent with the large temperature-independent susceptibility observed (K = 7.75(7) × 10−4 cm3 mol−1) which contributes ∼20% to the total susceptibility at 300 K.
On cooling below T = 45 K, La0.5Sr0.5Fe0.5Rh0.5O3 undergoes a transition to a spin-glass state. In order to adopt a spin-glass state a system must be crystallographically disordered, and subject to magnetic frustration. If we consider the nearest-neighbour super exchange couplings in La0.5Sr0.5Fe0.5Rh0.5O3 we observe FeIII–O–FeIII should be strongly antiferromagnetic, RhIV–O–RhIV should also be antiferromagnetic, but only weakly, and FeIII–O–RhIV should be ferromagnetic.8 If these are the only significant magnetic couplings, a disordered Fe/Rh array would not be subject to magnetic frustration, making the observed spin-glass behaviour of La0.5Sr0.5Fe0.5Rh0.5O3 a little puzzling.
Battle et al. considered the spin-glass states of SrFe0.5Ru0.5O3 and Sr2Fe0.5Ru0.5O4 which arise from disordered arrays of Fe3+ and Ru5+.39,40 The nearest-neighbour super exchange couplings in SrFe0.5Ru0.5O3 and Sr2Fe0.5Ru0.5O4 are directly analogous to those in La0.5Sr0.5Fe0.5Rh0.5O3: FeIII–O–FeIII is antiferromagnetic, RuV–O–RuV is antiferromagnetic and FeIII–O–RuV is ferromagnetic.
Battle et al. concluded that the order of coupling strengths is FeIII–O–FeIII > RuV–O–RuV ∼ FeIII–O–RuV ∼ FeIII–O–O–FeIII where this last term is the next-nearest-neighbour Fe–Fe coupling. In this situation, frustration arises because the ferromagnetic FeIII–O–RuV couplings align next-nearest neighbour Fe spins ferromagnetically, but the FeIII–O–O–FeIII super–super exchange coupling is antiferromagnetic and the two couplings have about the same strength. We propose that a similar frustration between nearest-neighbour FeIII–O–RhIV and next-nearest-neighbour FeIII–O–O–FeIII couplings is responsible for the spin-glass behaviour in La0.5Sr0.5Fe0.5Rh0.5O3.
It is interesting to note that the contrasting magnetic behaviour of La0.5Sr0.5Mn0.5Rh0.5O3 and La0.5Sr0.5Fe0.5Rh0.5O3 can be understood on the basis of the relative strengths of the different transition-metal couplings in the two systems – the dominance of 3d–3d couplings in La0.5Sr0.5Mn0.5Rh0.5O3 leading to ferromagnetism; the comparable strengths of 3d–3d, 3d–4d couplings in La0.5Sr0.5Fe0.5Rh0.5O3 resulting in spin-glass behaviour.
Conclusions
Hole doping of the perovskite phases LaMn0.5Rh0.5O3 and LaFe0.5Rh0.5O3 leads to the oxidation of Rh3+ to Rh4+ in the resulting La0.5Sr0.5Mn0.5Rh0.5O3 and La0.5Sr0.5Fe0.5Rh0.5O3 compounds. The stabilization of Rh4+ in the presence of the readily oxidizable Mn3+ and Fe3+ cations suggests that the 3d Mn/Fe states and 4d Rh states are of similar energy, so that if the electronic bandwidth of the phases could be increased, by decreasing the magnitude of the octahedral tilting distortion for example, or the 3d and 4d cations could be ordered, correlated electronic behaviour is likely, in line with computational predictions for high-valent rhodium oxides.41Conflicts of interest
There are no conflicts to declare.Acknowledgements
Experiments at the Diamond Light Source were performed as part of the Block Allocation Group award “Oxford Solid State Chemistry BAG to probe composition–structure–property relationships in solids” (EE13284). NH acknowledges funding from the “State Programme on Education of Azerbaijani Youth Abroad in 2007–2015” by the Ministry of Education of Azerbaijan. The XPS data collection was performed at the EPSRC National Facility for XPS (“HarwellXPS”), operated by Cardiff University and UCL, under Contract No. PR16195.Notes and references
- R. J. D. Tilley, Perovskites: Structure-Property Relationships, John Wiley and Sons, Chichester, 2016 Search PubMed.
- J. B. Goodenough and J.-S. Zhou, Chem. Mater., 1998, 10, 2980–2993 CrossRef.
- T. Saha-Dasgupta, Mater. Res. Express, 2020, 7, 014003 CrossRef.
- A. Halder, P. Sanyal and T. Saha-Dasgupta, Phys. Rev. B, 2019, 99, 020402(R) CrossRef.
- K. L. Kobayashi, T. Kimura, H. Sawada, K. Terakura and Y. Tokura, Nature, 1998, 395, 677–680 CrossRef.
- K. I. Kobayashi, T. Kimura, Y. Tomioka, H. Sawada, K. Terakura and Y. Tokura, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 11159–11162 CrossRef.
- H. Kato, T. Okuda, Y. Okimoto, Y. Tomioka, Y. Takenoya, A. Ohkubo, M. Kawasaki and Y. Tokura, Appl. Phys. Lett., 2002, 81, 328–330 CrossRef.
- J. B. Goodenough, Magnetism and the chemical bond, Wiley, New York, 1963 Search PubMed.
- S. Kanungo, B. H. Yan, M. Jansen and C. Felser, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 214414 CrossRef.
- X. D. Ou, Z. W. Li, F. R. Fan, H. B. Wang and H. Wu, Sci. Rep., 2014, 4(7542), 7541–7544 Search PubMed.
