Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Organometallic nucleoside analogues: effect of the metallocene metal atom on cancer cell line toxicity†
Media K.
Ismail
 a,
Katie A.
Armstrong
b,
Samantha L.
Hodder
b,
Sarah L.
Horswell
a,
Katie A.
Armstrong
b,
Samantha L.
Hodder
b,
Sarah L.
Horswell
 a,
Louise
Male
a,
Louise
Male
 a,
Huy V.
Nguyen
a,
Huy V.
Nguyen
 a,
Edward A.
Wilkinson
a,
Edward A.
Wilkinson
 a,
Nikolas J.
Hodges
a,
Nikolas J.
Hodges
 *b and
James H. R.
Tucker
*b and
James H. R.
Tucker
 *a
*a
aSchool of Chemistry, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK. E-mail: j.tucker@bham.ac.uk
bSchool of Biosciences, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK. E-mail: n.hodges@bham.ac.uk
First published on 16th December 2019
Abstract
A new chiral organometallic nucleoside analogue containing ruthenocene is reported, in which alkylthymine and alkylhydroxyl groups are attached in adjacent positions on one cyclopentadienyl ring. The synthetic procedures for this metallocene derivative and two control compounds are described, along with their characterisation by cyclic voltammetry and X-ray crystallography. Their biological activities in a human pancreatic cancer cell line (MIA-Pa-Ca-2) were significantly lower than those of three previously reported analogous ferrocene compounds, indicating that the choice of metallocene metal atom (Fe or Ru) plays a pivotal role in determining the anticancer properties of these nucleoside analogues, which in turn suggests a different mode of action from that of a conventional nucleoside analogue.
Introduction
A topical area within the field of metal-based anticancer drug research involves examining the effect of incorporating organometallic moieties into known organic drugs and related biological molecules.1,2 Ferrocene is a popular choice in this respect as a so-called bioisosteric group2 because of its stability and well-understood reactivity and electrochemistry. Its incorporation into the breast cancer drug tamoxifen to form the ferrocifen family of compounds3,4 has revealed potent activities in different cell lines compared to the parent compound. This suggests novel modes of action related to the redox properties of the ferrocene unit, which may help combat drug resistance in the clinic.Nucleoside and nucleobase analogues are an important class of chemotherapeutic agents, with 5-fluorouracil (5-FU)5 and gemcitabine (Gem)6 being two examples of leading drugs on the market. This presents a similar opportunity to decorate and derivatise the components of DNA/RNA with organometallic groups to give a range of new biologically active and medicinally relevant compounds.7,8 As part of our work in this area, we previously reported the thymidine analogue 1-(S,Rp)-Fe in which the five-membered Cp ring of ferrocene replaced the five-membered sugar ring of the nucleoside (Fig. 1).9 This compound demonstrated excellent anticancer activities in a range of human cancer cell lines, with both the hydroxyl linker and the nucleobase moiety required for optimal cytotoxicity. A subsequent structure–activity relationship (SAR) study found a correlation between the IC50 values in cancer cells and the length of the hydroxyalkyl linker in these so-called ferronucleosides.10 In continuation of this line of enquiry, we next decided to consider the role played by the metal atom in the lead compound 1-(S,Rp)-Fe, the subject of this report. In metallocene-based drug discovery, changing the metal from iron to ruthenium is a worthwhile endeavour, given the stability of ruthenocene and its amenability to functionalisation. Furthermore, and of particular relevance from an SAR point of view, ruthenocenes have different redox properties to ferrocenes, having more positive oxidation potentials and less reversible electrochemistry.11 It follows that any difference in biological activity between the two metallocenes could indicate the role of redox processes in the mode of action. Indeed, work on ruthenocifen derivatives has indicated different anticancer activities to the ferrocifens, with their biological behaviour being more similar to the parent organic compound tamoxifen.3,12 However, despite these findings, other reports on the biological activities of ruthenocene compounds13 or organoruthenium nucleobase derivatives8a are relatively rare. Here we report the synthesis and anticancer properties of 1-(S,Rp)-Ru, the direct ruthenocene analogue of 1-(S,Rp)-Fe, and two related control compounds (Fig. 2). Our findings do indeed suggest an important role of the metal atom in controlling the anticancer activities of these metallocene-containing nucleoside analogues.
