Cu carbonyls enhance the performance of Ru-based SILP water–gas shift catalysts: a combined in situ DRIFTS and DFT study†
Dominik
Blaumeiser‡
a,
Robert
Stepić‡
bd,
Patrick
Wolf
c,
Christian R.
Wick
be,
Marco
Haumann
 c,
Peter
Wasserscheid
cf,
David M.
Smith
d,
Ana-Sunčana
Smith
bde,
Tanja
Bauer
c,
Peter
Wasserscheid
cf,
David M.
Smith
d,
Ana-Sunčana
Smith
bde,
Tanja
Bauer
 *a and
Jörg
Libuda
*a and
Jörg
Libuda
 a
a
aLehrstuhl für Katalytische Grenzflächenforschung, Erlangen Catalysis Resource Center, Friedrich-Alexander-Universität Erlangen-Nürnberg, Egerlandstraße 3, D-91058 Erlangen, Germany. E-mail: tanja.tb.bauer@fau.de
bPULS Group, Physics Department and Interdisciplinary Center for Nanostructured Films, Friedrich-Alexander-Universität Erlangen-Nürnberg, Staudtstraße 7, D-91058 Erlangen, Germany
cLehrstuhl für Chemische Reaktionstechnik, Friedrich-Alexander-Universität Erlangen-Nürnberg, Egerlandstraße 3, D-91058 Erlangen, Germany
dGroup for Computational Life Sciences, Division of Physical Chemistry, Ruđer Bošković Institute, Bijenička cesta 54, HR-10002 Zagreb, Croatia
eCentral Institute for Scientific Computing (ZISC), Friedrich-Alexander-Universität Erlangen-Nürnberg, Martensstraße 5a, D-91058 Erlangen, Germany
fForschungszentrum Jülich, Helmholtz-Institute Erlangen-Nürnberg for Renewable Energy (IEK 11), Egerlandstraße 3, D-91058 Erlangen, Germany
First published on 27th November 2019
Abstract
Ru-based supported ionic liquid phase (SILP) catalysts efficiently promote the water–gas shift reaction (WGSR). The addition of CuCl further improves the performance. This is attributed to the formation of labile Cu carbonyl species, which act as CO shuttles in the ionic liquid (IL) phase. We apply in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) to monitor the formation of these Cu carbonyls in an imidazolium-based SILP system. To aid the identification of the relevant Cu carbonyls, we perform density functional theory (DFT) calculations. In the present study, we investigate [Ru(CO)3Cl2]2/CuCl/[C4C1C1Im]Cl/Al2O3 and CuCl/[C4C1C1Im]Cl/Al2O3 with various CuCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL ratios. The relevant Cu carbonyls are identified as neutral CuCl monomers and dimers, as well as monoanionic monomers and dimers. Moreover, we demonstrate that the ratio of the individual Cu carbonyl species depends on the CuCl
IL ratios. The relevant Cu carbonyls are identified as neutral CuCl monomers and dimers, as well as monoanionic monomers and dimers. Moreover, we demonstrate that the ratio of the individual Cu carbonyl species depends on the CuCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL ratio.
IL ratio.
1. Introduction
The water–gas shift reaction (WGSR) is used in a large number of technical applications. Prominent examples are ammonia synthesis and the production of high purity H2, where the purpose is to reduce the amount of CO and to increase the amount of H2 produced.1–3 The WGSR is the mildly exothermic (≃41 kJ mol−1), reversible conversion of H2O and CO into CO2 and H2.3,4 The most prominent catalysts applied at intermediate temperatures (∼300–450 °C) are based on supported Fe oxides. Catalysts based on supported Cu oxides are usually employed in the WGSR at lower temperatures (∼200–250 °C).3–5 Moreover, the application of supported metals such as Au,6 Pt,7 or Ru5 was also reported in the literature. Due to the exothermic nature of the WGSR, it is desirable to shift the reaction conditions to even lower temperatures to obtain a product gas with maximum H2 and minimal CO contents.A decade ago, Ru-based supported ionic liquid phase (SILP) systems were shown to catalyze the WGSR at ultra-low temperatures (<200 °C) and under ambient conditions.8 Werner et al. demonstrated improved catalytic activity of a RuCl3 precursor dissolved in the ionic liquid (IL) 1-butyl-2,3-dimethylimidazolium trifluoromethane sulfonate ([C4C1C1Im][OTf]) supported on silica as compared to that of a commercial Cu-based catalyst.8 However, this improvement was observed only after an induction period (∼80 h). The induction period was attributed to the formation of active Ru chloro carbonyl species during the first stages of the reaction.9 Indeed, the system immediately reached full catalytic activity by using [Ru(CO)3Cl2]2 as an active complex in the SILP. Thus, Werner et al. concluded that the active species is based on a Ru chloro carbonyl.10
In a follow-up study, Ru carbonyls formed from a [Ru(CO)3Cl2]2 precursor in a 1-butyl-2,3-dimethylimidazolium chloride ([C4C1C1Im]Cl)-based SILP catalyst were investigated by in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS). Bauer et al. assigned different Ru carbonyls by comparison with spectra calculated from density functional theory (DFT). Additionally, they demonstrated that an equilibrium of different carbonyl–chloro–ruthenium species is present in the IL.11 Under realistic reaction conditions, the equilibrium was found to be dominated by a monomeric [Ru(CO)3Cl3]− species. A reaction mechanism was suggested based on this species for this very efficient WGSR catalyst.12
Recently, we showed that the performance of the [Ru(CO)3Cl2]2 SILP system can be further improved with metal chloride additives.13 The screening of different additives showed that the highest enhancement of the catalytic activity was achieved with CuCl. This was attributed to the higher availability of CO in the IL phase caused by the transfer of CO to the active Ru-species via a Cu-carbonyl acting as a ‘CO shuttle’. The presence of different [CuxCly]z species in ILs,14–19 as well as their capability to bind CO, was reported previously.15,18 Indeed, we were able to prove the formation of several Cu carbonyls from chlorocuprates by the use of in situ DRIFTS and DFT calculations in our recent work.13
We expect that the formation of a certain Cu carbonyl species from [CuxCly]z will depend on the amount of Cl− in the SILP system. In the present work, we focus on the effect of varying the CuCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL ratio and the corresponding changes in activity. In particular, we aim to understand the role and the nature of Cu carbonyl complexes in different SILP environments. To this end, we initially compare the catalytic activity of [Ru(CO)3Cl2]2/CuCl/[C4C1C1Im]Cl/Al2O3 samples (CuCl
IL ratio and the corresponding changes in activity. In particular, we aim to understand the role and the nature of Cu carbonyl complexes in different SILP environments. To this end, we initially compare the catalytic activity of [Ru(CO)3Cl2]2/CuCl/[C4C1C1Im]Cl/Al2O3 samples (CuCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL = 0.2, 0.5, 1.2 and a CuCl-free benchmark) during the WGSR. In the in situ DRIFTS experiments, the corresponding Ru-free samples were investigated under a CO atmosphere, as the presence of carbonyl–chloro–ruthenium complexes leads to broad vibrational features. These may cover the peaks originating from the Cu carbonyls. Furthermore, a specific data treatment procedure was applied to subtract the CO gas phase signals from the IR spectra and reveal the carbonyl peaks of the CO-containing complexes. The carbonyl bands were assigned with the help of IR spectra obtained from DFT. The combination of our experimental and theoretical investigations allows us to identify neutral Cu carbonyl monomers and dimers, as well as monoanionic Cu carbonyl monomers and dimers in [C4C1C1Im]Cl and to connect the presence of these complexes with the changes in catalytic performance.
