Open Access Article
Open Access ArticleInclusion and release of ant alarm pheromones from metal–organic frameworks†
Harina
Amer Hamzah
 a,
Daniel
Rixson
a,
Daniel
Rixson
 a,
Joseph
Paul-Taylor
a,
Joseph
Paul-Taylor
 a,
Huan V.
Doan
a,
Huan V.
Doan
 a,
Christopher
Dadswell
b,
Gavin W.
Roffe
b,
Arun
Sridhar
a,
Christopher
Dadswell
b,
Gavin W.
Roffe
b,
Arun
Sridhar
 c,
Claire L.
Hobday
c,
Claire L.
Hobday
 c,
Charlie
Wedd
c,
Charlie
Wedd
 c,
Tina
Düren
c,
Tina
Düren
 c,
William O. H.
Hughes
c,
William O. H.
Hughes
 d,
John
Spencer
d,
John
Spencer
 b and
Andrew D.
Burrows
b and
Andrew D.
Burrows
 *a
*a
aDepartment of Chemistry, University of Bath, Claverton Down, Bath BA2 7AY, UK. E-mail: a.d.burrows@bath.ac.uk
bChemistry Department, School of Life Sciences, University of Sussex, Falmer, Brighton, East Sussex BN1 9QJ, UK
cCentre for Advanced Separations Engineering, Department of Chemical Engineering, University of Bath, Claverton Down, Bath BA2 7AY, UK
dSchool of Life Sciences, University of Sussex, Falmer, Brighton, East Sussex BN1 9QJ, UK
First published on 21st July 2020
Abstract
Zinc(II) and zirconium(IV) metal–organic frameworks show uptake and slow release of the ant alarm pheromones 3-octanone and 4-methyl-3-heptanone. Inclusion of N-propyl groups on the MOFs allows for enhanced uptake and release over several months. In preliminary field trials, leaf cutting ants show normal behavioural responses to the released pheromones.
With over one billion undernourished people in the world, the need to develop novel crop protection strategies that are easy to implement and transferable to end users cannot be overemphasised. The sustainable production of food and other natural resources is one of the grand challenges facing society, and insect pests are a significant impediment to achieving this objective.1 In the face of a growing population, shrinking resources and pesticide resistance, there is an increasing need for the development of better targeted and more environmentally friendly strategies for the integrated management of insect pests.
Porous materials such as metal–organic frameworks (MOFs) offer a novel mechanism of achieving these goals as the materials allow for slow or environment-specific release of compounds. Since the first report, in 2006, that bioactive molecules could be encapsulated within the pores of MOFs and released slowly,2 there has been a focus on using MOFs to control drug delivery.3–5 In these studies, the MOF acts as a source of the drug molecule, with the controlled delivery typically related to either slow diffusion of the guest drug molecule from the pores or decomposition of the framework. Despite considerable advances in this area, there has been little attention paid to using MOFs for the delivery of other biologically active molecules. As such, the potential of MOFs for pest management remains essentially untapped. Zeolites have been used previously to dispense pheromones,6,7 but the greater synthetic control, structural diversity and ease of functionalisation of MOFs make this type of porous system especially attractive.8
Leaf-cutting ants are major pest species of agriculture and forestry in many areas of the Neotropics causing an estimated $8 billion damage each year to Eucalyptus forestry in Brazil alone.9 Attempts to control them are limited by environmental conditions which degrade traditional pesticides, and a lack of target specificity that results in substantial wastage of pest control products, environmental contamination and non-target impacts.10 The use of a pheromone to attract pest social insects to an insecticide bait is an attractive strategy to resolve these problems, but it is more challenging than it might initially appear. Trail pheromones, used by most ants and termites to organise their foraging, naturally act as short-range signals in a trail, whereas in a control strategy the pheromone is required to act as a long-range attractant to a bait-associated point source.11,12 The use of an alarm pheromone as an attractant is counter-intuitive, but leaf-cutting ants (Atta and Acromyrmex species) typically show an ‘aggressive’ alarm response, in which workers are attracted by the alarm pheromone to attack the source of the disturbance.12–14 Previous work has demonstrated that the most behavioural active components of the alarm pheromones of Acromyrmex and Atta are 3-octanone and 4-methyl-3-heptanone respectively,15,16 and that these compounds can attract leaf-cutting ants to an insecticide bait, and then increase bait harvest by the ants as their alarm reaction dissipates to be replaced by foraging behaviour.17,18
The high volatility of these compounds makes their use for pest control impractical without a method to bind and then release the compounds in a targeted manner. In this paper, we report our initial findings on the inclusion of ant alarm pheromones as guests into MOFs based on the IRMOF and UiO-66 isoreticular series as well as the coordinatively-unsaturated MOF-74 and their subsequent slow release. Framework structures of the MOFs employed in this study are shown in Fig. 1.