 | ||
| Fig. 1 Structures of the nucleoside thymidine (left), a generic nucleoside analogue (middle), and a ferronucleoside drug candidate 1-(S,Rp)-Fe (right). | ||
 | ||
| Fig. 2 Structures of the main target compound 1-(S,Rp)-Ru (middle) and the two control compounds 2-(S) and 3 (left and right) respectively. | ||
Results and discussion
Synthesis
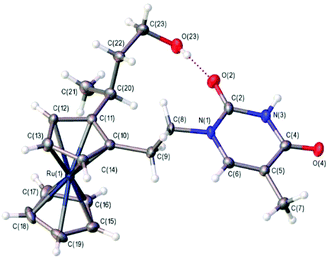
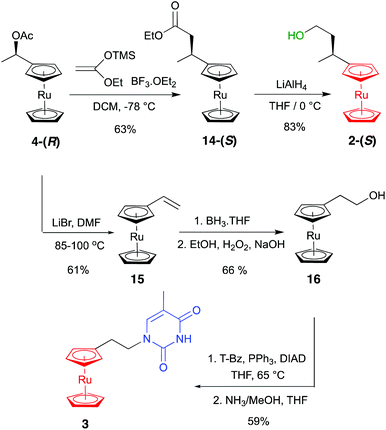
We considered that the synthesis of 1-(S,Rp)-Ru and the two control compounds 2-(S) and 3 would allow a direct comparison with the three analogous ferrocene compounds previously reported9 and also enable similar synthetic routes to be followed. In the case of the main target 1-(S,Rp)-Ru, this meant building the compound up from the known acetoxy derivative 4-(R)14 (Scheme 1), which itself was prepared via a two-step route from acetylruthenocene (see ESI†). This was then treated with NHMe2 to give the ruthenocene version of Ugi's amine 5-(R),14 whose chiral purity was found to be greater than 98%, as evidenced by chiral HPLC (see ESI†). Its X-ray structure was determined for the first time from crystals grown from a solution of the racemate in DCM layered with hexane (see ESI†).‡ The next step was to introduce planar chirality through the diastereoselective synthesis of 6-(R,Sp)via treatment with n-BuLi in diethylether and then quenching with iodine in THF. This compound was then converted to the acetoxy derivative 7-(R,Sp) by heating at 50 °C for two hours in acetic anhydride. A short reaction time and a relatively low temperature were used to avoid the elimination of the amine group to give the alkene. The arm was then extended to three carbon atoms by reacting with freshly prepared 1-ethoxyvinyloxy trimethylsilane to give the ethyl ester product 8-(S,Sp). The ester was reduced to the corresponding alcohol 9-(S,Sp) using the mild reducing agent DIBAL-H, before being protected with the TBDPS group to give compound 10-(S,Sp). This compound was then formylated in dry ether in two steps in situ by reacting with n-BuLi in a lithium–halogen exchange reaction followed by addition of DMF to give compound 11-(S,Sp). A Wittig reaction of the aldehyde added another carbon atom to give the alkene 12-(S,Rp), which was then converted to the primary alcohol 13-(S,Rp) by hydroboration–oxidation with BH3·THF. Finally, a Mitsunobu coupling reaction of the alcohol 13-(S,Rp) with benzoyl-protected thymine gave the fully protected product, which was treated first with TBAF to remove the silyl group and then with ammonia in methanol to remove the benzoyl group, giving the target compound 1-(S,Rp)-Ru. HPLC analysis of this compound confirmed its formation in high chiral purity (>98%, see ESI†). Crystals suitable for X-ray crystallography were grown from an acetonitrile solution of the racemate at 0 °C (Fig. 3).‡ An internal O–H⋯O H bond is formed between the hydroxyl hydrogen atom and one carbonyl oxygen atom in the thymine base. Intramolecular H-bonding has previously been observed within other bioorganometallic compounds.8d,25The synthesis of control compounds 2-(S) and 3 also started from compound 4-(R) and proceeded through the routes depicted in Scheme 2. The chiral alcohol was obtained via a linker extension reaction using 1-ethoxyvinyloxy trimethylsilane, followed by reduction of the ester 14-(S) with LiAlH4. Achiral 3 was obtained in four steps, first involving elimination of the acetoxy group to give the vinyl ruthenocene 15. Crystals of this compound suitable for X-ray diffraction were successfully grown from a solution of DCM layered with hexane (see ESI†). A hydroboration–oxidation reaction then yielded the anti-Markovnikov product 16 containing the desired hydroxyethyl linker. The X-ray structure of this compound was also obtained from crystals grown using the same conditions (see ESI†). The route was completed using the same methodology described earlier via a Mitsunobu coupling reaction with the protected thymine base to give the protected product 17, which was then deprotected with ammonia to give the target compound 3. Crystals of the latter were grown by slow evaporation from a solution of ethyl acetate layered with hexane. The resulting X-ray structure, showing the correct bond connectivity, is depicted in Fig. 4.
Electrochemistry
The electrochemistry of ruthenocene is more complicated than that of ferrocene. The 17-electron ruthenocenium cation is considerably more unstable and reactive than its ferrocene counterpart with Ru(VI) products formed from both the chemical15,16 and electrochemical11,16,17 oxidation of ruthenocene. The appearance of the cyclic voltammograms (CVs) of ruthenocene and those of its derivatives is highly dependent on the type of solvent and electrolyte.18–20 In non-coordinating electrolyte systems and in the presence of non-coordinating boron-containing electrolytes that do not form ion pairs, the cation has been reported to form two different dimers in a temperature-dependent ratio.20,21 In coordinating solvents and in the presence of more conventional electrolytes,18,19,22 the behaviour is different again. For example, a two-electron oxidation has been reported in acetonitrile using [NBu4][PF6] as a supporting electrolyte.18,23 with the cation binding to acetonitrile to form [MeCN-RuCp2]+, which then undergoes further oxidation to give [MeCN-RuCp2]2+ followed by a double reduction back to ruthenocene. Given these previous findings, it was decided to conduct CV experiments on the three target compounds 1-(S,Rp)-Ru, 2-(S) and 3 in acetonitrile in the presence of [NBu4][PF6] and compare them with 1-(S,Rp)-Fe under the same conditions. The experiments were performed in the presence of decamethylferrocene (dmfc) as an internal reference, as reported previously.10All three ruthenocene compounds showed a similar EC (electrochemical-chemical) oxidation process at a positive potential value, with no return wave observed under the conditions used. Voltammograms of 1-(S,Rp)-Ru at different scan rates are displayed in Fig. 5, with those of 2-(S) and 3 presented in the ESI.† The Epa data for all three compounds are presented in Table 1, along with the corresponding value for the ferrocene analogue 1-(S,Rp)-Fe, which is considerably more negative (Epa = 455 mV, E1/2 = 424 mV (ref. 10)).
