Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The first amino acid bound manganese–calcium clusters: a {[MnIII3Ca]2} methylalanine complex, and a [MnIII6Ca] trigonal prism†
Thomais G.
Tziotzi
a,
Evangelos K.
Andreou
a,
Eirini
Tzanetou
a,
Dimitris A.
Kalofolias
 a,
Daniel J.
Cutler
b,
Marek
Weselski
c,
Milosz
Siczek
a,
Daniel J.
Cutler
b,
Marek
Weselski
c,
Milosz
Siczek
 c,
Tadeusz
Lis
c,
Euan K.
Brechin
c,
Tadeusz
Lis
c,
Euan K.
Brechin
 b and
Constantinos J.
Milios
b and
Constantinos J.
Milios
 *a
*a
aDepartment of Chemistry, The University of Crete, Voutes, 71003, Herakleion, Greece. E-mail: komil@uoc.gr
bEaStCHEM School of Chemistry, The University of Edinburgh, David Brewster Road, Edinburgh, EH9 3FJ, UK
cFaculty of Chemistry, University of Wroclaw, 50-283 Wroclaw, Poland
First published on 17th July 2020
Abstract
An amino acid containing octanuclear heterometallic {[MnIII3Ca]2} cluster has been synthesized, alongside a structurally-related trigonal prismatic [MnIII6Ca]2+ cage.
Perhaps one of the most stimulating events in the course of bioinorganic chemistry was the discovery of a pentanuclear heterometallic manganese–calcium cluster in the active center of Photosystem II (PSII). The Oxygen Evolving Center (OEC) of PSII is responsible for the photosynthetic process, more specifically for the oxidative splitting of water to molecular dioxygen, four protons and 4 electrons (per two water molecules), by sunlight. Molecular oxygen is consequently released into the atmosphere, while the protons produced are used to generate ATP chemical energy coins.1 Yet, the complete nature and chemical composition of PSII has been puzzling bioinorganic chemists and crystallographers for several years, with details at the atomic level only appearing in the last two decades.2 The water splitting site is positioned at the heart of the OEC and consists of a pentanuclear [Mn4Ca] complex, whose structure has been described as a cubane-like [Mn3CaO4]n+ moiety linked to a fourth Mnn+ atom via oxo-bridges. The structure is held in position by the carboxylate groups of the side chains of amino acids (D1-Asp170, D1-Glu333, D1-Asp342, CP43-Glu354), as well as the carboxy-terminal residue of D1-Ala344, responsible for the intra-cubane linkage of the calcium ion to a manganese ion. The role of the pentametallic cluster is to serve as an e− donor during consequent cycles through a procedure that involves four oxidation steps, S0–4 (Kok cycle). At the end of the catalytic cycle the cluster, short of 4 e−, “engages” two water molecules oxidizing them to O2, protons and e−, in order to regenerate.3 During the last years numerous compounds have been synthesized and characterized as potential synthetic models of the active center of the enzyme. More specifically, compounds have been reported displaying structural, spectroscopic, and/or catalytic similarity to the OEC. Most of these compounds are polynuclear manganese clusters with the metal ions in various oxidation states.4 However, only a few examples of mixed metal manganese–calcium clusters (i.e. those containing both of the metal centers present in the active site) displaying structural similarities and/or similar metal-content to the OEC have been reported.5
We previously reported the first examples of polynuclear manganese clusters containing amino acid ligands, in which the manganese ions existed in moderate-high oxidation states.6 This motivated us to investigate whether amino acids could also be used to build heterometallic Mn/Ca clusters, and herein report the synthesis, structure and magnetic properties of the first two clusters of this new family, namely {[MnIII3CaO(OH)(mAla)(O2CPh)3(HLa)2(MeOH)]2}·6.2MeCN·0.9H2O (1·6.2MeCN·0.9H2O) and [MnIII6CaO3Lb6(H2O)3](ClO4)2·14.1H2O (2·14.1H2O), using the amino acid methylalanine, mAlaH, and either the naphthalene-based triol ligand 2-(β-naphthalideneamino)-2-hydroxyethyl-1-propanol, H3La, or the Schiff-base ligand created in situ upon the condensation of mAlaH and salicylaldehyde, H2Lb, as co-ligands (Scheme S1†). To the best of our knowledge compounds 1 and 2 are the first examples of any heterometallic Mn/Ca clusters containing amino acids as ligands, with obvious relation to OEC, mimicking not only the metallic content of the active center, but also the amino acid moiety bridging the Mn and Ca ions.