IL = 0.2, 0.5, 1.2 and a CuCl-free benchmark) during the WGSR. In the in situ DRIFTS experiments, the corresponding Ru-free samples were investigated under a CO atmosphere, as the presence of carbonyl–chloro–ruthenium complexes leads to broad vibrational features. These may cover the peaks originating from the Cu carbonyls. Furthermore, a specific data treatment procedure was applied to subtract the CO gas phase signals from the IR spectra and reveal the carbonyl peaks of the CO-containing complexes. The carbonyl bands were assigned with the help of IR spectra obtained from DFT. The combination of our experimental and theoretical investigations allows us to identify neutral Cu carbonyl monomers and dimers, as well as monoanionic Cu carbonyl monomers and dimers in [C4C1C1Im]Cl and to connect the presence of these complexes with the changes in catalytic performance.
2. Sample preparation and experimental methods
2.1 Preparation of the SILP systems under investigation
The SILP systems were prepared in a standard Schlenk flask under an argon atmosphere at ambient pressure and room temperature. In the first step, [C4C1C1Im]Cl (Merck KGaA, LOT: 99/818) was dissolved in dichloromethane (DCM; Sigma-Aldrich, LOT: SZBG073AV) and stirred for 10–15 min. For Ru-based SILP catalysts, the Ru precursor complex [Ru(CO)2Cl3]2 (Alfa Aesar, LOT: X06C034) was added to the mixture dissolved in DCM and stirred until full dissolution (approx. 10–15 min). For all Ru-free samples, this step was skipped.In the case of CuCl addition, CuCl (Merck KGaA: CuCl, LOT: A999739833, ≥97.0%) was added with an additional 10 mL of DCM and the resulting mixture was stirred for 60–90 min. In the next step, alumina (γ-Al2O3, Sasol Germany GmbH, LOT: B39598) was added as a support material to the mixture and the suspension was stirred shortly for 2–5 min to avoid mechanical stress of the support material. In the last step, the solvent was removed using a rotary evaporator (step 1: 3 h, 40 °C, 900 mbar; step 2: 1 h, 40 °C, <3 mbar). Finally, a free-flowing SILP catalyst was obtained. The Ru-loading of the Ru-based SILP catalysts was wRu = 0.02 gRu gsupport−1. The pore filling grade of the support was α = 0.34, which describes the IL volume related to the total pore volume of the support material (VIL Vsupport−1).
The catalytic tests were performed with the Ru-based SILP systems. Here, the molar amount of CuCl to IL (in molCuCl LIL−1) was varied between 0.5 and 8 M equaling to a molar ratio χ (in molCuCl molIL−1) in the range of 0.04–1.4. In the following, we refer to the molar ratio as χ = n(CuCl)/n(IL). For the IR measurements, Ru-free samples were used with a molar amount of CuCl to IL between 2 and 6 M, equaling to a molar ratio χ in the range of 0.3–1. In addition, pristine CuCl/Al2O3 was used as a reference.
2.2 CO pulse chemisorption
To investigate the CO uptake, a pulse chemisorption setup (AutoChem 2920 device from Micromeritics®) was used. Approximately 0.3–0.4 g of the Ru-based SILP catalysts (with and without CuCl) was placed in a U-bend quartz glass tube. With the help of a bed of glass wool, a possible discharge of the catalyst was prevented. Placing the catalyst bed in the isothermal zone of an oven guaranteed a uniform temperature field. Prior to the chemisorption measurements, the sample was heated to 130 °C with a heating rate of 5 °C min−1 under a helium flow of 20 mLN min−1. Once the measurement conditions were reached, the sample was dried for 80 min before the pulse experiment was started with a sample loop volume of 0.5 mLN of CO. Following every single pulse, a lag time of 6 minutes was maintained. The obtained thermal conductivity detector (TCD) signal of the eluting CO (as area and peak height) can directly be related to the amount of CO adsorbed by the sample under standard conditions (cmN,CO3 gsample−1). The measurement is considered complete when two sequential peaks have the same area and height.2.3 Catalyst testing
The evaluation of the SILP catalysts was performed in a fixed-bed reactor setup (1.4571 stainless steel) with an inner diameter of 10 mm and a total volume of 26 mL. The reactor together with its peripheral structures is depicted elsewhere.13 CO (Linde, >99.97%) and N2 (Linde, >99.999%) were dosed via mass flow controllers (MFCs) from Bronkhorst. H2O was dosed with a liquid flow controller and mixed with N2 utilizing a controlled evaporator mixer (CEM) unit from Bronkhorst. The gas mixture consisting of N2, CO and H2O can either be directed through a bypass-line (for reference measurement purposes) or through the fixed-bed reactor. The catalyst samples were placed on a 0.5 μm frit to avoid particles from entering the downstream section. After the reactor or the bypass-line, respectively, the gas mixture passes a condenser unit (T = 1.5 °C) to remove excess moisture from the stream. Finally, the gas composition was analyzed by an Emerson X-Stream gas analyzer based on IR technology, which allows for in situ analysis of the gas mixture. A detailed explanation of the evaluation of the raw data can be found elsewhere.13Due to the fact that the amount of IL is fixed with the pore filling grade, the amount of CuCl is related to that of the IL and given as molarity value M. For a better understanding, the conversion between molarities and the molar ratio CuCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL χ is given in Table 1. Additionally, the molar ratio CuCl
IL χ is given in Table 1. Additionally, the molar ratio CuCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ru ξ is calculated as well. To account for the catalytic activity of the different samples, the turnover frequency (TOF) for the WGSR was calculated for each sample. We use the data from a temperature dependent experiment at a constant feed flow and composition. Each temperature was held for 5 h before returning to the reference temperature of 120 °C afterwards.20 This allows us to observe possible deactivation phenomena. Normally, the TOF would be calculated as TOF = ṅproduct/ncatalyst. However, the molar flow of the product cannot be determined with high accuracy. This is due to the condensation of water before the dry gas flow passes the online IR analyzer. Thus, we calculated the TOF based on the CO flow as TOF = ṅsubstrate × X/ncatalyst, where X is the conversion. For the molar amount of catalyst, we used the total amount of ruthenium immobilized on the catalyst. Copper is not considered as it was not catalytically active in the SILP, as shown recently.13 The data points (one data point per 30 s) were averaged over a stable operation regime of 30 minutes to obtain the TOF at the respective temperature.
