Open Access Article
Open Access ArticleIn situ anion-doped epitaxial strontium titanate films†
M.
Tyunina
 *ab,
O.
Pacherova
b,
N.
Nepomniashchaia
*ab,
O.
Pacherova
b,
N.
Nepomniashchaia
 b,
V.
Vetokhina
b,
S.
Cichon
b,
T.
Kocourek
b and
A.
Dejneka
b
b,
V.
Vetokhina
b,
S.
Cichon
b,
T.
Kocourek
b and
A.
Dejneka
b
aMicroelectronics Research Unit, Faculty of Information Technology and Electrical Engineering, University of Oulu, P. O. Box 4500, FI-90014, Finland. E-mail: marina.tjunina@oulu.fi
bInstitute of Physics of the Czech Academy of Sciences, Na Slovance 2, 18221 Prague, Czech Republic
First published on 8th October 2020
Abstract
Misfit strains arising from a film–substrate mismatch can induce novel phases and properties in the epitaxial films of perovskite oxides. Here we employ yet another effect, namely, strain-assisted formation of oxygen vacancies. We demonstrate the misfit-promoted presence of oxygen vacancies and related substitutional incorporation of anion dopants in the epitaxial films of archetypal perovskite oxide SrTiO3. Both the oxygen vacancies and hydrogen or nitrogen dopants are introduced in situ during the pulsed-laser deposition of the films using compressive substrates. The films exhibit peculiar chemical expansion and optical properties, which are consistent with substitutional anion doping.
Introduction
Strontium titanate (SrTiO3, STO) is an archetypal representative of technologically important perovskite oxide ferroelectrics. Unstressed pure STO crystals are wide-bandgap insulators and adopt a cubic paraelectric state at temperatures above 105 K, and a tetragonal antiferrodistortive phase at lower temperatures. Substitution of Sr can induce ferroelectricity and raise the electrical conductivity in STO.In addition to cationic doping, substitution of oxygen with anions such as F, H, or N has also been achieved in ferroelectrics. These mixed-anion compounds include high-performance oxyfluoride piezoelectrics, non-toxic oxynitride pigments, and especially valuable oxyhydrides, which can enable solid-state hydride ion conductivity and fast hydrogen exchange and are vital for applications in energy and catalysis (see the review in ref. 1 and references therein).
Because of their metastability, the synthesis of mixed-anion perovskites is a complex process. The most successful method employs the preparation of stoichiometric oxides with the subsequent introduction of anion dopants during high-temperature processing in an anion-containing gas atmosphere. In this work we present the in situ synthesis of stable mixed-anion perovskites via the epitaxial growth of misfit-strained films.
Cube-on-cube-type epitaxial perovskite oxide films experience misfit strain that arises from a film–substrate mismatch in lattice parameters. This strain is a powerful tool to create new phases and properties in epitaxial films.2 For instance, the state-of-the-art strain-temperature phase diagram of epitaxial STO films suggests the room-temperature tetragonal antiferrodistortive or ferroelectric phases, which are not available in STO crystals.3
Another recently revealed essential consequence of misfit strain is the reduction of the formation energy for oxygen vacancies (VO) in perovskite oxides.4–7 These oxygen vacancies may offer unoccupied lattice sites for anion dopants, therefore, the misfit-promoted formation of oxygen vacancies may facilitate the substitutional incorporation of anion dopants in epitaxial films. Moreover, as recently found in epitaxial BaTiO3 films,8,9 a misfit strain can control the orientation of the Ti–VO–Ti complexes and determine the specific locations of the vacancies in selected atomic planes. Hence, misfit-assisted anion doping may lead to specific spatial distribution of dopants as well. Here we experimentally demonstrate such doping in representative STO films.
We employed in-plane compressive misfit strains and varied the gas atmosphere to introduce oxygen vacancies, hydrogen, or nitrogen in situ, during the pulsed-laser deposition (PLD) of epitaxial STO films. We observed a strong out-of-plane chemical expansion that indicates the selective location of dopants. We inspected the optical properties of the films and found dopant-specific near-edge absorption, which points to substitutional incorporation of anion dopants. Furthermore, we identified the main interband optical transitions, which suggest minor if any interstitial incorporation of dopants. Finally, we propose that such strain-assisted in situ anion doping can be successfully applied to a broad class of perovskite oxides.