Mn(ClO4)2·6H2O (181 mg, 0.5 mmol), Ca(O2CPh)2·2H2O (318 mg, 1 mmol), mAlaH (51 mg, 0.5 mmol) and H3La (130 mg, 0.5 mmol) were stirred in MeCN/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20 ml) in the presence of excess base, NEt3, for 2 hours to form a dark brown suspension. The precipitate was removed by filtration and the dark-brown solution was layered with Et2O (20 ml) to form dark brown crystals of {[MnIII3CaO(OH)(mAla)(O2CPh)3 (HLa)2(MeOH)]2}·6.2MeCN·0.9H2O (1· 6.2MeCN·0.9H2O) after 4 days in ∼30% yield. For 2, Mn(ClO4)2·6H2O (181 mg, 0.5 mmol), Ca(NO3)2·4H2O (354 mg, 1.5 mmol), mAlaH (25 mg, 0.5 mmol), salicylaldehyde (0.0552 mL, 0.5 mmol) and NBu4MnO4 (MeCN solution, 0.05 M, 1 ml, 0.05 mmol) were stirred in MeCN (20 ml) in the presence of excess base NEt3, for 30 minutes to form a dark brown solution, which was left to slowly evaporate, forming dark brown crystals of [MnIII6CaO3Lb6(H2O)3](ClO4)2·14.1H2O (2·14.1H2O) after ∼2 days, in ∼40% yield‡
1, 20 ml) in the presence of excess base, NEt3, for 2 hours to form a dark brown suspension. The precipitate was removed by filtration and the dark-brown solution was layered with Et2O (20 ml) to form dark brown crystals of {[MnIII3CaO(OH)(mAla)(O2CPh)3 (HLa)2(MeOH)]2}·6.2MeCN·0.9H2O (1· 6.2MeCN·0.9H2O) after 4 days in ∼30% yield. For 2, Mn(ClO4)2·6H2O (181 mg, 0.5 mmol), Ca(NO3)2·4H2O (354 mg, 1.5 mmol), mAlaH (25 mg, 0.5 mmol), salicylaldehyde (0.0552 mL, 0.5 mmol) and NBu4MnO4 (MeCN solution, 0.05 M, 1 ml, 0.05 mmol) were stirred in MeCN (20 ml) in the presence of excess base NEt3, for 30 minutes to form a dark brown solution, which was left to slowly evaporate, forming dark brown crystals of [MnIII6CaO3Lb6(H2O)3](ClO4)2·14.1H2O (2·14.1H2O) after ∼2 days, in ∼40% yield‡
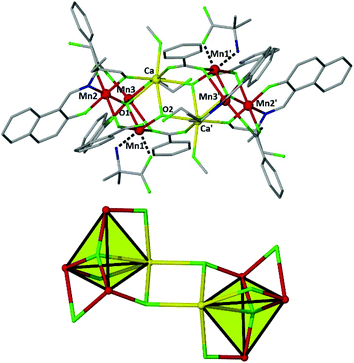
Compound 1 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , with the asymmetric unit (ASU) comprising half the molecule. Its structure (Fig. 1) consists of two {MnIII3CaIIμ4-O} tetrahedra linked together via two μ3-OH ions (O1 and symmetry equivalent (s.e.)), which also bridge to Mn1 and s.e. The basal plane of the tetrahedron comprises an oxo-centered [MnIII3O]7+ triangle in which the O2− ion (O2 and s.e.) resides 0.233 Å ‘above’ the [MnIII3] plane, towards the center of the cage, bonding to the Ca2+ ion at a distance of ∼2.63 Å. The benzoate ligands are of three types: one μ- bridges between Ca1 and Mn1′ (and s.e.), linking the two halves of the molecule; one μ- bridges between Mn2 and Mn3 (and s.e.) in the basal plane of the tetrahedron; and one is terminally bonded to Mn1 (and s.e.), the non-bonded arm H-bonding to the protonated arm of the neighbouring triol ligand (O2C⋯(H)–O15F, 2.67 Å). Each η2:η1:μ-HLa 2− ligand connects a MnIII ion in the basal plane of the tetrahedron to the CaII ion in the upper vertex, with the chelating mAla ligand being bound to Mn1 (and s.e.) forming a five-membered ring through the N and one Ocarboxylate atoms. The manganese ions are all in the 3+ oxidation state (BVS = 3.04, 2.94 and 2.89, for Mn1, Mn2 and Mn3, respectively) displaying Jahn–Teller elongated octahedral geometries (O1C–Mn1–O2A, 2.170–2.210 Å; O1B–Mn2–O1C, 2.138–2.498 Å; O2B–Mn3–O2A, 2.142–2.509 Å). The JT axes all lie within the [MnIII3] plane. The CaII ions are seven-coordinate adopting capped octahedral geometries (SHAPE analysis7), the last coordination site being occupied by a MeOH molecule. The Mn⋯Mn distances fall in the range ∼3.2–3.4 Å, while the Mn⋯Catetrahedron distance are in the narrow range ∼3.4–3.5 Å. Intermolecular interactions are mediated via the π–π stacking of the naphthyl groups of the HL2− and benzoate ligands (C⋯C, ∼3.4 Å).