Ru ξ is calculated as well. To account for the catalytic activity of the different samples, the turnover frequency (TOF) for the WGSR was calculated for each sample. We use the data from a temperature dependent experiment at a constant feed flow and composition. Each temperature was held for 5 h before returning to the reference temperature of 120 °C afterwards.20 This allows us to observe possible deactivation phenomena. Normally, the TOF would be calculated as TOF = ṅproduct/ncatalyst. However, the molar flow of the product cannot be determined with high accuracy. This is due to the condensation of water before the dry gas flow passes the online IR analyzer. Thus, we calculated the TOF based on the CO flow as TOF = ṅsubstrate × X/ncatalyst, where X is the conversion. For the molar amount of catalyst, we used the total amount of ruthenium immobilized on the catalyst. Copper is not considered as it was not catalytically active in the SILP, as shown recently.13 The data points (one data point per 30 s) were averaged over a stable operation regime of 30 minutes to obtain the TOF at the respective temperature.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL (χ) and CuCl
IL (χ) and CuCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ru (ξ)
Ru (ξ)
| Molarity M | CuCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL (χ) IL (χ) |
CuCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ru (ξ) Ru (ξ) |
|---|---|---|
| molCuCl LIL−1 | molCuCl molIL−1 | molCuCl molRu−1 |
| 0 (benchmark) | 0.00 | 0.00 |
| 1 | 0.17 | 0.77 |
| 3 | 0.52 | 2.31 |
| 5 | 0.87 | 3.86 |
| 7 | 1.22 | 5.40 |
2.4 DRIFTS setup and experimental procedures
All DRIFTS measurements were performed using a Bruker Vertex 80v Fourier transform infrared (FTIR) spectrometer. This spectrometer is equipped with a KBr beam splitter and a liquid-N2 cooled HgCdTe-detector. The DRIFTS measurements were conducted in a home-built reactor system, which was described previously.11,21 The system is equipped with a high temperature reactor chamber and Praying Mantis diffuse reflectance accessory (both from Harrick). The reactor system is placed in the sample compartment of the spectrometer and supplies all the electrical and gas dosing feedthroughs. Thus, the sample compartment and the optical path are evacuated during the IR measurements. This results in an outstanding stability, also throughout long-term experiments.The DRIFTS reactor is equipped with two CaF2 windows and a type K thermocouple, which is in direct contact with the powder sample. All DRIFT spectra were recorded with an acquisition time of 1 min and a spectral resolution of 2 cm−1. A gas dosing system is connected to the DRIFTS reactor and allows dosage of five different gases. In the present study, Ar (Linde, >99.999%) and CO (Linde, >99.997%) were dosed. Ar was used without further purification. CO was fed through a carbonyl trap (Gaskleen® II Purifier from Pall Corporation) to supply a gas feed, which is free of metal carbonyls. Mass flow controllers (MFCs, Bronkhorst) control the gas dosing at flow rates up to 20 mLN min−1. The pressure in the gas lines can be adjusted by three pressure controllers (PCs, Bronkhorst) between 1 mbar and 20 bar. Finally, all devices run in a remote-controlled fashion, which enables us to perform long-term experiments with high reproducibility.
An identical experimental procedure was applied to all samples. Prior to the measurements, the samples were purged with Ar (2 mLN min−1, 1 bar) overnight at 30 °C to remove water residues. Under these conditions, a background spectrum (acquisition time: 2 min) was recorded. Subsequently, the reactor was evacuated (∼1 mbar, 1 min) and the procedure was started. The complete procedure is illustrated in Fig. 1.
 | ||
| Fig. 1 Schematic illustration of the experimental procedure: two consecutive heating/cooling cycles including CO dosing, evacuation and Ar purging at every temperature step. | ||
Two identical and consecutive heating/cooling cycles were performed from 30 °C to 120 °C and back to 30 °C in steps of 30 °C. Only one heating/cooling cycle was performed for the samples with molar ratios χ = 0.3 and 0.7. At each temperature step, the sample was exposed to CO (1 bar, 9 min), evacuated (∼1 mbar, 1 min) and purged with Ar (1 bar, 10 min). The Ar atmosphere was maintained while heating/cooling (3 °C min−1) to the next temperature step.
2.5 Post-data treatment
All IR spectra presented below were treated identically. Heating of a sample causes changes in the reflectivity. Thus, all spectra were normalized to compensate for this effect.22 Subsequently, the spectra were baseline (BL) corrected. In a previous work, we observed that the Cu carbonyls formed are highly labile and decompose rapidly upon evacuation.13 To account for this, we use a specific data treatment procedure to subtract the CO gas phase signals from the spectra. This procedure has already been described and successfully applied in previous publications.21,23 The procedure is illustrated in Fig. 2 and the details are given in the following. In summary, this procedure enables us to study Cu carbonyls in the presence of a CO gas phase and under steady state conditions. Please note that the top left panel in Fig. 2 includes spectra recorded during evacuation and purging where carbonyl bands are even visible without the data treatment procedure.In the first step of the procedure, the as-recorded spectra and a suitable reference spectrum are Fourier transformed. The reference system should be similar to the system investigated and CO must not adsorb. Thus, we chose Al2O3 as a reference system. After Fourier transformation (FT), a low-pass filter (LPF) is applied. The filter removes the high frequency components, i.e. the fine structure of the CO gas phase features.21,23 This results in spectra, which resemble the contour of the gas phase signals. In the following step, these spectra are normalized and the reference is subtracted. Finally, BL correction yields the resulting spectra, where the carbonyl peaks are clearly visible. The same procedure was applied to all IR spectra shown in this work and is outlined schematically in Fig. 2.
