Isolation of the novel example of a monomeric organotellurinic acid†
Abstract
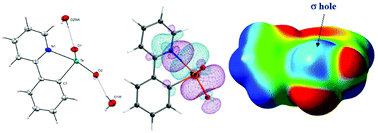
The stoichiometrically controlled alkaline hydrolysis of 4, (ppy)TeCl3, [ppy = 2-(2′-pyridyl)phenyl] afforded the partially hydrolyzed μ-oxo-bridged dinuclear telluroxane 5, [(ppyTeCl2)2(μ-O)] and a novel example of an Intramolecular Chalcogen Bonding (IChB) stabilized, monomeric organotellurinic acid 6, (ppy)Te(O)OH. The oxidation of diaryl ditelluride 7, (ppyTe)2 using H2O2 resulted in the isolation of μ-oxo-bridged dimethyl ester 8, [(ppy)Te(O)(OH)(OMe)]2(O). The molecular structures of 4–6 and 8 are unambiguously authenticated by single crystal X-ray diffraction studies. The electronic structure of monomeric tellurinic acid 6 is investigated using DFT calculations. The Natural Bond Order (NBO) analysis, in corroboration with Atoms in Molecules (AIM) analysis reveals that tellurinic acid 6 is stabilized by σ-hole participation of the tellurium atom with the pyridyl N-atom resulting in strong electrostatic interactions between the N and Te atoms.


 Please wait while we load your content...
Please wait while we load your content...