, with the asymmetric unit (ASU) comprising half the molecule. Its structure (Fig. 1) consists of two {MnIII3CaIIμ4-O} tetrahedra linked together via two μ3-OH ions (O1 and symmetry equivalent (s.e.)), which also bridge to Mn1 and s.e. The basal plane of the tetrahedron comprises an oxo-centered [MnIII3O]7+ triangle in which the O2− ion (O2 and s.e.) resides 0.233 Å ‘above’ the [MnIII3] plane, towards the center of the cage, bonding to the Ca2+ ion at a distance of ∼2.63 Å. The benzoate ligands are of three types: one μ- bridges between Ca1 and Mn1′ (and s.e.), linking the two halves of the molecule; one μ- bridges between Mn2 and Mn3 (and s.e.) in the basal plane of the tetrahedron; and one is terminally bonded to Mn1 (and s.e.), the non-bonded arm H-bonding to the protonated arm of the neighbouring triol ligand (O2C⋯(H)–O15F, 2.67 Å). Each η2:η1:μ-HLa 2− ligand connects a MnIII ion in the basal plane of the tetrahedron to the CaII ion in the upper vertex, with the chelating mAla ligand being bound to Mn1 (and s.e.) forming a five-membered ring through the N and one Ocarboxylate atoms. The manganese ions are all in the 3+ oxidation state (BVS = 3.04, 2.94 and 2.89, for Mn1, Mn2 and Mn3, respectively) displaying Jahn–Teller elongated octahedral geometries (O1C–Mn1–O2A, 2.170–2.210 Å; O1B–Mn2–O1C, 2.138–2.498 Å; O2B–Mn3–O2A, 2.142–2.509 Å). The JT axes all lie within the [MnIII3] plane. The CaII ions are seven-coordinate adopting capped octahedral geometries (SHAPE analysis7), the last coordination site being occupied by a MeOH molecule. The Mn⋯Mn distances fall in the range ∼3.2–3.4 Å, while the Mn⋯Catetrahedron distance are in the narrow range ∼3.4–3.5 Å. Intermolecular interactions are mediated via the π–π stacking of the naphthyl groups of the HL2− and benzoate ligands (C⋯C, ∼3.4 Å).