2.6 DFT calculations
The DFT calculations were carried out using the ORCA24 software package. The RI-BP8625–27 functional was used in combination with D3 dispersion corrections28 and the def2-TZVPPD29,30 basis set, which incorporates diffuse functions required for calculations on anionic species. This approach has been successfully applied to similar systems.12 The resolution of identity approximation was used to speed up the calculations of the Coulomb part in combination with the def2/J auxiliary basis set.31 Optimizations were done without any constraints using the CPCM32 model with a dielectric constant of 12 to represent the IL.33,34 Vibrational frequencies were determined using numerical derivatives of the energy as implemented in ORCA. The bond order was extracted using the Mayer35,36 model.3. Results and discussion
3.1 Catalytic tests and CO chemisorption studies
To compare the catalytic activity of the different SILP materials, the WGSR TOF was calculated for each system. A temperature variation experiment at constant feed flows and compositions is the basis for this evaluation. Fig. 3 shows selected results from different catalyst samples with various CuCl loadings. In this plot, the benchmark system without CuCl is indicated with a dashed line for better comparability.There is a strong correlation between the CuCl loading of the IL film and the catalytic activity with pronounced activation effects for a wide range of CuCl loadings (Fig. 3). While the 1 M catalyst sample (with molar CuCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL ratio χ = 0.17) shows improvements in activity of +9% at 120 °C and +10% at 140 °C, the 3 M SILP catalyst (χ = 0.52) leads to improvements of +27% at 120 °C and +18% at 140 °C. Further addition of CuCl, however, results in the loss of the beneficial effect, as visible for the 7 M (χ = 1.22) sample. Here, we observed losses of activity of −64% at 120 °C and −42% at 140 °C.
IL ratio χ = 0.17) shows improvements in activity of +9% at 120 °C and +10% at 140 °C, the 3 M SILP catalyst (χ = 0.52) leads to improvements of +27% at 120 °C and +18% at 140 °C. Further addition of CuCl, however, results in the loss of the beneficial effect, as visible for the 7 M (χ = 1.22) sample. Here, we observed losses of activity of −64% at 120 °C and −42% at 140 °C.
The observed trend can be attributed to a change of the Lewis acid–base behavior of the different chlorocuprate species, which are formed upon CuCl addition to the IL. Depending on the molar CuCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL ratio χ, different complexes evolve, which are in dynamic equilibrium with each other.15 SILP catalysts with comparatively low CuCl loading contain primarily Lewis-basic species, such as [CuCl2]− or [CuCl3]2−. On the other hand, higher CuCl concentrations lead to the formation of Lewis-acidic species, e.g. [Cu2Cl3]− or [Cu3Cl4]−.14–17,37 Such equilibria, generating either chloride-rich (Lewis-basic) or chloride-poor (Lewis-acidic) environments, have been reported previously for a selection of other halometallate IL systems.37–39 The catalytic tests showcased in Fig. 3 and our previous results indicate that a Lewis basic environment is beneficial to the performance of the WGSR SILP catalysts.13
IL ratio χ, different complexes evolve, which are in dynamic equilibrium with each other.15 SILP catalysts with comparatively low CuCl loading contain primarily Lewis-basic species, such as [CuCl2]− or [CuCl3]2−. On the other hand, higher CuCl concentrations lead to the formation of Lewis-acidic species, e.g. [Cu2Cl3]− or [Cu3Cl4]−.14–17,37 Such equilibria, generating either chloride-rich (Lewis-basic) or chloride-poor (Lewis-acidic) environments, have been reported previously for a selection of other halometallate IL systems.37–39 The catalytic tests showcased in Fig. 3 and our previous results indicate that a Lewis basic environment is beneficial to the performance of the WGSR SILP catalysts.13
In order to compare the CO uptakes of the SILP catalysts with various CuCl loadings, CO pulse chemisorption experiments were performed. The results depicted in Fig. 4 reveal a maximum CO uptake at χ = 0.7 (4 M), which matches the maximum of the activity improvement reported in our previous work.13 The benchmark system showed an initial CO sorption of 0.92 mLN g−1, whereas the value of the maximum uptake can be nearly tripled to 2.71 mLN g−1 for χ = 0.7 (4 M). This affirms our assumption that the predominance of the Lewis-basic chlorocuprate species leads to increased availability of CO in the IL phase. This is most likely due to the formation of Cu carbonyl species, which efficiently shuttle CO to the active Ru species in the SILP.
3.2 CO adsorption studied by DRIFTS
We apply DRIFTS to identify Cu carbonyls present in the CuCl/[C4C1C1Im]Cl/Al2O3 SILP systems with χ = 0.3, 0.5, 0.7, 1.0 and a pristine CuCl/Al2O3 sample. In the IR measurements, we investigate exclusively the Ru-free SILP systems because the presence of Ru carbonyls results in strong CO adsorption peaks, which interfere with the assignment of the Cu carbonyl bands. | ||
| Fig. 5 Results of the first heating/cooling cycle: a–e: offset DRIFT spectra; f: integrated peak areas during cooling from 120 to 30 °C, supported with guide to the eye curves. | ||
In Fig. 5e, the spectra obtained from the pristine CuCl/Al2O3 sample are displayed. They show a different behavior during the experiment. At 30 °C, the same peaks as compared to those of the other samples are visible (2132, 2121, 2080, 2072 and 2044 cm−1) and decrease slightly upon heating. However, no increase of the peak areas at <2100 cm−1 is observed upon cooling back to room temperature. Instead, the peak areas remain the same. The most pronounced peaks obtained from the CuCl/Al2O3 sample are the features located at >2100 cm−1 (highlighted in green). The peak intensities for all samples are affected by the temperature but the peaks are, nevertheless, clearly visible at all temperatures.
We assign the peaks on the basis of literature and DFT calculations. The variety of peaks in the IR spectra indicates that different [CuxCly]z carbonyls are present in the liquid phase of the WGSR catalyst, which is in good agreement with the literature.14–18 Huang et al. investigated CuCl dissolved in [C4C1Im]Cl by fast atom bombardment mass spectrometry (FAB-MS). They identified several monoanionic [CuxCly]− species, such as [CuCl2]−, [Cu2Cl3]− and [Cu3Cl4]−.16 David et al. investigated supported IL membranes (SILMs), which are based on CuCl dissolved in [C6C1Im]Cl and are suitable for the recovery of CO from a gas stream.15 They gave an overview about several [CuxCly]z− species, the stoichiometry of which depends on the molar ratio χ between CuCl and IL.14–17,19 They concluded that [CuCl3]2− is the predominant species at χ = 0–1 and, moreover, that this species is responsible for the binding of CO.