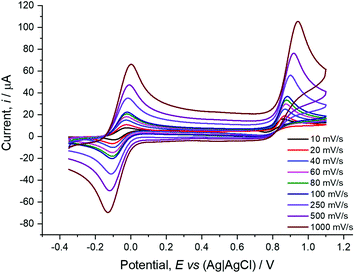 | ||
| Fig. 5 Cyclic voltammograms of 1-(S,Rp)-Ru (1.0 mM) at various scan rates in dry acetonitrile with an electrolyte of [NBu4][PF6] (0.1 M) and dmfc (1.0 mM) as internal reference. | ||
| Compound | 1-(S,Rp)-Fe | 1-(S,Rp)-Ru | 2-(S) | 3 |
|---|---|---|---|---|
| a Mean values at scan rates 10–100 mV s−1versus the E1/2 of decamethylferrocene, at 1 mM concentration in dry acetonitrile containing 0.1 M TBAPF6, confidence limit = ±5 mV. b The mean from three independent biological experiments (n = 3), calculated using a variable slope 4 parameter model in Prism V8. Values in parentheses are the 95% CI. IC50 of cisplatin = 8.3 (5.1–17.0) μM. | ||||
| E pa | 455 | 932 | 948 | 968 |
| IC50b | 9.3 (7.3–12.1) | 46.0 (37.7–56.6) | >80 | >80 |
This difference in value reflects the large difference in the relative stabilities between the oxidized and reduced forms of the two metallocenes, with the ferrocene derivative clearly being thermodynamically much easier to oxidise than its ruthenocene counterpart. The ferrocene derivative also shows reversible electrochemistry. The more positive value of Epa for compounds 2-(S) and 3 compared with 1-(S,Rp)-Ru can be explained by a greater inductive effect (+I) as the number of electron donating groups on the Cp ring increases, giving more stability to the charged ruthenocenium ion. The same trend is observed for the analogous ferrocene control compounds of 2-(S) and 3, which have Epa values of 497 mV and 540 mV respectively vs. dmfc (see ESI†).
Biological studies
The three ruthenocene compounds were next tested for cytotoxic activity in the pancreatic ductal adenocarcinoma cell line MIA-Pa-Ca-2 and compared with the ferrocene counterpart 1-(S,Rp)-Fe as well as with cisplatin. Assays were performed after 4 days incubation time using crystal violet staining. Cell viabilities, expressed as a percentage of a negative control, were plotted against concentration (μM) as shown in Fig. 6, with the resulting IC50 values presented in Table 1. As found previously for other cancer cell lines,9 the IC50 value for the ferrocene derivative 1-(S,Rp)-Fe is in the low micromolar range, with a value similar to that of cisplatin. However, the five-fold reduction in the toxicity of the ruthenocene analogue clearly shows that the identity of the metal ion has a significant impact on cytotoxicity.§ It is worth noting that the control compounds 2-(S) and 3 were even less toxic than 1-(S,Rp)-Ru, with IC50 values of >80 μM. This agrees with our previous findings on analogous and related ferrocene compounds,9,10 in that those metallocenes that are more electron rich, for example by having two groups attached to one cyclopentadienyl ring, are more cytotoxic. Indeed the previously published ferrocene analogues of 2-(S) and 3, which display more positive Epa values, are less toxic than 1-(S,Rp)-Fe.9 Overall the trend in the biological data supports the hypothesis that there is a significant relationship between the redox properties of the metallocene units in this series and cancer cell line toxicity.Conclusion
A ruthenocene-containing nucleoside analogue and two control compounds have been synthesised and fully characterized by a combination of spectroscopic, X-ray crystallography and electrochemical measurements. Their oxidation potentials were affected by the type and number of linker groups attached to the ruthenocene unit. All three compounds showed very low biological activities towards MIA-Pa-Ca-2 pancreatic ductal adenoma carcinoma cells, with IC50 values for the two mono-functionalised controls higher than that for the bis-functionalised target compound. The main finding of this study is the five-fold difference in cytotoxicity between 1-(S,Rp)-Ru and its ferrocene counterpart 1-(S,Rp)-Fe. Given their otherwise identical chemical structures and stereochemistries, this difference can confidently be attributed to the change in the metal atom from iron to ruthenium. While such a change would make little difference to a metallocene's size or lipophilicity, it clearly does affect its redox properties. The ferrocenes in this series demonstrate more reversible electrochemistry than their ruthenocene counterparts, with their oxidised forms accessible at significantly lower potentials. These differences in electrochemical behaviour signify an important role of the iron atom in determining the anticancer activity of the lead compound 1-(S,Rp)-Fe. This in turn suggests a mode of action different from that of a conventional nucleoside analogue, one that points more towards intra-cellular redox-triggered and ROS-mediated pathways leading to cell death. This line of enquiry is currently under investigation in our laboratory.Experimental
Synthesis
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H), 2963 (CH2), 2923 (CH2), 2851 (CH2), 1100 (C–H), 1453 (CH3), 1370 (CH3), 1261, 1156, 918 (CH
C–H), 2963 (CH2), 2923 (CH2), 2851 (CH2), 1100 (C–H), 1453 (CH3), 1370 (CH3), 1261, 1156, 918 (CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 806 (CH Ar). [α]20D = +27(±2) (c = 0.25 in CHCl3).