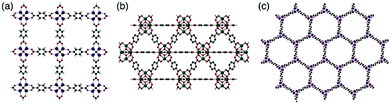 | ||
| Fig. 1 Framework structures of (a) [Zn4O(bdc)3] (IRMOF-1),19 (b) [Zr6O4(OH)4(bdc)6] (UiO-66),20 and (c) [Zn2(dobdc)] (MOF-74)21 (bdc = 1,4-benzenedicarboxylate, dobdc = 2,5-dioxo-1,4-benzenedicarboxylate). | ||
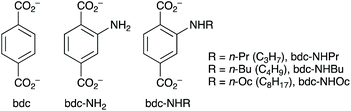
The IRMOF system was initially selected for study due to its ease of functionalisation and high crystallinity. We postulated that the presence of either a hydrogen bond donor, to interact with the carbonyl group of the pheromone, or an alkyl group, to provide a hydrophobic pore environment, would be beneficial, and prepared IRMOFs based on the linkers shown in Fig. 2.
The MOFs prepared using previously reported methods were [Zn4O(bdc)3] (IRMOF-1),19,22 [Zn4O(bdc-NH2)3] (IRMOF-3),19,23 [Zn4O(bdc-NHPr)3] (IRMOF-NHPr), [Zn4O(bdc-NHBu)3] (IRMOF-NHBu) and [Zn4O(bdc-NHOc)3] (IRMOF-NHOc).24 3-Octanone was selected as the pheromone for initial inclusion studies. Based on a space-filling model using van der Waals radii of the atoms, this molecule has approximate dimensions of 13.0 Å × 5.5 Å × 4.2 Å, so is readily able to access IRMOF pores (approximate pore window 11.0 Å × 11.0 Å in IRMOF-1).
Prior to uptake, crystalline samples of the MOFs were washed with chloroform and isolated by decantation of the solvent. Preliminary investigations revealed that optimum uptake of 3-octanone from DMF could be achieved using four equivalents of 3-octanone per MOF formula unit at room temperature over a period of three days. Following uptake, the crystals were washed once with DMF, dried, then digested in DCl/D2O and DMSO-d6 for analysis by 1H NMR spectroscopy. The degree of 3-octanone inclusion within the MOF was calculated by comparing the integrals of the aromatic signals with those for the methylene group β- to the carbonyl group. To gauge how easy it was to remove 3-octanone, samples were washed with DMF a further once or twice and re-analysed by 1H NMR spectroscopy following digestion. The results demonstrate poor inclusion and retention of 3-octanone into IRMOF-1, but much higher inclusion into IRMOF-3, IRMOF-NHPr and IRMOF-NHBu (Table 1) despite their lower BET areas.24
This suggests the presence of an amino group is important for 3-octanone inclusion. Furthermore, uptake is considerably higher for both IRMOF-NHPr and IRMOF-NHBu than for IRMOF-3, with the guest exhibiting better retention on washing in the secondary amine-containing MOFs. This implies that a hydrophobic pore environment is important for 3-octanone inclusion and retention. It is, however, notable that essentially no inclusion was observed under identical conditions using IRMOF-NHOc, presumably because the large octyl groups take up too much pore space to allow 3-octanone inclusion. The highest uptake was observed with IRMOF-NHPr, though IRMOF-NHBu retains the pheromone guest better on washing.
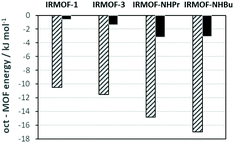
In order to shed light on the differences in uptake and retention, the interaction of the 3-octanone molecules with the framework was studied using molecular dynamics simulations at low loading. While the amine group leads to increased electrostatic interactions as expected, the dispersion interaction is at least five times larger than the electrostatic interaction and the alkyl chains of the bdc-NHPr and bdc-NHBu linkers result in a significant increase of the dispersive interactions (Fig. 3). These interactions in turn lead to very different diffusion behaviour with 3-octanone molecules being able to move freely in IRMOF-1 and being much more confined in IRMOF-NHPr and IRMOF-NHBu.