The DFT calculations build on our previous work,13 but this time we also estimate the influence of the IL on the CO stretching frequency by using a continuum solvation model (CPCM).40 With this, a high level of accuracy is achieved for the calculated peak positions (Δν(exp. − cal.) < ±20 cm−1). For a detailed assignment of the bands, we refer to Table 2 where a summary of all calculated and experimental peak positions is given. For the assignment, we use the spectra at 30 °C after cooling because here the peaks are the largest and individual contributions are visible the best.
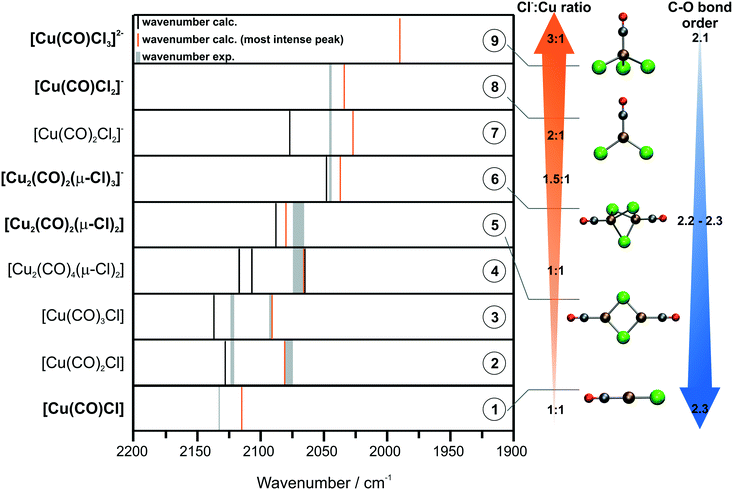
| Species | ν(CO) (DFT)/cm−1 | ν(CO) (exp.)/cm−1 | Δν (exp. − calc.)/cm−1 | |
|---|---|---|---|---|
| 1 | [Cu(CO)Cl] | 2115 | 2132–2131 | ∼17 |
| 2 | [Cu(CO)2Cl] | 2128, 2081 | 2121–2120, 2092–2090, 2080–2073 | ∼8, ∼11, ≃8 |
| 3 | [Cu(CO)3Cl] | 2137, 2091 | 2121–2120, 2092–2090, 2080–2073 | ≃17, ∼1, ≃18 |
| 4 | [Cu2(CO)4(μ-Cl)2] | 2117, 2107, 2066, 2065 | 2073–2064 | ∼7 |
| 5 | [Cu2(CO)2(μ-Cl)2] | 2088, 2080 | 2073–2064 | ≃16 |
| 6 | [Cu2(CO)2(μ-Cl)3]− | 2048, 2037 | 2044–2042 | ∼7 |
| 7 | [Cu(CO)2Cl2]− | 2077, 2027 | 2044–2042 | ∼17 |
| 8 | [Cu(CO)Cl2]− | 2034 | 2044–2042 | ∼10 |
| 9 | [Cu(CO)Cl3]2− | 1990 | — | — |
According to the work of Haakansson and Jagner, we attribute the band at 2132–2131 cm−1 to CO bound to a neutral CuCl monomer (species 1).41 Based on DFT calculations, the peaks at 2121–2120 cm−1, 2092–2090 cm−1 and 2080–2073 cm−1 are assigned to neutral monomers with two or three CO molecules bound, respectively (species 2 and 3). The peaks at 2073–2064 cm−1 are attributed to CO bound to neutral dimers with bridging chloride ligands (species 4 and 5). Finally, we assign the contributions at 2044–2042 cm−1 to monoanionic monomers and dimers, which bind CO (species 6–8). Huang et al. already identified the corresponding chlorocuprates (species 6–8; without CO) by FAB-MS.16 Based on the assumptions of David et al., we also derived the peak position for [Cu(CO)Cl3]2−.15 However, the calculated peak position of 1990 cm−1 (species 9) agrees only poorly with the experimental data. Thus, we exclude the possibility that this carbonyl is present in our systems. The color code introduced in Fig. 5 illustrates the carbonyl speciation in each sample. According to our assignment and Table 2, features at >2100 cm−1 (highlighted in green) correspond to neutral CuCl monomers with up to three CO ligands (species 1–3). In contrast, neutral CuCl dimers and monoanionic monomers and dimers (species 4–8) correspond to the peaks labeled in blue.
For the sake of clarity, the peak assignments are depicted schematically in Fig. 6. The Cu carbonyls are listed by the Cl−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio in the complex. The peak positions derived by DFT are indicated by black and orange lines, where orange indicates the most intense peak. The assigned, experimentally observed peak positions are shown as grey lines. Concerning the peak positions of the different CuCl species, a clear trend is visible in Fig. 6. Both experimental and calculated peak positions undergo a shift to lower wavenumbers with increasing Cl−
Cu ratio in the complex. The peak positions derived by DFT are indicated by black and orange lines, where orange indicates the most intense peak. The assigned, experimentally observed peak positions are shown as grey lines. Concerning the peak positions of the different CuCl species, a clear trend is visible in Fig. 6. Both experimental and calculated peak positions undergo a shift to lower wavenumbers with increasing Cl−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio in the Cu complex. This is rationalized by electronic effects arising from the interplay of the Cl− and CO ligands: Cl− is considered as a weak ligand exhibiting a π-donor effect. In contrast, CO is considered as a strong ligand and exhibits a π-accepting effect.42 An increasing Cl−
Cu ratio in the Cu complex. This is rationalized by electronic effects arising from the interplay of the Cl− and CO ligands: Cl− is considered as a weak ligand exhibiting a π-donor effect. In contrast, CO is considered as a strong ligand and exhibits a π-accepting effect.42 An increasing Cl−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio indicates a higher partial charge on the Cu center and, hence, a higher tendency for backbonding from Cu to the available CO ligands.
Cu ratio indicates a higher partial charge on the Cu center and, hence, a higher tendency for backbonding from Cu to the available CO ligands.
According to the Blyholder model, more π-backbonding to CO results in weakening of the C–O bond. This is due to the donation of electron density to the antibonding 2π* orbital of the CO molecule.43 The actual amount of backbonding strongly depends on the electronic environment of the Cu(I) center. This can even lead to the so-called nonclassical Cu carbonyl species, which show ν(CO) values higher than those in free CO.44,45 A useful indicator to discern chemical bonding in inorganic and organometallic systems based on quantum mechanical calculations is the Mayer bond order (MBO).35,46,47 In order to obtain an insight into the CO bond strength of the species 1–9 and to underline our general assumptions based on the Blyholder model, we computed the MBO at the same level of theory for the optimized structures of the model species listed in Fig. 6. A more detailed summary of the MBOs and associated bond distances can be found in the ESI.† Indeed, the MBOs of the CO bonds roughly follow the trend of decreasing bond orders for increasing Cl−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratios in the Cu complexes. While the lowest MBO of 2.1 is predicted for the singly and doubly negatively charged species (7 and 9), the highest MBO across the series is computed to be 2.3 for the neutral monomers (1–3). Furthermore, we observe a smooth transition between those extremes moving along the series of dimeric species (4–6). However, the computed bond orders are in a very narrow range and should only be considered as purely qualitative indicators. Nevertheless, they indicate weakening of the CO bond presumably due to increasing π-backbonding. This effect can be observed in a shift of the C–O stretching frequency to lower wavenumbers,43 which is in line with our experimental and theoretical findings.