CH), 806 (CH Ar). [α]20D = +27(±2) (c = 0.25 in CHCl3).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 91.9 (ipso Cp), 78.2 (Cp), 73.8 (Cp), 71.7 (Cp), 69.4 (CH), 68.9 (Cp), 39.9 (ipso Cp), 21.2 (CH3), 19.4 (CH3). HRMS (ES) (m/z) calcd for C14H15O2Na102Ru127I 466.9058 found 466.9054. Vmax/cm−1 3098 (
O), 91.9 (ipso Cp), 78.2 (Cp), 73.8 (Cp), 71.7 (Cp), 69.4 (CH), 68.9 (Cp), 39.9 (ipso Cp), 21.2 (CH3), 19.4 (CH3). HRMS (ES) (m/z) calcd for C14H15O2Na102Ru127I 466.9058 found 466.9054. Vmax/cm−1 3098 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H), 2979 (CH2), 2929 (CH2), 2818 (CH2), 1727 (C
C–H), 2979 (CH2), 2929 (CH2), 2818 (CH2), 1727 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1449 (CH3), 1367 (CH3), 1229 (C–O), 1044, 1018, 806 (CH
O), 1449 (CH3), 1367 (CH3), 1229 (C–O), 1044, 1018, 806 (CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 752 (CH–Ar). [α]20D = +25(±2) (c = 0.25 in CHCl3).
CH), 752 (CH–Ar). [α]20D = +25(±2) (c = 0.25 in CHCl3).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 98.6 (ipso Cp), 76.9 (Cp), 73.4 (Cp), 70.8 (Cp), 68.0 (Cp), 60.3 (CH2), 43.0 (CH2), 40.3 (ipso Cp), 30.6 (CH), 20.6 (CH3), 14.4 (CH3). HRMS (ES) (m/z) calcd for C16H19O2Na102Ru127I 494.9371, found 494.9373. Vmax/cm−1 3097 (
O), 98.6 (ipso Cp), 76.9 (Cp), 73.4 (Cp), 70.8 (Cp), 68.0 (Cp), 60.3 (CH2), 43.0 (CH2), 40.3 (ipso Cp), 30.6 (CH), 20.6 (CH3), 14.4 (CH3). HRMS (ES) (m/z) calcd for C16H19O2Na102Ru127I 494.9371, found 494.9373. Vmax/cm−1 3097 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H), 2973 (CH2), 2929 (CH2), 2823 (CH2), 1728 (C
C–H), 2973 (CH2), 2929 (CH2), 2823 (CH2), 1728 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1459 (CH3), 1369 (CH3), 1173, 1030 (C–O), 1100, 998 (CH
O), 1459 (CH3), 1369 (CH3), 1173, 1030 (C–O), 1100, 998 (CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 805 (CH–Ar). [α]20D = −6(±2) (c = 0.25 in CHCl3).
CH), 805 (CH–Ar). [α]20D = −6(±2) (c = 0.25 in CHCl3).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H Fc), 2955 (CH2), 2924 (CH2), 2852 (CH2), 1524, 1458 (CH2), 1374 (CH3), 1054, 997 (C–O), 805 (C
C–H Fc), 2955 (CH2), 2924 (CH2), 2852 (CH2), 1524, 1458 (CH2), 1374 (CH3), 1054, 997 (C–O), 805 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). [α]20D = +10(±3) (c = 0.2 in CHCl3). m.p.: 98–100 °C.
C). [α]20D = +10(±3) (c = 0.2 in CHCl3). m.p.: 98–100 °C.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH Fc), 2956 (CH2), 2928 (CH2), 2855(CH2), 1472 (CH2), 1387 (CH3), 1307, 1187, 1108, 821 (CH–Ar TBDPS), 701 (C
CH Fc), 2956 (CH2), 2928 (CH2), 2855(CH2), 1472 (CH2), 1387 (CH3), 1307, 1187, 1108, 821 (CH–Ar TBDPS), 701 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). [α]20D = +15(±2) (c = 0.25 in CHCl3).
C). [α]20D = +15(±2) (c = 0.25 in CHCl3).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 135.57 (Ar), 1334.0 (ipso Ar), 133.8 (ipso Ar), 129.6 (Ar), 127.6 (Ar), 101.8 (ipso Cp), 82.4 (ipso Cp), 72.7 (Cp), 72.4 (Cp), 72.4 (Cp), 70.6 (Cp), 62.0 (CH2), 42.9 (CH2), 27.5 (CH), 26.9 (CH3), 20.9 (CH3), 19.2 (ipso t-Bu). HRMS (ES) (m/z) calcd for C31H36O2Na102Ru28Si 593.1432, found 593.1426. Vmax/cm−1 3096 (C–H Fc), 2958, 2931, 2856 (CH2), 1678 (C
O), 135.57 (Ar), 1334.0 (ipso Ar), 133.8 (ipso Ar), 129.6 (Ar), 127.6 (Ar), 101.8 (ipso Cp), 82.4 (ipso Cp), 72.7 (Cp), 72.4 (Cp), 72.4 (Cp), 70.6 (Cp), 62.0 (CH2), 42.9 (CH2), 27.5 (CH), 26.9 (CH3), 20.9 (CH3), 19.2 (ipso t-Bu). HRMS (ES) (m/z) calcd for C31H36O2Na102Ru28Si 593.1432, found 593.1426. Vmax/cm−1 3096 (C–H Fc), 2958, 2931, 2856 (CH2), 1678 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1427 (CH2), 1388 (CH3), 1109, 998 (C–O), 820, 739 (CH Ar), 703 (C
O), 1427 (CH2), 1388 (CH3), 1109, 998 (C–O), 820, 739 (CH Ar), 703 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). [α]20D = +7(±2) (c = 0.2 in CHCl3).
C). [α]20D = +7(±2) (c = 0.2 in CHCl3).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C Ar), 1471 (CH2), 1388 (CH3), 1108, 1086 (C–O), 821, 806 (C–H Ar), 700 (vinyl/C
C Ar), 1471 (CH2), 1388 (CH3), 1108, 1086 (C–O), 821, 806 (C–H Ar), 700 (vinyl/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). [α]20D = +52(±3) (c = 0.2 in CHCl3).