1H NMR spectroscopic analysis of the digested 3-octanone-loaded MOFs revealed that the samples still retained some DMF, which had been incompletely substituted by 3-octanone. In order to determine maximum loading, samples of IRMOF-3 and IRMOF-NHPr were activated by solvent exchange with chloroform followed by evacuation. The samples were then soaked in neat 3-octanone for 3 days. On removal from the 3-octanone, the samples were tacky, though following hexane washing they became free-flowing powders, suggesting that the only guest present was that included within the pores. Analysis of the 1H NMR spectra of the digested MOFs revealed that the uptake of 3-octanone had increased, with values of 75 wt% and 62 wt% observed for IRMOF-3 and IRMOF-NHPr respectively. The greater uptake for IRMOF-3 under these conditions is consistent with its greater BET surface area.
Decomposition of MOFs within the IRMOF isoreticular series in the presence of water is well established25,26 though not necessarily a problem for pheromone delivery, since framework collapse will be accompanied by guest release. However, decomposition could make release difficult to control and compromise shelf-life. Consequently, it was decided to investigate 3-octanone inclusion into a derivative of UiO-66, a well-known, moisture-stable zirconium(IV) MOF.20 Given that work with the IRMOF series had shown bdc-NHPr to be a good ligand for uptake, [Zr6O4(OH)4(bdc-NHPr)6] (UiO-66-NHPr) was identified as a good potential host. UiO-66-NHPr was prepared from ZrCl4 and H2bdc-NHPr under analogous conditions to those employed in the synthesis of [Zr6O4(OH)4(bdc-NH2)6] (UiO-66-NH2).27 PXRD analysis of UiO-66-NHPr showed a similar powder pattern to those of UiO-66 and UiO-66-NH2, confirming that it is isoreticular. A sample of UiO-66-NHPr was treated with neat 3-octanone for three days then washed with DMF. UiO-66 and its analogues are stable in acids, so cannot be digested in the same way as IRMOFs. Instead, samples were digested using aqueous ammonium fluoride solution,28 and analysed, following digestion, by 1H NMR spectroscopy. The analysis revealed that the sample had included 35.1 wt% 3-octanone so UiO-66-NHPr exhibits a lower capacity than IRMOF-NHPr using neat 3-octanone. Samples of the loaded MOF were further washed with DMF and analysed by 1H NMR spectroscopy. The spectra revealed that washing once or twice more with DMF reduced the 3-octanone content to 17.9 wt% and 6.4 wt% respectively, suggesting that the pheromone is less tightly bound in UiO-66-NHPr than in IRMOF-NHPr.
The molecular dynamics simulations showed that 3-octanone preferentially occupies positions close to the metal centres in the IRMOF-3 structure. This prompted an investigation into [Zn2(dobdc)] (MOF-74, dobdc = 2,5-dioxo-1,4-benzenedicarboxylate)21 as a host for the pheromone as it contains coordinatively unsaturated metal sites. An activated sample of Zn-MOF-74 was treated with neat 3-octanone for three days and washed with n-hexane. 1H NMR analysis of the digested MOF showed an uptake of 29.0 wt% of 3-octanone.
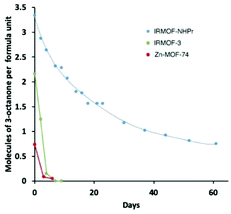
Although analysis of pheromone uptake into MOFs is important, in order to be useful the MOFs also need to be able to release the included pheromones in a slow and controlled manner. 1H NMR spectroscopy studies were carried out on samples of IRMOF-3, IRMOF-NHPr and MOF-74 that had been loaded with 3-octanone by solvent exchange. The pheromone-loaded MOFs were left open to the air for a period of nine weeks, and samples taken every few days were digested and analysed spectroscopically, with the results shown in Fig. 4. IRMOF-3 and MOF-74 released nearly all the guest 3-octanone within the first week. In contrast, IRMOF-NHPr released 3-octanone at a steady rate, and was still releasing 3-octanone at 61 days. This suggests that the hydrophobicity of the alkyl chain is key to the retention of the 3-octanone for a prolonged length of time suitable for application in a lure trap.
Further studies on samples of IRMOF-NHPr that were activated and then loaded with 3-octanone showed the samples continued to release 3-octanone for over 100 days. Head space analysis, conducted using gas chromatography, revealed that 3-octanone could be observed above samples of 3-octanone-loaded IRMOF-NHPr, with the amount detected proportional to the mass of the sample under ambient conditions.