Experimental
Epitaxial STO films were grown by pulsed laser deposition on (001) (LaAlO3)0.3(SrAl0.5Ta0.5O3)0.7 (LSAT) substrates (MTI Corp.). For both reference and comparison, polycrystalline STO films were also simultaneously grown on Si substrates covered with an amorphous native SiO2 layer (Si/SiO2). Compared to epitaxy-enabling LSAT substrates, the SiO2 layer prevents the epitaxial growth of STO. Dense stoichiometric STO ceramics were used as a target. Substrates were kept at a temperature of 973 K during deposition and the temperature was lowered at a rate of 5 K min−1 during post-deposition cooling. The gas pressure was varied for different samples (kept constant during deposition and post-deposition cooling): 20 Pa oxygen for regular films (marked here by O), 5 Pa oxygen for introducing oxygen vacancies (VO), and 5 Pa hydrogen or nitrogen for hydrogen or nitrogen doping, respectively (H or N). The thickness of the films was ∼80 nm. Additionally, a 200 nm-thick VO-film was prepared for structural investigations.The room-temperature crystal structure of the films was studied by high-resolution X-ray diffraction on a D8 DISCOVER diffractometer (Bruker corporation) using Cu Kα radiation. Θ–2Θ scans in the range of 2Θ = (10–130) deg and reciprocal space maps (RSM) in the vicinity of the perovskite (002) and (303) diffractions were acquired. The in-plane (parallel to the substrate surface) and out-of-plane (normal to the substrate surface) lattice parameters were estimated from the positions of the diffraction maxima using LSAT as a reference. The thickness of the films was determined using Laue satellites in the Θ–2Θ scans and by X-ray reflectometry on the diffractometer. The diffraction and reflectometry data were fitted using LEPTOS software.
The chemical composition of the films and the valence state of the constituent elements were inspected by X-ray photoelectron spectroscopy using a NanoESCA photoelectron emission spectroscopy and microscopy instrument (Oxford Instruments Omicron Nanoscience). The sample excitation was performed using monochromatic Al Kα1,2 (1486.7 eV), He I (21.2 eV), and Hg (5.2 eV) radiation. The measured binding energies were referenced to the Fermi level EF = 0 eV. The acquired spectra were processed using CasaXPS software.10 The peaks’ backgrounds were convoluted using Shirley functions. The elemental composition was estimated using Scofield's relative sensitivity factors.
The optical properties of the films were examined using variable angle spectroscopic ellipsometry. The measurements were performed on a J. A. Woollam VUV ellipsometer at room temperature, in a dry nitrogen atmosphere, and at photon energies of (0.75–8.8) eV. Ellipsometric spectra were acquired at five angles of incidence (55° to 80° with 5° step) with an energy step of 0.02 eV. The optical constants of the films were extracted from the spectra of ellipsometric angles using a commercial WVASE32 software package. More details on the ellipsometric procedure can be found elsewhere.10,11
Results and discussion
Uniaxial chemical expansion
XRD analyses showed a cube-on-cube-type epitaxial growth of the STO films on LSAT [Fig. 1(a–i)]. The STO/LSAT films have a perovskite-structure and possess epitaxial relationships [100](001)STO‖[100](001)STO. The VO-, H-, and N-films were perfectly coherent to LSAT [Fig. 1(f–h)], whereas partial relaxation was found in the O-film [Fig. 1(e)].The lattice parameter of cubic LSAT (aLSAT = 3.868 Å) is smaller than that of cubic STO (aSTO = 3.905 Å). Correspondingly, the theoretical biaxial in-plane STO/LSAT misfit strain is compressive: sa = (aLSAT/aSTO − 1) ≈ −0.95%. For the coherent strained (001)STO film on (001)LSAT, the in-plane lattice parameters should be similar and equal to a = aLSAT and the out-of-plane lattice parameter c should be elongated, respectively: c = aSTO·[1 − (2c12/c11)·sa] ≈ 3.927 Å. The elastic constants of STO are c11 = 3.48 × 1011 N m−2 and c12 = 1.03 × 1011 N m−2, respectively.12 As found recently,3 such tetragonal fully strained STO/LSAT films can grow to a thickness of <100 nm, above which the misfit relaxation occurs. In agreement with ref. 3, the 80 nm-thick O-film is tetragonal. The lattice parameters are c ≈ 3.926 Å and a ≈ 3.870 Å, nearly equal to the theoretically estimated ones.