Cu ratios in the Cu complexes. While the lowest MBO of 2.1 is predicted for the singly and doubly negatively charged species (7 and 9), the highest MBO across the series is computed to be 2.3 for the neutral monomers (1–3). Furthermore, we observe a smooth transition between those extremes moving along the series of dimeric species (4–6). However, the computed bond orders are in a very narrow range and should only be considered as purely qualitative indicators. Nevertheless, they indicate weakening of the CO bond presumably due to increasing π-backbonding. This effect can be observed in a shift of the C–O stretching frequency to lower wavenumbers,43 which is in line with our experimental and theoretical findings.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL molar ratios χ. In the spectra shown in Fig. 5a–d, we clearly observe that the peaks at <2100 cm−1 (highlighted in blue) grow for the IL-containing samples upon cooling to 30 °C, which is not the case for the pristine CuCl/Al2O3 sample (Fig. 5e). In Fig. 5f, the integration of the peak areas at wavenumbers <2100 cm−1 is shown for all samples during cooling from 120 to 30 °C. This demonstrates again that the peak areas for all IL-containing samples increase upon cooling, whereas the peak area remains constant during CO dosing on CuCl/Al2O3. Thus, the formation of these Cu species is clearly related to the presence of [C4C1C1Im]Cl. In the IL phase, CuCl molecules bind Cl− ions and/or form dimers. This promotes the formation of the Cu carbonyls (species 4–8) assigned above, which are in equilibrium with each other. A similar dynamic equilibrium was already reported for a CuCl-free [Ru(CO)3Cl2]2/[C4C1C1Im]Cl/Al2O3 WGS catalyst.11
IL molar ratios χ. In the spectra shown in Fig. 5a–d, we clearly observe that the peaks at <2100 cm−1 (highlighted in blue) grow for the IL-containing samples upon cooling to 30 °C, which is not the case for the pristine CuCl/Al2O3 sample (Fig. 5e). In Fig. 5f, the integration of the peak areas at wavenumbers <2100 cm−1 is shown for all samples during cooling from 120 to 30 °C. This demonstrates again that the peak areas for all IL-containing samples increase upon cooling, whereas the peak area remains constant during CO dosing on CuCl/Al2O3. Thus, the formation of these Cu species is clearly related to the presence of [C4C1C1Im]Cl. In the IL phase, CuCl molecules bind Cl− ions and/or form dimers. This promotes the formation of the Cu carbonyls (species 4–8) assigned above, which are in equilibrium with each other. A similar dynamic equilibrium was already reported for a CuCl-free [Ru(CO)3Cl2]2/[C4C1C1Im]Cl/Al2O3 WGS catalyst.11
Moreover, the peak growth upon cooling is rationalized by the exothermic nature of the CO complexation.15 However, the ratio of the two major contributions that increase in intensity upon cooling is different throughout the IL-containing samples. This becomes apparent by comparison of the bottom spectra recorded at 30 °C in Fig. 5a–e. This set of spectra shows that the ratio of the peaks at 2073–2064 cm−1 and 2044–2042 cm−1 varies systematically with the CuCl to [C4C1C1Im]Cl molar ratio χ. For χ = 0.3, the peaks at 2073–2064 cm−1 and 2044–2042 cm−1 have approximately the same peak height. With increasing molar ratio χ, the peak at 2073–2064 cm−1 becomes the most prominent one.
According to our DFT calculations, the band at 2044–2042 cm−1 is assigned to the monoanionic monomers and dimers (species 6–8). Among the set of samples, this band is most pronounced for χ = 0.3, where the highest excess of Cl−-containing IL is present with respect to CuCl. This means that the formation of anions, which requires binding of Cl−, is more likely in environments where more Cl− is available per CuCl. Vice versa, an increase of the molar ratio χ leads to an enhanced contribution of neutral dimers (2073–2064 cm−1; species 4 and 5). This is due to a decrease of the Cl− content per available Cu metal center, which promotes the formation of neutral dimers as compared to anionic Cu species. The observation that the nature of the Cu carbonyls depends on the relative amount of Cl− in the SILP confirms our idea of an equilibrium existing between the anionic and neutral Cu carbonyl species.
Additionally, the IR features of the Cu carbonyls observed are apparent at all temperatures investigated. This is important because the highest temperatures are similar to those under real reaction conditions during the WGSR. Thus, we assume that the Cu carbonyls are present under the experimental conditions of the WGSR and are responsible for the improved catalytic activity. In our previous work, we demonstrated that the highest TOFs are obtained at a molar ratio of χ = 0.7.13 A comparison of the IR spectra reveals that the most intense peak at <2100 cm- 1 at a molar ratio χ = 0.7 is assigned to the neutral CuCl dimers (species 4 and 5) independent of the temperature. This observation suggests that the presence of neutral CuCl carbonyl dimers (species 4 and 5) is beneficial for the performance of [Ru(CO)3Cl2]2/CuCl/[C4C1C1Im]Cl/Al2O3 as a WGS catalyst.
Stepić et al.12 investigated the mechanism of the WGSR over Ru-based SILP catalysts in a computational and experimental study. They suggested a five-step mechanism of the WGSR, which starts with the activation of the Ru catalyst by addition of H2O. Upon evolution of HCl, a metallocarboxylic acid is formed. Thereafter, a metal hydride is formed upon release of CO2. The metal hydride recombines with HCl to form a dihydride complex. Finally, the H2 dissociates and the catalyst is regenerated by the addition of CO.12 Thus, the availability of CO in the IL phase is crucial for the regeneration of the catalyst. The CO shuttles increase the availability of CO, which is decisive for the completion of the catalytic cycle of the WGSR.
 | ||
| Fig. 7 1st and 2nd heating/cooling cycles: a, representative IR spectra of the sample with molar ratio χ = 0.5; b, integrated peak areas for selected samples, supported with guide to the eye curves. | ||
Referring to the integrated peak areas at <2100 cm−1, striking differences are observed between the pure CuCl and the IL-containing samples. As one can see in Fig. 7b, the integrated peak area of the CuCl/Al2O3 reference remains constant throughout the experiment. In contrast, both IL-containing samples show the same behavior. The peak area stays constant upon heating to 120 °C in the first cycle. This is followed by an increase of the peak area during cooling to 30 °C. In the subsequent heating ramp of the second cycle, the peaks vanish nearly completely but reappear during cooling back to 30 °C.