C). [α]20D = +52(±3) (c = 0.2 in CHCl3).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H Fc), 2955 (CH2), 2927 (CH2), 1589, 1470 (CH3), 1427 (CH2), 1388 (CH3), 1361, 1109, 997 (C–O), 822, 738 (C–H Ar), 702 (
C–H Fc), 2955 (CH2), 2927 (CH2), 1589, 1470 (CH3), 1427 (CH2), 1388 (CH3), 1361, 1109, 997 (C–O), 822, 738 (C–H Ar), 702 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H). [α]20D = +45(±3) (c = 0.25 in CHCl3).
C–H). [α]20D = +45(±3) (c = 0.25 in CHCl3).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 151.1 (C
O), 151.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 140.4 (CH thymine), 111.0 (ipso thymine), 95.3 (ipso Cp), 80.7 (ipso Cp), 69.3 (Cp), 67.6 (Cp), 65.9 (Cp), 65.3 (Cp), 60.2 (CH2), 49.8 (CH2), 43.2 (CH2), 27.9 (CH2), 27.1 (CH), 19.2 (CH3 thymine), 12.3 (CH3). HRMS (ES) (m/z) calcd for C21H26N2O3NaFe 433.1191, found 433.1182. Vmax/cm−1 3462 br (OH), 3097 (
O), 140.4 (CH thymine), 111.0 (ipso thymine), 95.3 (ipso Cp), 80.7 (ipso Cp), 69.3 (Cp), 67.6 (Cp), 65.9 (Cp), 65.3 (Cp), 60.2 (CH2), 49.8 (CH2), 43.2 (CH2), 27.9 (CH2), 27.1 (CH), 19.2 (CH3 thymine), 12.3 (CH3). HRMS (ES) (m/z) calcd for C21H26N2O3NaFe 433.1191, found 433.1182. Vmax/cm−1 3462 br (OH), 3097 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H Fc), 2954 (CH2), 2926 (CH2), 1669 (C
C–H Fc), 2954 (CH2), 2926 (CH2), 1669 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1468, 1425 (CH3), 1425 (CH2), 1383 (CH3), 1353 (C–N) 1100, 1036, 997 (C–O), 805, 759 (C–H Ar), 690, 669 (
O), 1468, 1425 (CH3), 1425 (CH2), 1383 (CH3), 1353 (C–N) 1100, 1036, 997 (C–O), 805, 759 (C–H Ar), 690, 669 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H). [α]20D = +42(±3) (c = 0.1 in CH3CN).
C–H). [α]20D = +42(±3) (c = 0.1 in CH3CN).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 98.3 (ipso Cp), 70.5 (Cp), 70.1 (Cp), 69.5 (Cp), 68.9 (Cp), 60.3 (CH2), 43.7 (CH2), 29.6 (CH), 21.5 (CH3), 14.3 (CH3). HRMS (ES) (m/z) calcd for C16H19O2102Ru 346.0507, found 346.0508. Vmax/cm−1 3095 (C–H), 2964, 2929 (CH2), 1731 (C
O), 98.3 (ipso Cp), 70.5 (Cp), 70.1 (Cp), 69.5 (Cp), 68.9 (Cp), 60.3 (CH2), 43.7 (CH2), 29.6 (CH), 21.5 (CH3), 14.3 (CH3). HRMS (ES) (m/z) calcd for C16H19O2102Ru 346.0507, found 346.0508. Vmax/cm−1 3095 (C–H), 2964, 2929 (CH2), 1731 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1369 (CH3), 1030 (C–O), 817 (
O), 1369 (CH3), 1030 (C–O), 817 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H). [α]20D = +10.5(±2) (c = 0.1 in CHCl3).
C–H). [α]20D = +10.5(±2) (c = 0.1 in CHCl3).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H). [α]20D = +13(±2) (c = 0.1 in CHCl3).
C–H). [α]20D = +13(±2) (c = 0.1 in CHCl3).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 805, 753 (
C), 805, 753 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H).
C–H).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H Fc), 2926, 2874 (CH2), 1037, 999 (C–O), 813 (C–H).
C–H Fc), 2926, 2874 (CH2), 1037, 999 (C–O), 813 (C–H).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 150.6 (C
O), 150.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 140.9 (CH–thymine), 110.2 (ipso thymine), 87.2 (ipso-Cp), 70.9 (Cp), 70.7 (Cp), 70.0 (Cp), 50.8 (CH2), 28.8 (CH2), 12.2 (CH3). HRMS (m/z) calcd for C17H18N2O2102Ru23Na 407.0309, found 407.0311. Vmax/cm−1 3210 (NH), 3089 (
O), 140.9 (CH–thymine), 110.2 (ipso thymine), 87.2 (ipso-Cp), 70.9 (Cp), 70.7 (Cp), 70.0 (Cp), 50.8 (CH2), 28.8 (CH2), 12.2 (CH3). HRMS (m/z) calcd for C17H18N2O2102Ru23Na 407.0309, found 407.0311. Vmax/cm−1 3210 (NH), 3089 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H Fc), 2954 (CH2), 2921 (CH2), 2850 (CH2), 1685, 1672 (C
C–H Fc), 2954 (CH2), 2921 (CH2), 2850 (CH2), 1685, 1672 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1462, 1385 (CH3), 1353 (C–N), 803, 759 (C
O), 1462, 1385 (CH3), 1353 (C–N), 803, 759 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C).
C).