Guest inclusion can stabilise a MOF with respect to decomposition29 and the effects of 3-octanone inclusion on the stability of IRMOF-NHPr were assessed through PXRD analyses. Activated samples of IRMOF-NHPr lost much of their crystallinity within 30 min on exposure to air. In contrast, the PXRD patterns for 3-octanone-loaded samples of IRMOF-NHPr remained unaltered after exposure to air for at least 5 days. This demonstrates that 3-octanone inclusion stabilises the MOF towards hydrolysis, with the presence of the hydrophobic guest presumably preventing access of water molecules to the metal-containing secondary building units.
Preliminary uptake studies were also undertaken with 4-methyl-3-heptanone (mhp). This is a structural isomer of 3-octanone, but differs in being shorter and wider (approximate dimensions 11.5 Å × 6.1 Å × 5.5 Å), though it is still able to access IRMOF pore windows. IRMOF-NHPr was treated with either two equivalents of mhp in hexane or neat mhp, in both cases for 3 days at room temperature. The samples were washed once with hexane to remove mph from the external crystal surfaces, then the samples were digested in DCl/D2O and DMSO-d6 for analysis by 1H NMR spectroscopy. Comparison of the integrals for the digested framework and guest revealed an mhp uptake of 9.5 wt%, corresponding to [Zn4O(bdc-NHPr)3]·0.7 mhp, from the hexane solution, and an uptake of 13.6 wt%, corresponding to [Zn4O(bdc-NHPr)3]·mhp, from the neat liquid. Thus the uptake of 4-methyl-3-heptanone into this MOF is lower than that of 3-octanone, which may be related to the shapes of the pheromone molecules, with the pore windows becoming smaller with increasing size of the bdc substituent.
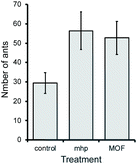
In order to assess whether pheromone-loaded MOFs are biologically active, samples of [Zn4O(bdc-NHPr)3]·mhp were tested in field bioassays in which the numbers of Atta sexdens leaf-cutting ants attracted to citrus pulp baits were compared between bait alone (control) and baits enhanced with either [Zn4O(bdc-NHPr)3]·mhp or pure mhp. Both [Zn4O(bdc-NHPr)3]·mhp and pure mhp were found to enhance the attractiveness of the bait compared to control, and did so to a similar extent (χ2 = 6.37, df = 2, P = 0.041, Fig. 5). This demonstrates that leaf-cutting ants show the normal behavioural responses to the released pheromone when it is included within the MOF, including most importantly being attracted to the source.
In conclusion, we have demonstrated that the leaf-cutting ant alarm pheromones 3-octanone and 4-methyl-3-heptanone can be included as guests within the IRMOF, UiO-66 and MOF-74 systems, and that secondary amine functionalities within the pores aid both uptake and guest retention, facilitating 3-octanone release over a period of over 100 days in the case of IRMOF-NHPr. The alkyl chain plays an important role in slowing down 3-octanone release. Field trials using [Zn4O(bdc-NHPr)3]·mhp have shown that leaf-cutting ants respond as expected to pheromones released from the MOF, thereby showing immense promise for this approach. We are currently undertaking wider studies with these and other semiochemicals in order to assess the generality of these observations.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Leverhulme Trust, the EPSRC and the Universities of Bath and Sussex for financial support and acknowledge the Material and Chemical Characterisation Facility (MC2) at University of Bath for technical support and assistance. We also thank Juliane Lopes, Julia Jones, Chris Tranter and Tatiane Sales for assistance with fieldwork.Notes and references
- H. C. J. Godfray, J. R. Beddington, I. R. Crute, L. Haddad, D. Lawrence, J. F. Muir, J. Pretty, S. Robinson, S. M. Thomas and C. Toulmin, Science, 2010, 327, 812–818 CrossRef CAS PubMed
.
- P. Horcajada, C. Serre, M. Vallet-Regí, M. Sebban, F. Taulelle and G. Férey, Angew. Chem., Int. Ed., 2006, 45, 5974–5978 CrossRef CAS PubMed
.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2012, 112, 1232–1268 CrossRef CAS PubMed
.
- C. He, D. Liu and W. Lin, Chem. Rev., 2015, 115, 11079–11108 CrossRef CAS PubMed
.
- A. D. Burrows, M. Jurcic, L. L. Keenan, R. A. Lane, M. F. Mahon, M. R. Warren, H. Nowell, M. Paradowski and J. Spencer, Chem. Commun., 2013, 49, 11260–11262 RSC
.