For oxygen vacancy VO in STO, the neighboring Ti ions are displaced away from the vacancy and the lattice locally stretches along the Ti–VO–Ti direction.13–17 This local lattice anisotropy averages out on the macroscopic level for a conventional random spatial distribution of the vacancies; the corresponding chemical lattice expansion is usually isotropic. However, in the epitaxial STO/LSAT films, the in-plane compression can promote the vacancy formation in the Sr–O planes parallel to the substrate (001) surface, i.e. in the [Sr–O](001) planes.7–9 This selective vacancy location is qualitatively comprehensible because the in-plane Ti–VO–Ti elongations “work” against the in-plane misfit compression that is energetically unfavorable. The strain-assisted favorable out-of-plane alignment of the local lattice Ti–VO–Ti elongations can result in a specific preferably out-of-plane macroscopic chemical expansion.
Here, the specific out-of-plane chemical expansion is evidenced by the increased out-of-plane lattice parameter cVO = 3.938 Å in the VO-film compared to the O-film, whereas the in-plane lattice parameters are unchanged therein [Fig. 1(e, f and i)]. Nevertheless, assuming that the large cVO originates from both the isotropic chemical expansion (due to cationic or oxygen off-stoichiometry) of the VO-film material and the film's in-plane compression by LSAT, the lattice parameter of the unstrained cubic VO-material should have been equal to aVO ≈ 3.913 Å, and the film–substrate misfit strain, correspondingly, saVO ≈ −1.1%. However, the increased magnitude of saVO suggests a partial strain relaxation and, hence, changes of the in-plane lattice parameters.3 Instead, the coherency of the VO-film to the LSAT substrate is improved compared to the O-film [Fig. 1(e, f and i)]. The better epitaxial quality of the VO-film is consistent with the out-of-plane alignment of the Ti–VO–Ti complexes that diminishes the total elastic energy.
In order to strongly prove the out-of-plane chemical expansion, we investigated a thicker VO-film (thickness ∼200 nm). In the absence of aligned Ti–VO–Ti complexes, the misfit strain was expected to relax.3 In contrast to this expectation, the film exhibits excellent coherency to LSAT, which evidences the lack of strain relaxation and solidly supports the out-of-plane Ti–VO–Ti orientation [Fig. S1, ESI†].
The out-of-plane chemical expansion is clearly present in the N-films and H-films [Fig. 1(c, d and g–i)], where the out-of-plane elongation is further enhanced: cN = 3.956 Å and cH = 3.966 Å. These observations are consistent with the out-of-plane alignment of the Ti–N–Ti and Ti–(H or H2)–Ti complexes and indicate substitutional lattice incorporation of anions.18–24 We emphasize that interstitial incorporation cannot produce such revealed anisotropic chemical expansion. We note that the deposition conditions do not affect the cationic composition of the films, where the Sr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti ratio is close to one [Fig. 1(j) and Fig. S2, ESI†]. In addition, we measured an approximately similar oxygen content in all films within the quantification accuracy of XPS, which suggests oxygen deficiency of δ ≤ 0.2 in SrTiO3−δ. However, compared to the O-film, the unit-cell volume is expanded by 0.3% in the VO-film, 0.8% in the N-film, and 1% in the H-film.
Ti ratio is close to one [Fig. 1(j) and Fig. S2, ESI†]. In addition, we measured an approximately similar oxygen content in all films within the quantification accuracy of XPS, which suggests oxygen deficiency of δ ≤ 0.2 in SrTiO3−δ. However, compared to the O-film, the unit-cell volume is expanded by 0.3% in the VO-film, 0.8% in the N-film, and 1% in the H-film.
