Additively manufactured RANEY®-type copper catalyst for methanol synthesis†
Christina
Heßelmann
a,
Torsten
Wolf
b,
Florian
Galgon
c,
Carolin
Körner
bc,
Jakob
Albert
 a and
Peter
Wasserscheid
a and
Peter
Wasserscheid
 *ad
*ad
aFriedrich-Alexander University Erlangen-Nürnberg, Institute for Chemical Reaction Engineering, Egerlandstr. 3, 91058 Erlangen, Germany. E-mail: peter.wasserscheid@fau.de
bFriedrich-Alexander University Erlangen-Nürnberg, Joint Institute of Advanced Materials and Processes, Dr.-Mack-Straße 81, 90762 Fürth, Germany
cFriedrich-Alexander University Erlangen-Nürnberg, Department of Materials Science, Chair of Materials Science and Technology for Metals, Martensstrasse 5, 91058 Erlangen, Germany
dForschungszentrum Jülich, Helmholtz-Institute Erlangen-Nürnberg fpr Renewable Energy (IEK 11), Egerlandstr. 3, 91058 Erlangen, Germany
First published on 25th November 2019
Abstract
We describe a novel approach to prepare cellular RANEY®-type catalysts by combining additive manufacturing (AM) of a Cu–Al alloy followed by selective leaching of Al with aqueous NaOH solutions. Three-dimensional cellular geometries of Cu–70Al were fabricated by laser-metal-deposition (LMD). After activation by an optimized leaching process, highly active and selective catalytic structures for CO hydrogenation to methanol were obtained.
It is well known that RANEY®-type catalysts (e.g. RANEY® nickel, RANEY® cobalt or RANEY® copper) are highly active in hydrogenation reactions and of high relevance for industrial hydrogenation processes.1 Traditional RANEY®-type catalysts are produced from fine aluminium-based alloy powders of the respective active metal. These powders are activated by alkaline leaching of aluminium from the bulk alloy.2 During the leaching process, a large surface area of the active metal is formed resulting in the catalytically active powder.
To date, the industrial application of RANEY®-type catalysts is typically limited to liquid phase hydrogenation in slurry reactors.1 For continuous gas phase reactions the powdery nature of traditional RANEY®-type catalysts leads to a prohibitively high pressure drop. There is, however, a strong interest to use cellular RANEY®-type catalyst structures as this approach would elegantly combine design freedom with effective heat and mass transport for industrially relevant gas phase hydrogenation processes. For this reason, several approaches for shaping RANEY®-type catalysts in macroscopically pelletized or cellular form were introduced in the last decades.
One published approach to synthesise RANEY®-type catalyst pellets combines pore producing agents (e.g. Distearylethylendiamid, Mg/Al stearates, methyl cellulose) and RANEY®-type metal powder as a binder material.3,4 After calcination, this mixture forms stable pellets that can subsequently be activated by alkaline leaching. Alternatively, polymer binders and alloy powders have been combined to synthesise extrudates.5–8 In this case only a part of the alloy grains can be activated by the basic treatment as the polymer matrix prevents access of the alkaline leaching solution to every alloy grain in the system. Another limitation of this latter approach is that the reaction temperature is limited to the softening temperature of the polymer. Calcination of the polymer extrudates is possible but would reduce the mechanical strength of the resulting material strongly. The literature also describes the preparation of supported RANEY®-type catalysts.9,10 By using different coating methods, such as e.g. thermal spraying, electron beam spraying or laser spraying, a thin layer of RANEY®-type alloy has been coated onto metallic or ceramic support structures. This method is limited, however, to the coating of geometrically relative simple supports, like corrugated foils. Coating of more complex support structures, such as monoliths, is not possible.10