Electrochemistry
Cyclic voltammetry measurements were performed using 1.0 mM solutions in dry and de-oxygenated MeCN containing tetrabutylammonium hexafluorophosphate (TBAPF6) electrolyte at a concentration of 0.1 M and 1.0 mM dfmc as internal reference. Data were measured with a BioAnalytical Systems Inc. (West Lafayette, IN) EC Epsilon potentiostat and with a C3 cell stand with a three-electrode unit. The cyclic voltammograms were recorded under argon at room temperature using a three-electrode cell. The electrodes were obtained from IJ Cambria (Llanelli, Wales). A platinum wire was used as a counter electrode (CE), a glassy carbon electrode with 3 mm diameter was used as a working electrode (WE) and an Ag|AgCl|3 M KCl electrode was used as a reference electrode (RE) and connected to the cell via a frit. Cleaning of all glassware was achieved by soaking overnight in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ammonia (35%) and hydrogen peroxide (30%), followed by multiple rinsing with ultrapure water (from a Millipore tandem Elix-A10 system, resistivity >18 MΩ cm, TOC < 5 ppb). The glassware was then left overnight in ultrapure water, then rinsed again and dried in an oven prior to use. The electrodes were cleaned as follows before their use: the RE was cleaned with dry acetonitrile and the CE was flame annealed. The WE was cleaned by polishing with aqueous slurries of successively finer grades of alumina (1.0 μm, 0.3 μm and 0.05 μm) and then rinsed with ultrapure water and MeCN, dried with a flow of argon, and then kept in an analyte solution.
1 ammonia (35%) and hydrogen peroxide (30%), followed by multiple rinsing with ultrapure water (from a Millipore tandem Elix-A10 system, resistivity >18 MΩ cm, TOC < 5 ppb). The glassware was then left overnight in ultrapure water, then rinsed again and dried in an oven prior to use. The electrodes were cleaned as follows before their use: the RE was cleaned with dry acetonitrile and the CE was flame annealed. The WE was cleaned by polishing with aqueous slurries of successively finer grades of alumina (1.0 μm, 0.3 μm and 0.05 μm) and then rinsed with ultrapure water and MeCN, dried with a flow of argon, and then kept in an analyte solution.
X-ray crystallography
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2), a = 12.1133(5) Å, b = 12.9420(7) Å, c = 15.0302(8) Å, α = 70.115(5)°, β = 84.171(4)°, γ = 84.068(4)°, V = 2198.4(2) Å3, Z = 4, T = 100.01(10) K, μ(CuKα) = 6.008 mm−1, Dcalc = 1.500 g cm−3, 15
(no. 2), a = 12.1133(5) Å, b = 12.9420(7) Å, c = 15.0302(8) Å, α = 70.115(5)°, β = 84.171(4)°, γ = 84.068(4)°, V = 2198.4(2) Å3, Z = 4, T = 100.01(10) K, μ(CuKα) = 6.008 mm−1, Dcalc = 1.500 g cm−3, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 146 reflections measured (7.284° ≤ 2Θ ≤ 136.502°), 8029 unique reflections (Rint = 0.0314, Rsigma = 0.0440) which were used in all calculations. The final R1 was 0.0498 (I > 2σ(I)) and wR2 was 0.1326 (all data). The structure contains two crystallographically-independent molecules and also two independent molecules of acetonitrile. The hydrogen atoms bonded to N(3), O(23), N(103) and O(123) were located in the electron density map and their positions refined, with N(3)–H(3) subjected to a bond distance restraint. All remaining hydrogen atoms were fixed as riding models and the isotropic thermal parameters (Uiso) of all hydrogen atoms were based on the Ueq of the parent atom.
146 reflections measured (7.284° ≤ 2Θ ≤ 136.502°), 8029 unique reflections (Rint = 0.0314, Rsigma = 0.0440) which were used in all calculations. The final R1 was 0.0498 (I > 2σ(I)) and wR2 was 0.1326 (all data). The structure contains two crystallographically-independent molecules and also two independent molecules of acetonitrile. The hydrogen atoms bonded to N(3), O(23), N(103) and O(123) were located in the electron density map and their positions refined, with N(3)–H(3) subjected to a bond distance restraint. All remaining hydrogen atoms were fixed as riding models and the isotropic thermal parameters (Uiso) of all hydrogen atoms were based on the Ueq of the parent atom.
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2), a = 5.9641(4) Å, b = 8.3166(7) Å, c = 15.9058(16) Å, α = 98.820(7)°, β = 95.605(6)°, γ = 104.953(6)°, V = 745.39(11) Å3, Z = 2, T = 100.01(10) K, μ(CuKα) = 8.584 mm−1, Dcalc = 1.708 g cm−3, 4176 reflections measured (11.206° ≤ 2Θ ≤ 136.494°), 4176 unique reflections (Rsigma = 0.0400) which were used in all calculations. The final R1 was 0.0369 (I > 2σ(I)) and wR2 was 0.0967 (all data). The crystal was a non-merohedral twin with the two domains related by 180° about the reciprocal direction [0 0 1] with the refined percentage ratio 59.4(1)
(no. 2), a = 5.9641(4) Å, b = 8.3166(7) Å, c = 15.9058(16) Å, α = 98.820(7)°, β = 95.605(6)°, γ = 104.953(6)°, V = 745.39(11) Å3, Z = 2, T = 100.01(10) K, μ(CuKα) = 8.584 mm−1, Dcalc = 1.708 g cm−3, 4176 reflections measured (11.206° ≤ 2Θ ≤ 136.494°), 4176 unique reflections (Rsigma = 0.0400) which were used in all calculations. The final R1 was 0.0369 (I > 2σ(I)) and wR2 was 0.0967 (all data). The crystal was a non-merohedral twin with the two domains related by 180° about the reciprocal direction [0 0 1] with the refined percentage ratio 59.4(1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40.6(1). The hydrogen atom bonded to N(3) was located in the electron density map and its position refined. All remaining hydrogen atoms were fixed as riding models and the isotropic thermal parameters (Uiso) of all hydrogen atoms were based on the Ueq of the parent atom.