- J. Muñoz-Pallares, A. Corma, J. Primo and E. Primo-Yufera, J. Agric. Food Chem., 2001, 49, 4801–4807 CrossRef PubMed
.
- S. M. Seo, J. M. Lee, H. Y. Lee, J. An, S. J. Choi and W. T. Lim, J. Porous Mater., 2015, 23, 557–562 CrossRef
.
- J. M. Moreno, I. Navarro, U. Díaz, J. Primo and A. Corma, Angew. Chem., Int. Ed., 2016, 55, 11026–11030 CrossRef CAS PubMed
.
- T. M. C. Della Lucia, L. C. Gandrab and R. N. C. Guedes, Pest Manage. Sci., 2014, 70, 14–23 CrossRef CAS
.
- R. A. Nascimento, D. B. O. Nunoo, E. Bizkarguenaga, L. Schultes, I. Zabaleta, J. P. Benskin, S. Spano and J. Leonel, Environ. Pollut., 2018, 242, 1436–1443 CrossRef CAS PubMed
.
-
E. Sunamura, in Chemical Ecology of Insects: Applications and Associations with Plants and Microbes, ed. J. Tabata, CRC Press, Boca Raton, FL, 2018, pp. 159–169 Search PubMed
.
-
B. Holldobler and E. O. Wilson, The Ants, Harvard University Press, 1990 Search PubMed
.
- M. R. V. Francelino, A. de Lima Mendonça, R. R. do Nascimento, F. A. C. Mendonça, E. L. da Silva, M. D. R. T. de Freitas, C. R. Cabral, C. E. da Silva, J. H. S. Ribeiro and A. E. G. Santana, Physiol. Entomol., 2008, 33, 37–42 CrossRef
.
- W. O. H. Hughes and D. Goulson, Behav. Ecol. Sociobiol., 2001, 49, 503–508 CrossRef
.
- W. O. H. Hughes, P. E. Howse, E. F. Vilela and D. Goulson, Phys. Ent., 2001, 26, 165–172 CrossRef CAS
.
- W. O. H. Hughes, P. E. Howse and D. Goulson, J. Chem. Ecol., 2001, 27, 109–124 CrossRef CAS PubMed
.
- W. O. H. Hughes and D. Goulson, Bull. Entomol. Res., 2002, 92, 213–218 CrossRef CAS PubMed
.
- W. O. H. Hughes, P. E. Howse, E. F. Vilela, J. J. Knapp and D. Goulson, J. Econ. Entomol., 2002, 95, 537–543 CrossRef CAS PubMed
.
- M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe and O. M. Yaghi, Science, 2002, 295, 469–472 CrossRef CAS PubMed
.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed
.
- N. Rosi, J. Kim, M. Eddaoudi, B. Chen, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 1504–1518 CrossRef CAS
.
- U. Mueller, M. Schubert, F. Teich, H. Puetter, K. Schierle-Arndt and J. Pastre, J. Mater. Chem., 2006, 16, 626–636 RSC
.
- K. K. Tanabe, Z. Wang and S. M. Cohen, J. Am. Chem. Soc., 2008, 130, 8508–8517 CrossRef CAS PubMed
.
- L. L. Keenan, H. Amer Hamzah, M. F. Mahon, M. R. Warren and A. D. Burrows, CrystEngComm, 2016, 18, 5710–5717 RSC
.
- L. Bellarosa, J. M. Castillo, T. Vlugt, S. Calero and N. López, Chem. – Eur. J., 2012, 18, 12260–12266 CrossRef CAS PubMed
.
- P. Guo, D. Dutta, A. G. Wong-Foy, D. W. Gidley and A. J. Matzger, J. Am. Chem. Soc., 2015, 137, 2651–2657 CrossRef CAS PubMed
.
- M. Kandiah, S. Usseglio, S. Svelle, U. Olsbye, K. P. Lillerud and M. Tilset, J. Mater. Chem., 2010, 20, 9848–9851 RSC
.
- H. Amer Hamzah, T. S. Crickmore, D. Rixson and A. D. Burrows, Dalton Trans., 2018, 47, 14491–14496 RSC
.
- N. C. Burtch, H. Jasuja and K. S. Walton, Chem. Rev., 2014, 114, 10575–10612 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Syntheses, 3-octanone loading and release studies, modelling studies. See DOI: 10.1039/d0dt02047h |
| This journal is © The Royal Society of Chemistry 2020 |