In this contribution, we present a new approach for generating cellular RANEY®-type catalyst structures in a two-step process. We apply first additive manufacturing to fabricate the macroscopic cellular structure in form of an alloy bulk material with an extremely high degree of design freedom. In the second step, alkaline leaching is applied to dissolve selectively the less-noble element of the alloy for creating the catalytic interface. We demonstrate our approach here for the preparation of a RANEY®-type copper catalyst structure. Consequently, we have prepared cellular structures from a CuAl-alloy11 by AM using laser-metal-deposition (LMD). LMD is a novel method for processing low amounts of metal powders into three-dimensional geometries. Our leaching process aims for only a partial dissolution of the surface-near aluminium.12–14 We target purposely a structure with a porous volume close to the external surface and a solid alloy core. The latter should provide mechanical stability and optimised heat transfer.15–17 With these characteristics, the obtained structure should be highly suitable for reactions with high heat consumption or high heat production.18–20
In this study, we apply our new AM-based preparation methodology for producing catalytic structures for methanol synthesis.21 This reaction counts among the most important transformations in industrial chemistry (production is ca. 110 million metric tons per year) and is also highly relevant in the context of chemical energy storage.22 Moreover, the reaction is highly exothermic and a major reason for catalyst deactivation to date is hot spot-formation in the applied fixed-bed reactors.23,24 Our study builds on former literature reports describing that zinc-promoted RANEY®-type Cu catalysts are potential replacement for the industrially used methanol catalyst which is a co-precipitated CuO/ZnO/Al2O3 system.25
The AM-structures applied in this study were fabricated from a commercial gas atomized powder supplied by Ecka Granules GmbH (Fürth, Germany). The powder consisted of 29.4 at% copper with a balance of aluminium and was characterised by a powder grain size distribution of 45 to 105 μm. From this alloy powder, cylindrical samples with a diameter of 9.8 mm and a height of 10 mm were fabricated by the LMD process using an InssTek MX600 machine in a custom inert gas enclosure (for processing details see ESI†). High resolution SEM-images and phase analysis were carried out using a Helios NanoLab 600i system. Both dendrite thickness and ligament diameters were determined using image processing software. The LMD-built Cu–70Al cylinders and Cu–70Al powder (for comparison) were leached in 4.9 M NaOH/0.5 M Na2Zn(OH)4 solution at room temperature until hydrogen evolution stopped. After activation, the so-obtained RANEY®-type catalyst was washed with water and stored under water. The catalyst composition was determined via inductively coupled plasma optical emission spectrometry (ICP-OES, Plasma 400 from Perkin-Elmer). To determine the composition of the RANEY®-type Cu cylinder, the alkaline leaching solution was analysed before and after the leaching process by ICP-OES. The structural properties of the tested catalysts were investigated with N2 sorption at 77 K. For calculation of the surface area, the Brunauer–Emmet–Teller (BET) method was used. The catalytically active Cu surface area was determined using isothermal nitrous oxide decomposition experiments (see ESI,† Fig. S1) according to the literature.26,27 Hydrogenation of CO to methanol was performed in a fixed-bed reactor with an inner tube diameter of 9.7 mm. The volume of the catalyst bed was 1.4 ml and kept constant for comparable experiments. The reaction was conducted at 75 bar and 250 °C with a total volume flow of 300 mlN min−1. The molar ratio of the reactants H2/CO was set to 2. For detailed information on the reaction procedure see ESI.†
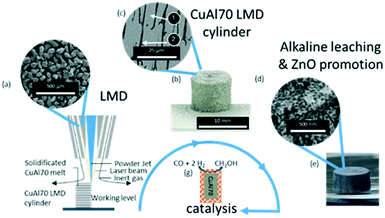
In Fig. 1, the conceptual approach of our research is shown. Gas-atomized CuAl70 powder (Fig. 1a) is produced and used to additively manufacture in an LMD process CuAl70 cylinders (Fig. 1b). The microstructure of the as-built cylinders is comprised of Θ-dendrites (Fig. 1c, ①) and an intermediate eutectic area of α-Al-, and Θ-lamellae (Fig. 1c, ②). The phase composition was determined by SEM micrographs and is exemplarily shown for the Cu–70Al cylinder in Table S2.† This microstructure is in good accordance with a literature report from Liu et al.28 The Θ-dendrites thickness ranges from 3 to 18 μm which can be attributed to the high cooling rate of the AM process.29–31 Dealloying this bulk material leads to no visible change in the macroscopic geometry besides the expected decrease in weight and change in colour (Fig. 1e). High resolution SEM-imaging (Fig. 1d) reveals that the surface-near volume turns into a nanoporous structure during dealloying with forming copper ligaments of 20 ± 5 nm in thickness.