40.6(1). The hydrogen atom bonded to N(3) was located in the electron density map and its position refined. All remaining hydrogen atoms were fixed as riding models and the isotropic thermal parameters (Uiso) of all hydrogen atoms were based on the Ueq of the parent atom.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45.8(3).
45.8(3).
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (no. 147), a = 30.3009(5) Å, c = 5.97018(11) Å, V = 4747.12(18) Å3, Z = 18, T = 100.00(10) K, μ(CuKα) = 11.714 mm−1, Dcalc = 1.733 g cm−3, 21
(no. 147), a = 30.3009(5) Å, c = 5.97018(11) Å, V = 4747.12(18) Å3, Z = 18, T = 100.00(10) K, μ(CuKα) = 11.714 mm−1, Dcalc = 1.733 g cm−3, 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 374 reflections measured (5.834° ≤ 2Θ ≤ 144.214°), 6213 unique reflections (Rint = 0.0387, Rsigma = 0.0342) which were used in all calculations. The final R1 was 0.0445 (I > 2σ(I)) and wR2 was 0.0953 (all data). The structure contains three crystallographically-independent molecules. The hydrogen atoms bonded to O(1), O(101) and O(201) were located in the electron density map and their positions and thermal parameters were freely refined.
374 reflections measured (5.834° ≤ 2Θ ≤ 144.214°), 6213 unique reflections (Rint = 0.0387, Rsigma = 0.0342) which were used in all calculations. The final R1 was 0.0445 (I > 2σ(I)) and wR2 was 0.0953 (all data). The structure contains three crystallographically-independent molecules. The hydrogen atoms bonded to O(1), O(101) and O(201) were located in the electron density map and their positions and thermal parameters were freely refined.
CCDC 1953303–1953305, 1953307 and 1953308 contain the supplementary crystallographic data for this paper.†
Biological studies
MIAPaCa2 (85062806) pancreatic ductal adenoma cancer cells were purchased from the European Collection of Authenticated Cell Cultures. Cell culture media and supplements were purchased from Gibco (Thermo Scientific). All plasticware was purchased from Greiner Bio-One. Cells were maintained at 37 °C in a 5% CO2 humidified incubator and grown in T75 tissue culture flasks in DMEM supplemented with 10% (v/v) foetal bovine serum, 100 U mL−1 penicillin, 100 μg mL−1 streptomycin, and 2 mM L-glutamine. Cells were sub-cultured twice weekly before confluency using a standard trypsin-EDTA protocol. Cell cultures were confirmed to be free from Mycoplasma sp. contamination using the EZ-PCR mycoplasma detection kit according to the manufacturer's instructions.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
J. H. R. T. acknowledges support from the Engineering and Physical Sciences Research Council (EPSRC) for the award of a Leadership Fellowship (grant number EP/G007578/1) and the Leverhulme Trust for a research grant entitled “Expanding the range and versatility of ferrocene nucleic acids” (RPG-2013-100). The Centre for Chemical and Materials Analysis at the University of Birmingham is acknowledged for technical support. M. K. I. acknowledges the Ministry of Higher Education & Scientific Research in Iraq and The University of Kirkuk for funding and support. We thank the EPSRC UK National Crystallography Service at the University of Southampton for the collection of the crystallographic data for 15.24References
- (a) A. Singh, I. Lumb, V. Mehra and V. Kumar, Dalton Trans., 2019, 48, 2840–2860 RSC; (b) M. M. Santos, P. Bastos, I. Catela, K. Zalewska and L. C. Branco, Mini-Rev. Med. Chem., 2017, 17, 771–784 CrossRef CAS PubMed; (c) G. Gasser, I. Ott and N. Metzler-Nolte, J. Med. Chem., 2011, 54, 3–25 CrossRef CAS PubMed.
- M. Patra and G. Gasser, Nat. Rev. Chem., 2017, 1, 0066 CrossRef CAS.
- G. Jaouen, A. Vessières and S. Top, Chem. Soc. Rev., 2015, 44, 8802–8817 RSC.
- A. Vessieres, S. Top, W. Beck, E. Hillard and G. Jaouen, Dalton Trans., 2006, 529–541 RSC.
- K. Miura, M. Kinouchi, K. Ishida, W. Fujibuchi, T. Naitoh, H. Ogawa, T. Ando, N. Yazaki, K. Watanabe, S. Haneda, C. Shibata and I. Sasaki, Cancers, 2010, 2, 1717–1730 CrossRef CAS PubMed.
- S. Noble and K. L. Goa, Drugs, 1997, 54, 447–472 CrossRef CAS PubMed.
- For examples of metallocene derivatives of DNA/RNA nucleobases, see: (a) M. Daniluk, W. Buchowicz, M. Koszytkowska-Stawińska, K. Jarząbek, K. N. Jarzembska, R. Kamiński, M. Piszcz, A. E. Laudy and S. Tyski, ChemistrySelect, 2019, 4, 11130–11135 CrossRef CAS; (b) K. Kowalski, Coord. Chem. Rev., 2016, 317, 132–156 CrossRef CAS and references therein. (c) P. James, J. Neudorfl, M. Eissmann, P. Jesse, A. Prokop and H. G. Schmalz, Org. Lett., 2006, 8, 2763–2766 CrossRef CAS PubMed; (d) M. Hocek, P. Štěpnička, J. Ludvík, I. Císařová, I. Votruba, D. Řeha and P. Hobza, Chem. – Eur. J, 2004, 10, 2058–2066 CrossRef CAS PubMed.