We emphasize that the large out-of-plane chemical expansion is achieved in the strained epitaxial STO/LSAT films, whereas the crystal structure and lattice parameters are independent of deposition conditions in polycrystalline films grown on Si/SiO2 [Fig. S3, ESI†]. These observations additionally corroborate the misfit-assisted formation of oxygen vacancies and anion doping. Next, we verify substitutional lattice incorporation of hydrogen or nitrogen by analyzing the optical properties of the films.
In-gap states
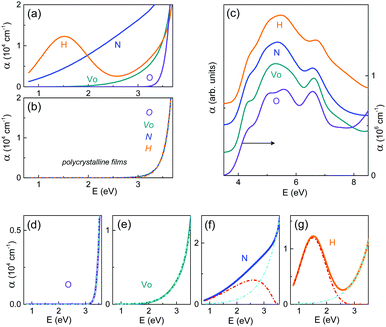
The oxygen vacancies and anion dopants at oxygen sites produce diverse in-gap states in STO.13–24 Because the optical transitions from/to these states are fingerprints of the vacancies and dopants, investigations of the optical absorption coefficient α can disentangle the vacancy formation and anion interstitial or substitutional incorporation.The measured optical absorption spectra α(E) reveal the onset of absorption at photon energy E > 3.5 eV in all films [Fig. 2(a–c)]. Remarkably, distinct dopant-dependent types of absorption behavior are clearly seen in the near-edge spectral range from 0.7 to ∼3.2 eV [Fig. 2(a and b)]. The regular epitaxial O-film [Fig. 2(a)] and all polycrystalline films [Fig. 2(b)] exhibit excellent transparency in this range. This behavior evidences a wide bandgap, in agreement with that in pure STO, and shows the absence of deep in-gap states. The absence of in-gap states is consistent with the absence of a chemical expansion, both of which indicate negligible, if any, concentration of vacancies or dopants.
For optical absorption in STO, a near-edge absorption tail is characteristic and obeys the Urbach rule: α ∝ exp[(σE)/(kBT)], where the parameter σ characterizes the tail steepness, kB is the Boltzmann constant, and T is the temperature.25 Good Urbach fits are obtained in the epitaxial O- and VO-films [Fig. 2(d and e)], whereas the Urbach tails are superimposed with dopant-specific absorption peaks in the N- and H-films [Fig. 2(f and g)]. The peaks indicate occupied in-gap states lying ∼2.5 eV below the conduction band in the N-film and ∼1.5 eV below the conduction band in the H-film.
According to the first-principles analyses,15,16 the in-gap VO-states are expected to be ∼0.2 eV and 0.5 eV below the conduction band and, hence, should not manifest themselves in the spectral range investigated here. The results in Fig. 2(e) are consistent with this expectation.
The deep in-gap states detected here in the N-film [Fig. 2(f)] were not found for the interstitial incorporation of nitrogen in nitrogen-doped (001)STO films.26 The optically evidenced absence of interstitial nitrogen in our films is confirmed by XPS data: the N 1s lines and shoulders of the Ti 2p lines, which correspond to interstitial nitrogen,26 are not detected therein. The wide bandgap and the deep in-gap states in the N-film agree well with theoretical calculations,18,19 which show that the substitution of N for O introduces in-gap states, whereas the mixing of N 2p and O 2p states is very weak and does not reduce the bandgap. Moreover, our observations of optical absorption at ∼2.5 eV are in excellent agreement with the calculations for substitutional N.20 The results in Fig. 2(f) thus solidly support the substitutional incorporation of nitrogen.
Considering theoretical analyses of hydrogen centers in STO, the observed concurrent strong out-of-plane lattice expansion and optical absorption at ∼1.5 eV in the H-film imply the incorporation of one and/or two hydrogen atoms at the oxygen-vacancy site.21,22 The two atoms can form stable hydrogen molecules (H2) and metastable (2H) complexes. Whereas H2 molecules can produce the strong lattice expansion, they cannot be responsible for the deep in-gap states. The optical absorption at ∼1.5 eV is likely caused by the excitation of metastable (2H) complexes.21 We note that other types of hydrogen-related centers, which are difficult to detect experimentally, may still be present in the H-film. Importantly, our observations reveal a profound substitutional incorporation of hydrogen.