Note, that applying the same dealloying procedure to the Cu–70Al powder with identical experimental parameters resulted in a ligament thickness of 17 ± 4 nm which is in very good agreement with earlier literature reports on dealloying CuAl powders.32 This proves that the AM-manufactured cylinders show a very similar dealloying behaviour compared to the bulk powder material.
It is well known that ZnO is an important ingredient of methanol synthesis catalysts. ZnO stabilises the Cu nanoparticles and protects the active copper centers from poisoning.33 Due to the relatively high vapour pressure of Zn, the processing of CuAlZn alloys in AM proved challenging though. Instead, we used a literature-known method to add ZnO to the RANEY®-type Cu catalyst during the leaching process.25,34 During Al leaching, the OH− concentration is reduced at the leaching front, which causes the sodium zincate to precipitate. The precipitated zinc hydroxide decomposes to ZnO if heated to 200 °C.34 Fig. S10† shows the positive influence on the methanol productivity if the leaching is carried out in sodium zincate.
| Na2Zn(OH)4 ⇌ Zn(OH)2 ↓ + 2NaOH | (1) |
| Zn(OH)2 ⇌ ZnO + H2O | (2) |
Table 1 shows the composition, the specific surface area SBET and the active copper surface area SCu,act of the so prepared catalyst cylinders.
| Catalyst | Compositiona/wt% | S BET /m2 g−1 | S Cu,act /m2 g−1 | ||
|---|---|---|---|---|---|
| Cu | Al | Zn | |||
| * Powder catalyst, ** powder was obtained by milling of the commercial pellet catalyst, ‘R-’ indicates a RANEY®-type catalyst material. a According to ICP-OES. b As determined by N2-sorption. c As determined by N2O decomposition. | |||||
| R-Cu* | 89.0 | 1.8 | 9.2 | 25 | 12 |
| R-Cu LMD cylinder (total) | 74.3 | 25.1 | 0.6 | ||
| R-Cu LMD cylinder (np-layer) | 96.1 | 1.4 | 2.5 | 29 | 9 |
| R-Cu* (grace) | 96.7 | 3.3 | 0 | 16 | 2 |
| CuO/ZnO/Al2O3** (Alfa Aesar) | 66.2 | 7.6 | 24.8 | 84 | 21 |
Note, that the zinc content of the dealloyed cylinder is with 2.5 wt% lower than the zinc content of equivalent RANEY®-type Cu powder catalyst. This can be explained by the different leaching times. As the LMD-manufactured Cu–70Al cylinder has a smaller outer surface area compared to the powder, the leaching reaction is restricted by the rate of diffusion with increasing leaching depths (see ESI,† Fig. S6). After 165 h, a leaching depth of around 1 mm was achieved (s. ESI,† Fig. S9), which indicates a leaching efficiency of 68%. In case of the powder, 98% of the aluminium was leached out already after 24 h (see ESI† for details). Note, that secondary dissolution processes of Zn(OH)2 are also known to affect the Zn content of the sample resulting from the precipitation process.12
N2 sorption measurements showed a specific surface area of 29 m2 g−1 for our as-prepared RANEY®-type Cu LMD cylinder (s. Table 1). This value is similar to the surface area of the RANEY®-type powder catalyst (25 m2 g−1) and in good agreement with earlier literature reports.1 For comparison, the commercial CuO/ZnO/Al2O3 catalyst is a supported co-precipitated catalyst, with a typical specific surface area of 84 m2 g−1.
Furthermore, we were interested in determining the active copper surface area. For this purpose, nitrous oxide decomposition experiments in a continuous flow reactor were carried out (see ESI,† Fig. S1) and the results were compared to known Cu-based heterogeneous catalysts. Among the studied Cu catalysts, our LMD-manufactured, RANEY®-type Cu catalyst showed an active Cu surface of 9 to 11 m2 g−1 similar to literature data for RANEY®-type Cu powders.34 The commercial Cu/ZnO/Al2O3 showed the highest active copper surface area among the tested systems with 21 m2 g−1 while the commercial RANEY®-type Cu catalyst from grace catalyst is characterised by a low active copper surface area of only 2 m2 g−1.