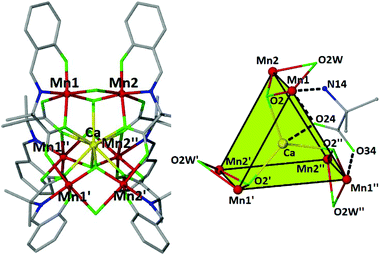
Compound 2 crystallizes in the trigonal space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c (with two crystallographically independent cluster cations). Their metallic skeleton consists of a {MnIII6} trigonal prism encapsulating a nine-coordinate CaII ion at its center (Fig. 2). Each triangular face describes an almost ideal equilateral triangle (MnIII⋯MnIII ∼ 5.26–5.28 Å), the Mn ions being bridged by one syn, anti carboxylate group of a mAla Schiff-base ligand (O23, O24, O33 and O34 and s.e.), which further bridges to the central divalent calcium ion in a η2:η1:η1:μ3-fashion. The two triangles are interconnected by three μ-H2O molecules (Mn–OW–Mn, 81.85°) and three μ3-O−2 ligands (O2 and s.e, Mn–O2–Mn, 114.28°) which also bind to the central CaII center. The approximate dimensions of the cage are 5.3 (Mn–Mn, triangular face) × 3.1 Å (Mn–Mn, upper-to-lower vertex), with the Mn–Ca distance being approximately 3.4 Å. The overall 2+ charge of the complex cation is compensated by the presence of perchlorate anions. The manganese centers are six-coordinate and in the +3 oxidation state (BVS = 2.93), adopting JT-elongated geometries, the JT axes being oriented along the OW–Mn–O(carboxylate) vectors, aligned midway between parallel and perpendicular to the [Mn3] plane. The central calcium ion is nine-coordinate with a tricapped trigonal prismatic {CaO9} geometry (SHAPE analysis7) with Ca–O distances in the range 2.446–2.511 Å. In the extended structure the clusters are surrounded by a large number of H2O molecules, several of which have partial occupancy, as well as several perchlorate anions. The solvate and coordinated μ-H2O molecules, along with the perchlorate anions participate in a large number of hydrogen bonding interactions. There is also a significant number of C–H⋯O (from H2O or ClO4− anions) interactions in the lattice that bridge the clusters together, creating a complicated 3D H-bonded framework (Fig. S1†). There are not any π–π or C–H⋯π interactions present.
c (with two crystallographically independent cluster cations). Their metallic skeleton consists of a {MnIII6} trigonal prism encapsulating a nine-coordinate CaII ion at its center (Fig. 2). Each triangular face describes an almost ideal equilateral triangle (MnIII⋯MnIII ∼ 5.26–5.28 Å), the Mn ions being bridged by one syn, anti carboxylate group of a mAla Schiff-base ligand (O23, O24, O33 and O34 and s.e.), which further bridges to the central divalent calcium ion in a η2:η1:η1:μ3-fashion. The two triangles are interconnected by three μ-H2O molecules (Mn–OW–Mn, 81.85°) and three μ3-O−2 ligands (O2 and s.e, Mn–O2–Mn, 114.28°) which also bind to the central CaII center. The approximate dimensions of the cage are 5.3 (Mn–Mn, triangular face) × 3.1 Å (Mn–Mn, upper-to-lower vertex), with the Mn–Ca distance being approximately 3.4 Å. The overall 2+ charge of the complex cation is compensated by the presence of perchlorate anions. The manganese centers are six-coordinate and in the +3 oxidation state (BVS = 2.93), adopting JT-elongated geometries, the JT axes being oriented along the OW–Mn–O(carboxylate) vectors, aligned midway between parallel and perpendicular to the [Mn3] plane. The central calcium ion is nine-coordinate with a tricapped trigonal prismatic {CaO9} geometry (SHAPE analysis7) with Ca–O distances in the range 2.446–2.511 Å. In the extended structure the clusters are surrounded by a large number of H2O molecules, several of which have partial occupancy, as well as several perchlorate anions. The solvate and coordinated μ-H2O molecules, along with the perchlorate anions participate in a large number of hydrogen bonding interactions. There is also a significant number of C–H⋯O (from H2O or ClO4− anions) interactions in the lattice that bridge the clusters together, creating a complicated 3D H-bonded framework (Fig. S1†). There are not any π–π or C–H⋯π interactions present.
The structural similarities of 1 and 2 to the actual OEC are: (i) the bridging mode of the carboxylate group of the amino acid ligand (D1-Ala344 in the OEC vs. mAla in 2 which bridges the Ca and Mn centers, (ii) the formation of a [Mn3Ca] tetrahedron, (iii) the presence of the sp2 N-atom of the mAla Schiff-base ligand in 2 mimicking the amide N-atoms present in the OEC, and (iv) the presence of six-coordinate Mn ions in all cases. In addition, the +3 oxidation state for all manganese centers may correspond to the dark stable S1 state as has been suggested previously,8 although it is still not clear whether S1 adopts a [MnIII4] or a [MnIV2MnIII2] state. Furthermore, the presence of the six bridging water molecules in 2 is of great biological importance, given the presence of the water molecules near the active site of the enzyme that serve as a substrate.