Interband transitions
For interstitial nitrogen, an uplift of the top valence band and the corresponding narrowing of the optical bandgap are expected.26 For interstitial hydrogen, the formation energy is low, whereas the related lattice expansion is negligible and not easily seen in chemical expansion. However, a sufficiently large concentration of interstitial hydrogen can lead to an apparent narrowing of the optical bandgap because of the lowering of the conduction band edge.22 Therefore, a decrease of the energies of the interband optical transitions can signify the presence of interstitial anion dopants in our films.Here, the near-edge absorption tails prevent from accurate analysis of the indirect bandgap, typical for STO. Good linear fits (αE)2 ∝ (E − Ed) reveal the lowest-energy direct transition being at Ed ≈ 4 eV in all films [Fig. 3(a–d)]. This energy is ∼0.2 eV larger than that (∼3.8 eV) in bulk STO.27 Such a substantial blueshift agrees well with the predicted uplift of the conduction band in epitaxial strained antiferrodistortive STO films.28 Remarkably, the blueshift increases with increasing out-of-plane elongation in the VO-, N-, and H-films, which also agrees with the predictions.28 There are no detectable indications of interstitial-induced bandgap narrowing.
The different shapes of the absorption spectra in Fig. 2(c) for different films may be caused by dopant-induced changes in the conduction and/or valence bands. To uncover such changes, we performed closer inspections of the interband transitions via critical-point (CP) analysis using the second derivatives of the dielectric functions.8,29,30 The types of the CP lines are essentially the same in all films [Fig. 3(e–g)]. The energies of the main CPs increase with increasing out-of-plane elongation (from the O-film to the VO-, N-, and H-film, respectively), in qualitative agreement with ref. 27. Additionally, compared to the O-film, the lines are smeared in the spectral range between 5 and 6 eV for all dopant types (VO, N, and H). This observation suggests an effect of oxygen deficiency or substitution rather than interstitial dopants.
Overall, the detected interband transitions are consistent with the effects of lattice strain and oxygen deficiency and/or substitution, while negating appreciable interstitial doping.
Conclusions
In conclusion, misfit-assisted in situ anion doping is demonstrated in the epitaxial films of archetypal perovskite oxide SrTiO3. Strong out-of-plane chemical expansion is evidenced in the presence of oxygen vacancies and/or nitrogen or hydrogen anion dopants in the cube-on-cube epitaxial films grown on compressive substrates. Substitutional incorporation of anion dopants is indicated by the optical behavior of the films. The observations imply the misfit-promoted formation of oxygen vacancies and oxygen substitution in the Sr–O planes parallel to the substrate surface. This demonstrated approach can be applied to other perovskite oxides such as titanates and rare-earth nickelates.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge support from the Czech Science Foundation (Grant No. 19-09671S) and the European Structural and Investment Funds and the Ministry of Education, Youth and Sports of the Czech Republic through Programme “Research, Development and Education” (Project No. SOLID21 – CZ.02.1.01/0.0/0.0/16_019/0000760).References
- H. Kageyama, K. Hayashi, K. Maeda, J. P. Attfield, Z. Hiroi, J. M. Rondinelli and K. R. Poeppelmeier, Nat. Commun., 2018, 9, 772 CrossRef.
- N. A. Pertsev, A. G. Zembilgotov and A. K. Tagantsev, Phys. Rev. Lett., 1998, 80, 1988 CrossRef CAS.
- T. Yamada, B. Wylie-van Eerd, O. Sakata, A. K. Tagantsev, H. Morioka, Y. Ehara, S. Yasui, H. Funakubo, T. Nagasaki and H. J. Trodahl, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 91, 214101 CrossRef.
- U. Aschauer, R. Pfenninger, S. M. Selbach, T. Grande and N. A. Spaldin, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 054111 CrossRef.
- U. Aschauer and N. A. Spaldin, Appl. Phys. Lett., 2016, 109, 031901 CrossRef.
- A. Herklotz, D. Lee, E.-J. Guo, T. L. Meyer, J. R. Petrie and H. N. Lee, J. Phys.: Condens. Matter, 2017, 29, 493001 CrossRef.