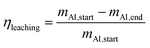
In the next set of experiments we applied our new LMD-prepared RANEY®-type Cu cylinder structure in catalytic CO hydrogenation studies for methanol synthesis. Fig. 2 shows the results of a long-term experiment for the RANEY®-type Cu cylinder. The methanol productivity based on the nanoporous copper mass (PCH3OH(mnp-Cu)) (see eqn (3)–(5)) and the equilibrium methanol productivity (PCH3OHeq.(mnp-Cu)) are shown over 110 h time-on-stream (TOS) with a temperature variation in the range between 200 and 260 °C. The nanoporous copper mass was estimated by eqn (3) keeping in mind that the starting alloy consists of 50 wt% copper balanced with aluminium. (For details of the calculation see ESI,† Fig. S8 and Table S2 with comments).
| mnp-Cu = ηleaching·mCu | (3) |
 | (4) |
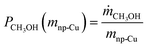 | (5) |
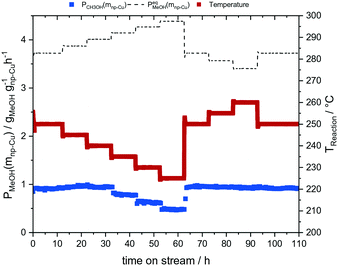 | ||
| Fig. 2 CO hydrogenation experiment with our LMD-manufactured RANEY®-type Cu cylinder structure; mcat = 3.5 g, p = 75 bar, T = 200–260 °C, residence time: 3 s, Vges = 300 mlN min−1 (H2/CO = 2/1). | ||
The methanol productivity follows the expected trend in the temperature variation. Only at higher temperatures (240–260 °C) a different behaviour was observed. A possible explanation is the consecutive reaction of methanol to dimethyl ether (DME) (s. ESI† Table S1, Fig. S5). By comparing the average methanol productivities of the reference point of 250 °C at the beginning (1–10 TOS) and at the end of the experiment (100–110 TOS), no deviation was determined. Thus, our new LMD-prepared RANEY®-type Cu cylinder achieved a stable methanol productivity of 0.92 ± 0.01 gCH3OH gnp-Cu−1 h−1. Moreover, we could confirm the mechanical stability of the used catalyst after methanol synthesis (see ESI,† Fig. S9).
To compare the results of our novel AM-based RANEY®-type Cu catalyst with commercial methanol synthesis catalysts, the two commercial catalysts shown in Table 1 were also tested in the same set-up (see ESI,† Fig. S4) and under identical conditions. Fig. 3 shows the comparison between the tested catalysts indicating the methanol productivity related to the mass of nanoporous copper (PCH3OH(mnp-Cu)) as calculated by eqn (3), and the obtained selectivity to methanol SCH3OH (for calculation details see ESI†).
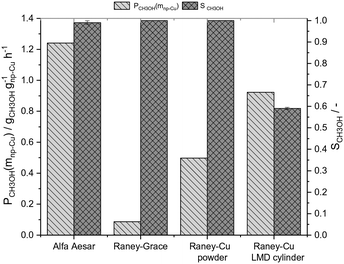 | ||
Fig. 3 Comparison of the catalytic activities for the tested catalysts (s. Table 1) in the CO hydrogenation to methanol at 75 bar and 250 °C (Vges = 300 mlN min−1, CO/H2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, t = 10–12 h, residence time: 3–4 s, Vcat = 1.4 ml). 2, t = 10–12 h, residence time: 3–4 s, Vcat = 1.4 ml). | ||
At 250 °C and 75 bar syngas pressure, the RANEY®-type Cu LMD cylinder showed with 0.92 gCH3OH gnp-Cu−1 h−1 a good productivity, only 26% lower than the productivity of the commercial CuO/ZnO/Al2O3 catalyst, which gave 1.24 gCH3OH gnp-Cu−1 h−1. The commercial RANEY®-type Cu Grace catalyst, however, shows with 0.09 gCH3OH gnp-Cu−1 h−1 a very low methanol productivity, which can be explained by the low active surface area and the lack of ZnO. The methanol selectivity for our RANEY®-type Cu cylinder catalyst was with 60% significantly lower than for all tested powder catalysts which all exceeded 95% selectivity. As seen from Fig. S5,† the LMD-manufactured, leached and ZnO-doped catalyst structure shows a pronounced activity for the consecutive reaction of methanol to DME, while for the RANEY®-type powder catalyst and the commercial catalysts only traces of DME are detected. We hypothesise that this