We interpret these results as follows. For the CuCl/Al2O3 sample, no peak growth at <2100 cm−1 upon cooling to 30 °C is apparent. This is due to the absence of the IL and, hence, the absence of a reaction medium and a Cl− reservoir. For the IL-containing samples, the first heating ramp to 120 °C is associated with an induction period, which is known from the literature.9,48–50 Thus, we attribute the induction period to reordering/mixing of the IL/CuCl phase including the formation of different [CuxCly]z species. In Fig. 7b, this induction period is highlighted in red. Upon cooling to 30 °C, the Cu carbonyl peaks become more pronounced due to the exothermic nature of the CO complexation reaction.15 During the second cycle, no further induction period is observed. Instead, the behavior resembles the first cycle after the induction period. Namely, we observe a decrease of the peak area at <2100 cm−1 upon heating and an increase of the peak area upon cooling to 30 °C. Thus, we conclude that the reordering/mixing of the IL/CuCl phase including the formation of CuCl species is completed after the first heating period.
Finally, we observe, in the presence of the chloride IL, only small Cu carbonyl peaks at temperatures close to the working conditions of the WGSR. In addition, these Cu carbonyls were demonstrated to be highly labile and they decompose rapidly upon evacuation or purging with Ar.13 This is further supported by the DFT-calculated Cu–C bond orders (Table S1†), which are all well below 1.0. These values contrast with the M–CO bond order for the carbonyl–chloro–ruthenium catalyst (1.20, as well as other well-known Ru carbonyl species with M–CO bond orders >1.25).51 The lability of the Cu carbonyls is crucial for them to work as efficient CO shuttles. Due to the fast kinetics of the Cu carbonyl decomposition, only a low concentration of these shuttles is necessary to have a strong influence on the performance of our [Ru(CO)3Cl2]2/CuCl/[C4C1C1Im]Cl/Al2O3 WGSR catalyst.
4. Conclusion
In this work, we investigated the formation and equilibrium between different Cu carbonyls in CuCl/[C4C1C1Im]Cl/Al2O3 by in situ DRIFTS. In the WGSR, these Cu species are assumed to take the role of CO shuttles in the Ru-based SILP system and were demonstrated to improve the performance of this specific SILP system. We identified the relevant Cu species based on their IR spectra with the help of DFT calculations. Our main results are the following:(1) CuCl additives improve the performance of Ru-based SILP systems. The Cu carbonyls act as CO shuttles, resulting in an increase of catalytic activity in the WGSR.
(2) We identify several relevant Cu carbonyls, namely neutral monomers ([Cu(CO)1–3Cl]) and dimers ([Cu2(CO)2,4(μ-Cl)2]), as well as monoanionic monomers ([Cu(CO)1,2Cl2]−) and dimers ([Cu2(CO)2(μ-Cl)3]−).
(3) Chloride ligands enhance π-backbonding from the Cu center to the carbonyl ligand, as revealed by the calculated C–O Mayer bond orders. The bond order decreases with increasing Cl−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio and, consequently, the corresponding CO bands shift to lower wavenumbers.
Cu ratio and, consequently, the corresponding CO bands shift to lower wavenumbers.
(4) The formation of specific Cu carbonyl species depends on the molar ratio between CuCl and the IL. The formation of anions is promoted by higher ratios of IL to CuCl due to an excess of Cl−. In contrast, neutral Cu dimers are dominant at lower IL to CuCl ratios.
(5) After an induction period, the peaks of different Cu carbonyl species increase in the presence of [C4C1C1Im]Cl. The induction period is attributed to reordering/mixing of the CuCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL phase.
IL phase.
(6) The formation of the Cu carbonyls is fully reversible. Their concentration in the supported IL increases upon cooling because of the exothermic nature of the CO complexation.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge funding from the European Commission within the Horizon 2020-SPIRE project ROMEO (grant agreement number 680395) as well as funding from the Deutsche Forschungsgemeinschaft (DFG) and the NIC project 11311 at the Jülich supercomputing facilities. Additional support by the DFG is acknowledged from the Excellence Cluster “Engineering of Advanced Materials” (project EXC 315) (Bridge Funding) and by the Free State of Bavaria through its funding for the Energie Campus Nürnberg (http://www.encn.de).References
- Y. Li, Q. Fu and M. Flytzani-Stephanopoulos, Appl. Catal., B, 2000, 27, 179–191 CrossRef CAS.
- D. Mendes, A. Mendes, L. Madeira, A. Iulianelli, J. Sousa and A. Basile, Asia-Pac. J. Chem. Eng., 2010, 5, 111–137 CrossRef CAS.
- D. S. Newsome, Catal. Rev.: Sci. Eng., 1980, 21, 275–318 CrossRef CAS.
- C. Rhodes, G. Hutchings and A. Ward, Catal. Today, 1995, 23, 43–58 CrossRef CAS.
- D. C. Grenoble, M. M. Estadt and D. F. Ollis, J. Catal., 1981, 67, 90–102 CrossRef CAS.
- D. Andreeva, V. Idakiev, T. Tabakova, A. Andreev and R. Giovanoli, Appl. Catal., A, 1996, 134, 275–283 CrossRef CAS.
- G. Jacobs, L. Williams, U. Graham, G. A. Thomas, D. E. Sparks and B. H. Davis, Appl. Catal., A, 2003, 252, 107–118 CrossRef CAS.
- S. Werner, N. Szesni, R. W. Fischer, M. Haumann and P. Wasserscheid, Phys. Chem. Chem. Phys., 2009, 11, 10817–10819 RSC.
- S. Werner, N. Szesni, A. Bittermann, M. J. Schneider, P. Härter, M. Haumann and P. Wasserscheid, Appl. Catal., A, 2010, 377, 70–75 CrossRef CAS.
- S. Werner, N. Szesni, M. Kaiser, R. W. Fischer, M. Haumann and P. Wasserscheid, ChemCatChem, 2010, 2, 1399–1402 CrossRef CAS.
- T. Bauer, R. Stepic, P. Wolf, F. Kollhoff, W. Karawacka, C. R. Wick, M. Haumann, P. Wasserscheid, D. M. Smith, A.-S. Smith and J. Libuda, Catal. Sci. Technol., 2018, 8, 344–357 RSC.