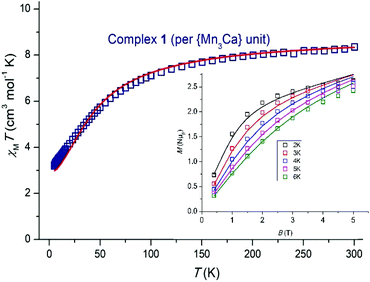
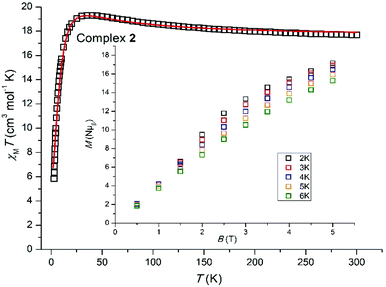
Variable temperature dc magnetic susceptibility data were collected for 1 and 2 in the temperature range 5.0–300 K under an applied field of 0.1 T, and are plotted as χMT versus T in Fig. 3 and 4. For 1, the room temperature value of 8.35 cm3 mol−1 K per {Mn3Ca} unit is slightly lower than the theoretical value of 9.00 cm3 mol−1 K expected for three non-interacting MnIII centers (g = 2.00). Upon cooling χMT decreases slowly to ∼7.49 cm3 mol−1 K at ∼130 K, before rapidly decreasing to a minimum value of 3.21 cm3 mol−1 K at 5 K. This suggests the presence of weak antiferromagnetic exchange between the three MnIII ions. For 2, the room temperature χMT value of 17.63 cm3 mol−1 K is very close to the theoretical value of 18.00 cm3 mol−1 K expected for six non-interacting MnIII centers (g = 2.00). Upon cooling χMT remains approximately constant until ∼150 K, below which it increases slowly to reach ∼19.32 cm3 mol−1 K at ∼35 K, before rapidly decreasing to a minimum value of 5.92 cm3 mol−1 K at 5 K. This behavior is indicative of the presence of both ferro- and antiferromagnetic exchange interactions.
The susceptibility and magnetization data for 1 can be simultaneously fitted through the use of the program PHI9 employing spin-Hamiltonian (1) and the exchange coupling scheme shown in Fig. S2,† where Ŝ is a spin-operator, J the pairwise exchange coupling constant, B the applied magnetic field, and D the axial zero field splitting parameter for the MnIII ion. The exchange coupling model assumes one exchange interaction (J1) between Mn1–Mn2 and Mn1–Mn3 mediated by one μ4-O2− bridge and one monoatomic-bridge benzoate ligand, and a second exchange interaction (J2) between Mn2–Mn3 mediated by a μ4-O2− bridge and a bridging η1:η1:μ2 benzoate. The best fit (Fig. 3) parameters are J1 = +0.50 cm−1, J2 = −7.00 cm−1 and DMn = −6.56 cm−1 with g fixed, gMn = 1.98. The fit can be improved with the inclusion of an intermolecular exchange interaction term, zJ′ = 0.12 cm−1. A fit of the susceptibility data on its own using only the isotropic exchange part of spin-Hamiltonian (1) affords J1 = +0.65 cm−1, J2 = −6.0 cm−1 with gMn = 1.98. These J values belong to a well-defined minimum for this system in the {0.0, +1.0 cm−1}(J1)–{−5.5, −7.5 cm−1}(J2) region, as shown by the contour error plot for the data of 1 as a function of J1 and J2 (Fig. S3†).
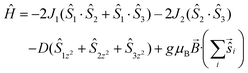 | (1) |
The weak ferromagnetic interaction J1 may be potentially attributed to the small Mn–Omonoatomic benz–Mn angles present (Mn–O–Mn: 85.22 and 87.67°, for Mn1–Mn2 and Mn1–Mn3, respectively), in addition to the in-plane arrangement of the JT axes with respect to the {Mn2O2} plane.10 In this simple “giant spin” model the ground-state of 1 is an S = 2 state, with the first S = 3 excited state located ∼10 cm−1 above.