- For recent examples of other organometallic derivatives of DNA/RNA nucleobases, see: (a) P. R. Florindo, D. M. Pereira, P. M. Borralho, M. F. M. Piedade, M. C. Oliveira, A. M. Dias, C. M. P. Rodrigues and A. C. Fernandes, New J. Chem., 2019, 43, 1195–1201 RSC; (b) M. I. P. S. Leitão, F. Herrera and A. Petronilho, ACS Omega, 2018, 3, 15653–15656 CrossRef PubMed; (c) A. Collado, M. Gómez-Gallego and M. A. Sierra, Eur. J. Org. Chem., 2018, 1617–1623 CrossRef CAS; (d) R. Kaczmarek, D. Korczynski, K. Krolewska-Golinska, K. A. Wheeler, F. A. Chavez, A. Mikus and R. Dembinski, ChemistryOpen, 2018, 7, 237–247 CrossRef CAS.
- H. V. Nguyen, A. Sallustrau, J. Balzarini, M. R. Bedford, J. C. Eden, N. Georgousi, N. J. Hodges, J. Kedge, Y. Mehellou, C. Tselepis and J. H. R. Tucker, J. Med. Chem., 2014, 57, 5817–5822 CrossRef CAS.
- J. L. Kedge, H. V. Nguyen, Z. Khan, L. Male, M. K. Ismail, H. V. Roberts, N. J. Hodges, S. L. Horswell, Y. Mehellou and J. H. R. Tucker, Eur. J. Inorg. Chem., 2017, 466–476 CrossRef CAS.
- S. Gubin, S. Smirnova, L. Denisovich and A. Lubovich, J. Organomet. Chem., 1971, 30, 243–255 CrossRef CAS.
- (a) P. Pigeon, S. Top, A. Vessieres, M. Huche, E. A. Hillard, E. Salomon and G. Jaouen, J. Med. Chem., 2005, 48, 2814–2821 CrossRef CAS PubMed; (b) H. Z. S. Lee, O. Buriez, E. Labbe, S. Top, P. Pigeon, G. Jaouen, C. Amatore and W. K. Leong, Organometallics, 2014, 33, 4940–4946 CrossRef CAS.
- For a recent example, see: S. Khanapure, M. Jagadale, P. Bansode, P. Choudhari and G. Rashinkar, J. Mol. Struct., 2018, 1173, 142–147 CrossRef CAS.
- T. Hayashi, A. Ohno, S.-J. Lu, Y. Matsumoto, E. Fukuyo and K. Yanagi, J. Am. Chem. Soc., 1994, 116, 4221–4226 CrossRef CAS.
- T. P. Smith, K. S. Kwan, H. Taube, A. Bino and S. Cohen, Inorg. Chem., 1984, 23, 1943–1945 CrossRef CAS.
- Y. S. Sohn, A. W. Schluete, D. N. Hendrick and H. B. Gray, Inorg. Chem., 1974, 13, 301–304 CrossRef CAS.
- (a) R.-J. Gale and R. Job, Inorg. Chem., 1981, 20, 42–45 CrossRef CAS; (b) T. Kuwana, D. E. Bublitz and G. Hoh, J. Am. Chem. Soc., 1960, 82, 5811–5817 CrossRef CAS.
- J. C. Swarts, A. Nafady, J. H. Roudebush, S. Trupia and W. E. Geiger, Inorg. Chem., 2009, 48, 2156–2165 CrossRef CAS PubMed.
- R. J. LeSuer, C. Buttolph and W. E. Geiger, Anal. Chem., 2004, 76, 6395–6401 CrossRef CAS.
- S. Trupia, A. Nafady and W. E. Geiger, Inorg. Chem., 2003, 42, 5480–5482 CrossRef CAS.
- (a) K. C. Kemp, E. Fourie, J. Conradie and J. C. Swarts, Organometallics, 2008, 27, 353–362 CrossRef CAS; (b) C. Amatore, M. Gareil and J. Savéant, J. Electroanal. Chem. Interfacial Electrochem., 1983, 147, 1–38 CrossRef CAS.
- S. Kukharenko, V. Strelets, A. Kudinov, A. Kreidlin, M. Peterleitner, L. Denisovich and M. Rybinskaya, J. Organomet. Chem., 1996, 519, 1–5 CrossRef CAS.
- (a) M. Watanabe, M. Sato, A. Nagasawa, M. Kai, I. Motoyama and T. Takayama, Bull. Chem. Soc. Jpn., 1999, 72, 715–723 CrossRef CAS; (b) K. Hashidzume, H. Tobita and H. Ogino, Organometallics, 1995, 14, 1187–1194 CrossRef CAS.
- S. J. Coles and P. A. Gale, Chem. Sci., 2012, 3, 683–689 RSC.
- C. Biot, N. Chavain, F. Dubar, B. Pradines, X. Trivelli, J. Brocard, I. Forfar and D. Dive, J. Organomet. Chem., 2009, 694, 845–854 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1953303–1953305, 1953307 and 1953308. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/C9DT04174E |
| ‡ The X-ray structures of the chiral compounds were determined from crystals grown from solutions of racemic mixtures (see the ESI† for more details). |
| § The enantiomer 1-(R,Sp)-Ru, isolated from a racemic batch of the target compound (see ESI†), was found to be even less toxic, with an IC50 of >80 μM, the highest concentration investigated. These data suggest that stereochemistry also plays a significant role in determining the anticancer activity of these ferronucleosides. This aspect is currently being investigated further and will be included in a future report. |
| This journal is © The Royal Society of Chemistry 2020 |