Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Enhancement of electron accepting ability of para-benzoquinone by a single water molecule
Golda
Mensa-Bonsu
 ,
Aude
Lietard
,
Aude
Lietard
 and
Jan R. R.
Verlet
and
Jan R. R.
Verlet
 *
*
Department of Chemistry, Durham University, Durham DH1 3LE, UK. E-mail: j.r.r.verlet@durham.ac.uk
First published on 3rd March 2020
Abstract
Correction for ‘Enhancement of electron accepting ability of para-benzoquinone by a single water molecule’ by Golda Mensa-Bonsu et al., Phys. Chem. Chem. Phys., 2019, 21, 21689–21692.
We recently reported the observation of an enhanced electron accepting ability of para-benzoquinone (pBQ) when solvated by a single water molecule in the published paper. The main experimental evidence for this was taken from the frequency-resolved (2D) photoelectron (PE) spectra. Unfortunately, we have since discovered that the presented spectra were erroneous. Specifically, in analysis of the PE images using the polar onion peeling routine,1 we had omitted to take into account the Jacobian that is required in the conversion from radius (velocity) to electron kinetic energy space. Hence, all the PE spectra presented require additional scaling by 1/r, where r is the radius of the image collected in the velocity map imaging experiment.
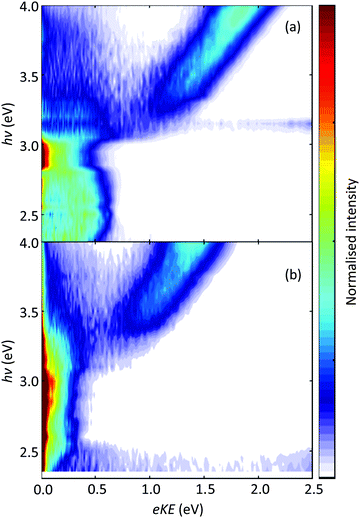
The corrected results are shown in Fig. 2 and should replace those shown in Fig. 2 of the published article. Fig. 2(a) and (b) show the 2D PE spectra for pBQ− and pBQ−(H2O), respectively, taken over the range 2.3 < eKE < 4.0 eV in 0.05 eV increments. The main conclusion of the previous study was that a single water molecule enhances the electron accepting ability of pBQ. This conclusion remains valid with the corrected data. The electron accepting ability is captured by the efficiency with which the ground electronic state is recovered when excited above the detachment threshold. The spectral signature of ground state recovery is the appearance of low-energy electrons. Fig. 2 clearly shows an overall enhancement of such low energy electrons over a broad range of photon energies and, hence, of the electron accepting ability in the presence of a water molecule. Fig. 3 shows the corrected 1D PE spectra, which should replace the erroneous Fig. 3 in the published article.
While the core conclusion is not affected by the correction, we also commented on the apparent discrepancy between the 2D PE spectra and a previous study from our group2,3 and we attributed the differences to the fact that the previous experiments were performed at 300 K instead of 10s of K in the molecular beam experiments. Having corrected the erroneous 2D PE spectra of pBQ−, we now conclude that, in fact, the temperature has very little, if any, effect on the overall dynamics. Hence, any discussions offered to explain the differences are not relevant and should be disregarded.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the EPSRC (EP/R023085/1).References
- G. M. Roberts, J. L. Nixon, J. Lecointre, E. Wrede and J. R. R. Verlet, Rev. Sci. Instrum., 2009, 80, 053104 CrossRef CAS PubMed.
- D. A. Horke, Q. Li, L. Blancafort and J. R. R. Verlet, Nat. Chem., 2013, 5, 711–717 CrossRef CAS PubMed.
- C. W. West, J. N. Bull, E. Antonkov and J. R. R. Verlet, J. Phys. Chem. A, 2014, 118, 11346–11354 CrossRef CAS PubMed.
| This journal is © the Owner Societies 2020 |