temperature-dependent activity for DME formation could be a function of the Al–Cu alloy material that forms in the leached cylinders at the interface of the massive core due to the incomplete leaching of the cylinder compared to the complete leaching of the RANEY®-type powder catalyst. Consequently, we have determined the number of surface acid sites by NH3-TPD of the used RANEY®-type catalysts in Fig. 3 (see ESI,† Table S3 and Fig. S11). The results clearly indicate that higher DME-selectivity is well in-line with increasing surface acidity of the used catalysts. This interpretation is supported by the finding that casted RANEY®-type Cu cylinders from the same alloy that are activated by an identical leaching process (see ESI,† Fig. S6) also show significant selectivity for DME (see ESI,† Fig. S7). A more detailed investigation of the reactivity of the Cu–Zn–Al-oxide interface that forms at the leaching frontline between the nanoporous, leached material and the massive alloy core is a topic of our on-going follow-up studies and the corresponding results will be published elsewhere.
In conclusion, we could show that additive manufacturing is a promising method to generate catalytically active, shaped or cellular RANEY®-type Cu catalysts for the methanol synthesis. We have demonstrated the printing of a stable three-dimensional geometry from Cu–70Al powder by using LMD. After catalytic activation by alkaline leaching the shape of the Cu–70Al LMD cylinder remained stable. Interestingly, the so-obtained core–shell material (solid core for structure stability; nonporous, catalytically active shell) shows comparable reactivity to the commercial CuO/ZnO/Al2O3 methanol synthesis catalyst. A significant higher selectivity for the consecutive DME production might add a very interesting feature to this new catalyst structures if DME is the desired product of the CO synthesis reaction. In any case opens the cellular, core–shell structure of the here-reported catalyst elements a novel way for hot-spot free operation of CO hydrogenation reactors with optimized hydrodynamics in the catalytic reactor.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Deutsche Forschungsgemeinschaft (DFG) for funding this work (projects LO 2030/1-1 and WA 1615/15-1). Additional infrastructural support by DFG through the Exzellenzcluster “Engineering of Advanced Materials” and through its instrument grant INST 90/987-1 FUGG (purchase of the LMD instrument) is gratefully acknowledged.Notes and references
- A. J. Smith and M. S. Wainwright, in Handbook of Heterogeneous Catalysis, Wiley-VCH Verlag GmbH & Co. KGaA, 2008, DOI:10.1002/9783527610044.hetcat0007.
- M. Raney, Ind. Eng. Chem., 1940, 32, 1199–1203 CrossRef CAS.
- P. Schütz, R. Burmeister, B. Despeyroux, H. Mösinger, H. Krause and K. Deller, US Pat., 5536694, 1996 Search PubMed.
- D. Ostgard, K. Möbus, M. Berweiler, B. Bender and G. Stein, US Pat., 6489521, 2002 Search PubMed.
- S. D. Robertson and R. B. Anderson, J. Catal., 1971, 23, 286–294 CrossRef CAS.
- W.-C. Cheng, C. B. Lundsager and R. M. Spotnitz, US Pat., 4826799, 1989 Search PubMed.
- M. Vicari, K. Flick, J.-P. Melder, W. Schnurr and J. Wulff-Doring, US Pat., 5733838, 1998 Search PubMed.
- A. Freund, M. Berweiler, B. Bender and B. Kempf, US Pat., 6262307, 2001 Search PubMed.
- M. Goldberger, US Pat., 3637437, 1972 Search PubMed.
- A. Wolf, T. Turek and L. Mleczko, Chem. Eng. Technol., 2016, 39, 1933–1938 CrossRef CAS.
- T. Wolf, Z. Fu and C. Körner, Mater. Lett., 2019, 238, 241–244 CrossRef CAS.
- H. E. Curry-Hyde, D. J. Young and M. S. Wainwright, Appl. Catal., 1987, 29, 31–41 CrossRef CAS.