- J. Xi, H. Xu, Y. Zhang and W. J. Weber, Phys. Chem. Chem. Phys., 2017, 19, 6264 RSC.
- M. Tyunina, D. Chvostova and A. Dejneka, Phys. Chem. Chem. Phys., 2019, 21, 7874 RSC.
- M. Tyunina, J. Peräntie, T. Kocourek, S. Saukko, H. Jantunen, M. Jelinek and A. Dejneka, Phys. Rev. Res., 2020, 2, 023056 CrossRef CAS.
- Casa Software, 2013, http://www.casaxps.com.
- A. Dejneka, D. Chvostova, O. Pacherova, T. Kocourek, M. Jelinek and M. Tyunina, Appl. Phys. Lett., 2018, 112, 031111 CrossRef.
- Landolt-Börnstein, Numerical Data and Functional Relationships in Science and Technology, New Series, Group III, Crystal and Solid State Physics, ed. K. H. Hellwege and A. M. Hellwege, Springer, Berlin, 1981 Search PubMed.
- J. Carrasco, F. Illas, N. Lopez, E. A. Kotomin, Yu. F. Zhukovskii, R. A. Evarestov, Yu. A. Mastrikov, S. Piskunov and J. Maier, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 064106 CrossRef.
- C. Lin, C. Mitra and A. A. Demkov, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 161102(R) CrossRef.
- D. Gryaznov, E. Blokhin, A. Sorokine, E. A. Kotomin, R. A. Evarestov, A. Bussmann-Holder and J. Maier, J. Phys. Chem. C, 2013, 117, 13776 CrossRef CAS.
- A. Janotti, J. B. Varley, M. Choi and C. G. Van de Walle, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 90, 085202 CrossRef.
- D. Lee, H. Wang, B. A. Noesges, T. J. Asel, J. Pan, J. W. Lee, Q. Yan, L. J. Brillson, X. Wu and C. B. Eom, Phys. Rev. Mater., 2018, 2, 060403(R) CrossRef.
- H. F. Liu, Solid State Commun., 2012, 152, 2063 CrossRef CAS.
- C. Zhang, Y. Jia, Y. Jing, Y. Yao, J. Ma and J. Sun, Comput. Mater. Sci., 2013, 79, 69 CrossRef CAS.
- P. Reunchan, N. Umezawa, A. Janotti, J. T-Thienprasert and S. Limpijumnong, Phys. Rev. B, 2017, 95, 205204 CrossRef.
- Y. Iwazaki, Y. Gohda and S. Tsuneyuki, APL Mater., 2014, 2, 012103 CrossRef.
- J. B. Varley, A. Janotti and C. G. Van de Walle, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 075202 CrossRef.
- T. U. Ito, A. Koda, K. Shimomura, W. Higemoto, T. Matsuzaki, Y. Kobayashi and H. Kageyama, Phys. Rev. B, 2017, 95, 020301(R) CrossRef.
- T. Misaki, I. Oikawa and H. Takamura, Chem. Mater., 2019, 31, 7178 CrossRef CAS.
- N. V. Kurik, Phys. Status Solidi A, 1971, 8, 9 CrossRef.
- Y. Y. Mi, Z. Yu, S. J. Wang, X. Y. Gao, A. T. S. Wee, C. K. Ong and C. H. A. Huan, J. Appl. Phys., 2007, 101, 063708 CrossRef.
- M. Rossle, C. N. Wang, P. Marsik, M. Yazdi-Rizi, K. W. Kim, A. Dubroka, I. Marozau, C. W. Schneider, J. Humlicek, D. Baeriswyl and C. Bernhard, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 104110 CrossRef.
- R. F. Berger, C. J. Fennie and J. B. Neaton, Phys. Rev. Lett., 2011, 107, 146804 CrossRef.
- P. Yu and M. Cardona, Fundamentals of Semiconductors, Springer, New York, 1996 Search PubMed.
- S. Zollner, A. A. Demkov, R. Liu, P. L. Fejes and R. B. Gregory, J. Vac. Sci. Technol., B, 2000, 18, 2242 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0cp03644g |
| This journal is © the Owner Societies 2020 |