The size of complex 2 precludes the use of an anisotropic spin-Hamiltonian through standard computational techniques, but the susceptibility data can be fitted by adopting isotropic spin-Hamiltonian (2) which describes a 2-J model with: (1) J1 between neighboring manganese centers located at the corners of the triangular faces, mediated by the syn, anti carboxylate group of the mAla Schiff-base ligand, and (ii) J2 responsible for the connection of the two triangular faces, mediated by one μ-H2O molecule and one μ3-O2− ligand (Fig. S4†).
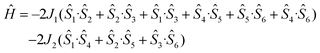 | (2) |
Best fit parameters (Fig. 4) are J1 = −0.35 cm−1, J2 = +1.94 cm−1 with g fixed, gMn = 1.98. This suggests the ground-state is a diamagnetic S = 0 state with the first S = 1 excited state located only ∼0.35 cm−1 above. Magnetization data are in agreement with this picture (Fig. 4) with M rising in a near linear fashion with B.
Conclusions
In conclusion, we have reported the synthesis and characterization of the first two examples of heterometallic Mn/Ca clusters containing an amino acid ligand, methylalanine. Both species are structurally related to the heterometallic cluster found in the active site of the OEC of PSII. Magnetic susceptibility and magnetization measurements reveal the presence of weak antiferro- and ferromagnetic exchange interactions in agreement with magneto-structural correlations developed for structurally similar MnIII clusters. Attempts are currently underway in order to probe the possibility of isolating heterometallic Mn/Ca species upon employment of the natural amino acids Asp, Glu and/or Ala, as a means of modelling the actual content of the OEC.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research work was supported by the Hellenic Foundation for Research and Innovation (HFRI) and the General Secretariat for Research and Technology (GSRT), under the HFRI PhD Fellowship grant (GA no. 4826). Part of this work was carried out in fulfillment of the requirements for the Master thesis of EKA according to the curriculum of the International Graduate Program in “Inorganic Biological Chemistry”, which operates at the University of Ioannina within the collaboration of the Departments of Chemistry of the Universities of Ioannina, Athens, Thessaloniki, Patras, Crete, and the Department of Chemistry of the University of Cyprus. EKB thanks the EPSRC for funding grants EP/N01331X/1 and EP/P025986/1.Notes and references
- Representative references and refs therein: (a) J. Barber, Chem. Soc. Rev., 2009, 38, 185 RSC; (b) S. Mukhopadhyay, S. K. Mandal, S. Bhaduri and W. H. Armstrong, Chem. Rev., 2004, 104, 3981 CrossRef CAS PubMed.
- (a) A. Zouni, H.–T. Witt, J. Kern, P. Fromme, N. Krauss, W. Saenger and P. Orh, Nature, 2001, 409, 739 CrossRef CAS PubMed; (b) K. N. Ferreira, T. M. Iverson, K. Maghlaoui, J. Barber and S. Iwata, Science, 2004, 303, 1831 CrossRef CAS PubMed; (c) A. Guskov, J. Kern, A. Gabdulkhakov, M. Broser, A. Zouni and W. Saenger, Nat. Struct. Mol. Biol., 2009, 16, 334 CrossRef CAS PubMed; (d) N. Kamiya and J. R. Shen, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 98 CrossRef CAS PubMed; (e) Y. Umena, K. Kawakami, J.-R. Shen and N. Kamiya, Nature, 2011, 473, 55 CrossRef CAS PubMed.
- B. Kok, B. Forbush and M. McGloin, Photochem. Photobiol., 1970, 11, 457 CrossRef CAS PubMed.
- See for example: (a) U. Bossek, H. Hummel, T. Weyhermüller, K. Wieghardt, S. Russell, L. Van der Wolf and U. Kolb, Angew. Chem., Int. Ed. Engl., 1996, 35, 1552 CrossRef CAS; (b) J. B. Vincent, H. –R. Chang, K. Folting, J. C. Huffman, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1987, 109, 5703 CrossRef CAS; (c) X. Li, D. P. Kessissoglou, M. L. Kirk, V. L. Pecoraro and C. J. Bender, Inorg. Chem., 1988, 27, 1 CrossRef CAS; (d) V. Tangoulis, D. A. Malamatari, G. A. Spyroulias, C. P. Raptopoulou, A. Terzis and D. P. Kessisoglou, Inorg. Chem., 2000, 39, 2621 CrossRef CAS PubMed; (e) J. B. Vincent, C. Christmas, H.-R. Chang, Q. Li, P. D. W. Boyd, J. C. Huffman, D. N. Hendrickson and G. Christou, J. Am. Chem. Soc., 1989, 111, 2086 CrossRef CAS; (f) W. F. Ruettinger, C. Campana and G. C. Dismukes, J. Am. Chem. Soc., 1997, 119, 6670 CrossRef CAS; (g) S. K. Chandra and A. Chakravorty, Inorg. Chem., 1991, 30, 3795 CrossRef CAS; (h) E. C. Sanudo, V. A. Grillo, M. J. Knapp, J. C. Bollinger, J. C. Huffman, D. N. Hendrickson and G. Christou, Inorg. Chem., 2002, 41, 2441 CrossRef CAS PubMed; (i) H. Chen, J. W. Faller, R.-H. Crabtree and G. W. Brudvig, J. Am. Chem. Soc., 2004, 126, 7345 CrossRef CAS PubMed.