- R. Stepić, C. R. Wick, V. Strobel, D. Berger, V. A. Nataša, M. Haumann, P. Wasserscheid, A. S. Smith and D. M. Smith, Angew. Chem., 2019, 131, 751–755 Search PubMed.
- P. Wolf, M. Aubermann, M. Wolf, T. Bauer, D. Blaumeiser, R. Stepic, C. R. Wick, D. M. Smith, A.-S. Smith, P. Wasserscheid, J. Libuda and M. Haumann, Green Chem., 2019, 21, 5008–5018 RSC.
- S. A. Bolkan and J. T. Yoke, J. Chem. Eng. Data, 1986, 31, 194–197 CrossRef CAS.
- O. C. David, G. Zarca, D. Gorri, A. Urtiaga and I. Ortiz, Sep. Purif. Technol., 2012, 97, 65–72 CrossRef CAS.
- C. Huang, B. Chen, J. Zhang, Z. Liu and Y. Li, Energy Fuels, 2004, 18, 1862–1864 CrossRef CAS.
- R. Lü, H. Tangbo and Z. Cao, J. Nat. Gas Chem., 2007, 16, 70–77 CrossRef.
- G. Zarca, I. Ortiz and A. Urtiaga, J. Membr. Sci., 2013, 438, 38–45 CrossRef CAS.
- J. Zhang, C.-P. Huang, B.-H. Chen, Y.-X. Li and C.-Z. Qiao, J. Fuel Chem. Technol., 2005, 33, 431 CAS.
- P. Wolf, M. Logemann, M. Schörner, L. Keller, M. Haumann and M. Wessling, RSC Adv., 2019, 9, 27732–27742 RSC.
- T. Bauer, S. Maisel, D. Blaumeiser, J. Vecchietti, N. Taccardi, P. Wasserscheid, A. Bonivardi, A. Görling and J. Libuda, ACS Catal., 2019, 9, 2842–2853 CrossRef CAS.
- T. Xu, M. Schwarz, K. Werner, S. Mohr, M. Amende and J. Libuda, Phys. Chem. Chem. Phys., 2016, 18, 10419–10427 RSC.
- A. Kaftan, A. Schönweiz, I. Nikiforidis, W. Hieringer, K. M. Dyballa, R. Franke, A. Görling, J. Libuda, P. Wasserscheid, M. Laurin and M. Haumann, J. Catal., 2015, 321, 32–38 CrossRef CAS.
- F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73–78 CAS.
- A. D. Becke, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 38, 3098 CrossRef CAS.
- J. P. Perdew, Phys. Rev. B: Condens. Matter Mater. Phys., 1986, 33, 8822 CrossRef PubMed.
- O. Vahtras, J. Almlöf and M. Feyereisen, Chem. Phys. Lett., 1993, 213, 514–518 CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef.
- A. Hellweg and D. Rappoport, Phys. Chem. Chem. Phys., 2015, 17, 1010–1017 RSC.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- F. Weigend, Phys. Chem. Chem. Phys., 2006, 8, 1057–1065 RSC.
- M. Cossi, N. Rega, G. Scalmani and V. Barone, J. Comput. Chem., 2003, 24, 669–681 CrossRef CAS PubMed.
- M. Sobota, S. Schernich, H. Schulz, W. Hieringer, N. Paape, P. Wasserscheid, A. Görling, M. Laurin and J. Libuda, Phys. Chem. Chem. Phys., 2012, 14, 10603–10612 RSC.
- H. Weingärtner, P. Sasisanker, C. Daguenet, P. J. Dyson, I. Krossing, J. M. Slattery and T. Schubert, J. Phys. Chem. B, 2007, 111, 4775–4780 CrossRef PubMed.
- I. Mayer, Chem. Phys. Lett., 1983, 97, 270–274 CrossRef CAS.
- I. Mayer, Int. J. Quantum Chem., 1984, 26, 151–154 CrossRef CAS.
- L. C. Brown, J. M. Hogg and M. Swadźba-Kwaśny, Ionic Liquids II, Springer, 2017, pp. 185–224 Search PubMed.
- J. Estager, P. Nockemann, K. R. Seddon, M. Swadźba-Kwaśny and S. Tyrrell, Inorg. Chem., 2011, 50, 5258–5271 CrossRef CAS.
- C. L. Hussey, Pure Appl. Chem., 1988, 60, 1763–1772 CAS.
- M. Cossi, M. Persico and J. Tomasi, J. Mol. Liq., 1994, 60, 87–105 CrossRef CAS.
- M. Haakansson and S. Jagner, Inorg. Chem., 1990, 29, 5241–5244 CrossRef CAS.
- A. F. Holleman, E. Wiberg and N. Wiberg, Lehrbuch der anorganischen Chemie, de Gruyter, Berlin, 102 edn, 2007 Search PubMed.
- K. W. Kolasinski, Surface science: foundations of catalysis and nanoscience, John Wiley & Sons, 2012 Search PubMed.
- R. D. Pike, Organometallics, 2012, 31, 7647–7660 CrossRef CAS.
- S. H. Strauss, J. Chem. Soc., Dalton Trans., 2000, 1–6, 10.1039/A908459B.
- A. J. Bridgeman, G. Cavigliasso, L. R. Ireland and J. Rothery, J. Chem. Soc., Dalton Trans., 2001, 2095–2108, 10.1039/B102094N.
- I. Mayer, J. Comput. Chem., 2007, 28, 204–221 CrossRef CAS.
- S. A. Forsyth, H. Q. N. Gunaratne, C. Hardacre, A. McKeown, D. W. Rooney and K. R. Seddon, J. Mol. Catal. A: Chem., 2005, 231, 61–66 CrossRef CAS.
- N. A. Hamill, C. Hardacre and S. J. McMath, Green Chem., 2002, 4, 139–142 RSC.
- I. Kim, K. Anas, S. Lee, C.-S. Ha and D.-W. Park, Catal. Today, 2008, 131, 541–547 CrossRef CAS.
- N. Fritsch, C. R. Wick, T. Waidmann, S. Pflock, P. O. Dral, J. Tucher, C. Steiner, T. E. Shubina, S. Maier, T. Clark and N. Burzlaff, Eur. J. Inorg. Chem., 2018, 2018, 54–61 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cy01852b |
| ‡ These authors share first authorship. |
| This journal is © The Royal Society of Chemistry 2020 |



![[V with combining dot above]](https://www.rsc.org/images/entities/i_char_0056_0307.gif)