- R. Morrow, M. A. McGuire, J. Q. Yan and P. M. Woodward, Inorg. Chem., 2018, 57, 2989–3001 CrossRef CAS.
- S. Vasala and M. Karppinen, Prog. Solid State Chem., 2015, 43, 1–36 CrossRef CAS.
- A. E. Smith, A. W. Sleight and M. A. Subramanian, Mater. Res. Bull., 2010, 45, 460–463 CrossRef CAS.
- P. D. Battle and J. F. Vente, J. Solid State Chem., 1999, 146, 163–167 CrossRef CAS.
- J. Ting, B. J. Kennedy, Z. Zhang, M. Avdeev, B. Johannessen and L. Y. Jang, Chem. Mater., 2010, 22, 1640–1646 CrossRef CAS.
- C. Schinzer, J. Phys. Chem. Solids, 2000, 61, 1543–1551 CrossRef CAS.
- K. Knizek, J. Hejtmanek, M. Marysko, Z. Jirak and J. Bursik, Phys. Rev. B, 2012, 85, 134401 CrossRef.
- J. Li, A. E. Smith, K. S. Kwong, C. Powell, A. W. Sleight and M. A. Subramanian, J. Solid State Chem., 2010, 183, 1388–1393 CrossRef CAS.
- S. V. Streltsov, V. V. Gapontsev and D. I. Khomskii, J. Phys.: Condens. Matter, 2016, 28, 086005 CrossRef.
- A. C. Larson and R. B. Von Dreele, Los Alamos National Laboratory Report LAUR 86-748, 2000.
- K. Lagarec and D. G. Rancourt, Recoil: Mössbauer spectral analysis software for windows, 1998 Search PubMed.
- J. P. Hodges, S. Short, J. D. Jorgensen, X. Xiong, B. Dabrowski, S. M. Mini and C. W. Kimball, J. Solid State Chem., 2000, 151, 190–209 CrossRef CAS.
- Y. Takeda, K. Kanno, T. Takada, O. Yamamoto, M. Takano, N. Nakayama and Y. Bando, J. Solid State Chem., 1986, 63, 237–249 CrossRef CAS.
- E. N. K. Glover, S. G. Ellington, G. Sankar and R. G. Palgrave, J. Mater. Chem. A, 2016, 4, 6946–6954 RSC.
- T. K. Le, D. Flahaut, H. Martinez, N. Andreu, D. Gonbeau, E. Pachoud, D. Pelloquin and A. Maignan, J. Solid State Chem., 2011, 184, 2387–2392 CrossRef CAS.
- M. C. Biesinger, B. P. Payne, A. P. Grosvenor, L. W. M. Lau, A. R. Gerson and R. S. Smart, Appl. Surf. Sci., 2011, 257, 2717–2730 CrossRef CAS.
- T. Yamashita and P. Hayes, Appl. Surf. Sci., 2008, 254, 2441–2449 CrossRef CAS.
- E. S. Ilton, J. E. Post, P. J. Heaney, F. T. Ling and S. N. Kerisit, Appl. Surf. Sci., 2016, 366, 475–485 CrossRef CAS.
- B. Bakowski, P. D. Battle, E. J. Cussen, L. D. Noailles, M. J. Rosseinsky, A. I. Coldea and J. Singleton, Chem. Commun., 1999, 2209–2210 RSC.
- N. Kamegashira, Y. Miyazaki and H. Yamamoto, Mater. Chem. Phys., 1984, 11, 187–194 CrossRef CAS.
- K. Kuroda, K. Shinozaki, K. Uematsu, N. Mizutani and M. Kato, J. Am. Ceram. Soc., 1980, 63, 109–110 CrossRef CAS.
- H. J. Gysling, J. R. Monnier and G. Apai, J. Catal., 1987, 103, 407–418 CrossRef CAS.
- K. Yamaura and E. Takayama-Muromachi, Phys. Rev. B, 2001, 64, 224424 CrossRef.
- C. Schinzer and G. Demazeau, J. Mater. Sci., 1999, 34, 251–256 CrossRef CAS.
- J. Blasco, J. Garcia, J. Campo, M. C. Sanchez and G. Subias, Phys. Rev. B, 2002, 66, 174431 CrossRef.
- E. J. Cussen, M. J. Rosseinsky, P. D. Battle, J. C. Burley, L. E. Spring, J. F. Vente, S. J. Blundell, A. I. Coldea and J. Singleton, J. Am. Chem. Soc., 2001, 123, 1111–1122 CrossRef CAS PubMed.
- J. Goodenough, R. J. Arnott, N. Menyuk and A. Wold, Phys. Rev., 1961, 124, 373–384 CrossRef CAS.
- M. T. Haque, H. Satoh and N. Kamegashira, J. Alloys Compd, 2005, 390, 115–121 CrossRef CAS.
- P. D. Battle, T. C. Gibb, C. W. Jones and F. Studer, J. Solid State Chem., 1989, 78, 281–293 CrossRef CAS.
- P. D. Battle, S. K. Bollen and A. V. Powell, J. Solid State Chem., 1992, 99, 267–275 CrossRef CAS.
- A. Halder, D. Nafday, P. Sanyal and T. Saha-Dasgupta, npj Quantum Mater., 2018, 3, 17 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Mn 2P and Fe 2P XPS from La0.5Sr0.5Mn0.5Rh0.5O3 and La0.5Sr0.5Fe0.5Rh0.5O3 respectively; additional fits to 57Fe Mössbauer data from La0.5Sr0.5Fe0.5Rh0.5O3. See DOI: 10.1039/d0dt02466j |
| This journal is © The Royal Society of Chemistry 2020 |