- (a) A. Mishra, W. Wernsdorfer, K. A. Abboud and G. Christou, Chem. Commun., 2005, 54 RSC; (b) I. J. Hewitt, J.-K. Tang, N. T. Mandhu, R. Clèrac, G. Buth, C. E. Anson and A. K. Powell, Chem. Commun., 2006, 2650 RSC; (c) S. Nayak, H. P. Nayek, S. Dehnen, A. K. Powell and J. Reedijk, Dalton Trans., 2011, 40, 2699 RSC; (d) Y. J. Park, J. W. Ziller and A. S. Borovik, J. Am. Chem. Soc., 2011, 133, 9258 CrossRef CAS PubMed; (e) V. Kotzabasaki, M. Siczek, T. Lis and C. J. Milios, Inorg. Chem. Commun., 2011, 14, 213 CrossRef CAS; (f) J. S. Kanady, P.-H. Lin, K. M. Carsch, R. J. Nielsen, M. K. Takase, W. A. Goddard III and T. Agapie, J. Am. Chem. Soc., 2014, 136, 14373 CrossRef CAS PubMed; (g) M. Kose, P. Goring, P. Lucas and P. Mckee, Inorg. Chim. Acta, 2015, 435, 232 CrossRef CAS; (h) J. S. Kanady, E. Y. Tsui, M. W. Day and T. Agapie, Science, 2011, 333, 733 CrossRef CAS PubMed; (i) E. S. Koumousi, S. Mukherjee, C. M. Beavers, S. J. Teat, G. Christou and T. C. Stamatatos, Chem. Commun., 2011, 47, 11128 RSC; (j) A. Mishra, J. Yano, Y. Pushkar, V. K. Yachandra, K. A. Abboud and G. Christou, Chem. Commun., 2007, 1538 RSC; (k) E. Y. Tsui, R. Tran, J. Yano and T. Agapie, Nat. Chem., 2013, 5, 293 CrossRef CAS PubMed; (l) C. Zhang, C. Chen, H. Dong, J.-R. Shen, H. Dau and J. Zhao, Science, 2015, 348, 690 CrossRef CAS PubMed; (m) A. A. Alaimo, D. Takahashi, L. Cunha-Silva, G. Christou and T. C. Stamatatos, Inorg. Chem., 2015, 54, 2137 CrossRef CAS PubMed; (n) R. O. Fuller, G. A. Koutsantonis, I. Lozic, M. I. Ogden and B. W. Skelton, Dalton Trans., 2015, 44, 2132 RSC; (o) C. Chen, C. Zhang, H. Dong and J. Zhao, Dalton Trans., 2015, 44, 4431 RSC; (p) S. Mukherjee, J. A. Stull, J. Yano, T. C. Stamatatos, K. Pringouri, T. A. Stich, K. A. Abboud, R. D. Britt, V. K. Yachandra and G. Christou, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 2257 CrossRef CAS PubMed; A. A. Alaimo, E. S. Koumousi, L. Cunha-Silva, L. J. McCormick, S. J. Teat, V. Psycharis, C. P. Raptopoulou, S. Mukherjee, C. Li, S. D. Gupta, A. Escuer, G. Christou and T. C. Stamatatos, Inorg. Chem., 2017, 56, 10760 Search PubMed (and refs therein); (q) A. Escuer, J. Mayans, M. Font-Bardia, M. Górecki and L. Di Bari, Dalton Trans., 2017, 46, 6514 RSC; (r) J. Mayans, M. Font-Bardia, L. Di Bari, M. Górecki and A. Escuer, Chem. – Eur. J., 2018, 24, 18705 CrossRef CAS PubMed.