- J. R. Mellor, N. J. Coville, A. C. Sofianos and R. G. Copperthwaite, Appl. Catal., A, 1997, 164, 171–183 CrossRef CAS.
- K. Shimazu, Y. Tateno, M. Magara, N. Okamoto, T. Ohshima, M. Nagasawa and H. Sakamura, US Pat., 6414201, 2002 Search PubMed.
- E. Bianchi, T. Heidig, C. G. Visconti, G. Groppi, H. Freund and E. Tronconi, Chem. Eng. J., 2012, 198–199, 512–528 CrossRef CAS.
- E. Bianchi, T. Heidig, C. G. Visconti, G. Groppi, H. Freund and E. Tronconi, Catal. Today, 2013, 216, 121–134 CrossRef CAS.
- I. Gräf, A.-K. Rühl and B. Kraushaar-Czarnetzki, Chem. Eng. J., 2014, 244, 234–242 CrossRef.
- C. Busse, H. Freund and W. Schwieger, Chem. Eng. Process., 2018, 124, 199–214 CrossRef CAS.
- G. Do, T. Stiegler, M. Fiegl, L. Adler, C. Körner, A. Bösmann, H. Freund, W. Schwieger and P. Wasserscheid, Ind. Eng. Chem. Res., 2017, 56, 13402–13410 CrossRef CAS.
- T. Stiegler, K. Meltzer, A. Tremel, M. Baldauf, P. Wasserscheid and J. Albert, Energy Technol., 2019, 7, 1900047 CrossRef.
- F. Enzenberger, R. Guschelbauerm, C. Körner, M. Lodes and P. Wasserscheid, WO2017137305A1, 2017.
- Methanol Institute, https://www.methanol.org/the-methanol-industry/, (accessed July 2019) Search PubMed.
- A. Montebelli, C. G. Visconti, G. Groppi, E. Tronconi, C. Ferreira and S. Kohler, Catal. Today, 2013, 215, 176–185 CrossRef CAS.
- P. L. Spath and D. C. Dayton, Preliminary Screening -- Technical and Economic Assessment of Synthesis Gas to Fuels and Chemicals with Emphasis on the Potential for Biomass-Derived Syngas, Report NREL/TP-510-34929, National Renewable Energy Lab., Golden, CO. US, 2003 Search PubMed.
- H. E. Curry-Hyde, M. S. Wainwright and D. J. Young, Appl. Catal., 1991, 77, 75–88 CrossRef CAS.
- J. W. Evans, M. S. Wainwright, A. J. Bridgewater and D. J. Young, Appl. Catal., 1983, 7, 75–83 CrossRef CAS.
- O. Hinrichsen, T. Genger and M. Muhler, Chem. Eng. Technol., 2000, 23, 956–959 CrossRef CAS.
- W. Liu, L. Chen, J. Yan, N. Li, S. Shi and S. Zhang, Corros. Sci., 2015, 94, 114–121 CrossRef CAS.
- D. Herzog, V. Seyda, E. Wycisk and C. Emmelmann, Acta Mater., 2016, 117, 371–392 CrossRef CAS.
- C. Körner, Int. Mater. Rev., 2016, 61, 361–377 CrossRef.
- M. Ramsperger, L. Mújica Roncery, I. Lopez-Galilea, R. F. Singer, W. Theisen and C. Körner, Adv. Eng. Mater., 2015, 17, 1486–1493 CrossRef CAS.
- W. B. Liu, S. C. Zhang, N. Li, J. W. Zheng and Y. L. Xing, Corros. Sci., 2011, 53, 809–814 CrossRef CAS.
- M. Behrens, F. Studt, I. Kasatkin, S. Kühl, M. Hävecker, F. Abild-Pedersen, S. Zander, F. Girgsdies, P. Kurr, B.-L. Kniep, M. Tovar, R. W. Fischer, J. K. Nørskov and R. Schlögl, Science, 2012, 336, 893–897 CrossRef CAS.
- L. Ma, D. L. Trimm and M. S. Wainwright, Top. Catal., 1999, 8, 271–277 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cy01657k |
| This journal is © The Royal Society of Chemistry 2020 |