- (a) C. Kozoni, E. Manolopoulou, M. Siczek, T. Lis, E. K. Brechin and C. J. Milios, Dalton Trans., 2010, 39, 7943 RSC; (b) C. Kozoni, M. Siczek, T. Lis, E. K. Brechin and C. J. Milios, Dalton Trans., 2009, 42, 9117 RSC.
- M. Llunell, D. Casanova, J. Girera, P. Alemany and S. Alvarez, SHAPE, version 2.0, Barcelona, Spain, 2010 Search PubMed.
- D. Kuzek and R. J. Pace, Biochim. Biophys. Acta, 2001, 1503, 123 CrossRef CAS.
- N. F. Chilton, R. P. Anderson, L. D. Turner, A. Soncini and K. S. Murray, J. Comput. Chem., 2013, 34, 1164 CrossRef CAS PubMed.
- See for example: (a) N. Berg, T. Rajeshkumar, S. M. Taylor, E. K. Brechin, G. Rajaraman and L. F. Jones, Chem. – Eur. J., 2012, 18, 5906 CrossRef CAS PubMed, and refs therein; (b) K. Mitra, D. Mishra, S. Biswas, C. R. Lucas and B. Adhikary, Polyhedron, 2006, 25, 1681 CrossRef CAS; (c) J. B. Vincent, H. L. Tsai, A. G. Blackman, S. Wang, P. D. W. Boyd, K. Folting, J. C. Huffman, E. B. Lobkovsky, D. N. Hendrickson and G. Christou, J. Am. Chem. Soc., 1993, 115, 12353 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1816563 and 1999601. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0dt01916j |
‡ Crystal data for 1·6.2MeCN·0.9H2O: C128.4H144.4Ca2Mn6N12.2O34.9, M = 2826.75, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 14.303(4) Å, b = 15.673(5) Å, c = 15.979(5) Å, α = 107.04(3)°, β = 99.23(3)°, γ = 97.21(3)°, V = 3323.5(19) Å3, Z = 1, T = 80 K, R1 (I > 2σ) = 0.067 and wR2 (all data) = 0.201 for 29 , a = 14.303(4) Å, b = 15.673(5) Å, c = 15.979(5) Å, α = 107.04(3)°, β = 99.23(3)°, γ = 97.21(3)°, V = 3323.5(19) Å3, Z = 1, T = 80 K, R1 (I > 2σ) = 0.067 and wR2 (all data) = 0.201 for 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 522 reflections collected, 12 522 reflections collected, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 519 observed reflections (I > 2σ(I)) of 18 519 observed reflections (I > 2σ(I)) of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 327 (Rint = 0.039) unique reflections, GOF = 1.01. Crystal data for 2·14.1H2O: C66H100.20CaCl2Mn6N6 O46.10, M = 2155.94, trigonal, space group R 327 (Rint = 0.039) unique reflections, GOF = 1.01. Crystal data for 2·14.1H2O: C66H100.20CaCl2Mn6N6 O46.10, M = 2155.94, trigonal, space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c, a = 21.029(4) Å, c = 143.48(6) Å, V = 54 c, a = 21.029(4) Å, c = 143.48(6) Å, V = 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 949(31) Å3, Z = 24, T = 100 K, R1 (I > 2σ) = 0.106 and wR2 (all data) = 0.208 for 40 949(31) Å3, Z = 24, T = 100 K, R1 (I > 2σ) = 0.106 and wR2 (all data) = 0.208 for 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 308 reflections collected, 6462 observed reflections (I > 2σ(I)) of 11 308 reflections collected, 6462 observed reflections (I > 2σ(I)) of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 186 (Rint = 0.099) unique reflections, GOF = 1.13. Full details can be found in the CIF files with CCDC reference numbers 1816563 and 1999601, for 1 and 2, respectively.† 186 (Rint = 0.099) unique reflections, GOF = 1.13. Full details can be found in the CIF files with CCDC reference numbers 1816563 and 1999601, for 1 and 2, respectively.† |
| This journal is © The Royal Society of Chemistry 2020 |