Theoretical prediction of T-graphene as a promising alkali-ion battery anode offering ultrahigh capacity†
Abstract
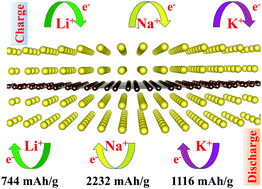
The pursuit of high capacity is one of the key challenges for the development of alkaline ion batteries (AIBs, namely Li/Na/K-ion batteries; LIBs, NIBs, or KIBs). Carbon-based anode materials represented by graphite have been widely used in secondary batteries; however, their storage capacity is generally not high. Graphene was once considered a promising candidate, but it has proven to be incapable of interacting strongly with alkali ions. Here, by first-principles calculations, we predict an allotrope of graphene that may soon be experimentally synthesized, called T-graphene, which is a promising anode material for AIBs. We find that when it is used as the anode for NIBs, its theoretical capacity can be as high as 2232 mA h g−1 (Na6C6), which is six times that of graphite. For LIB and KIB anodes, the specific capacities are 744 mA h g−1 and 1116 mA h g−1, corresponding to the Li2C6 and K3C6 chemical formula, respectively. We first demonstrate that the material is mechanically stable. We further show that the material has good electrical conductivity whether it is before or after adsorption of Li(Na or K). We also studied the diffusion of Li(Na or K) on its surface and found that their corresponding diffusion barriers are very low (Li, Na and K are about 0.37 eV, 0.35 eV and 0.25 eV, respectively), which means good rate performance. It is calculated that the average open circuit voltage of the corresponding three half-cells at full charge is also low (LIBs is about 0.20 V, NIBs is about 0.12 V, and KIBs are about 0.37 V), which is beneficial for increasing the operating voltage of the full battery. In addition, during the adsorption of lithium, sodium and potassium, the lattice change of the material is very small (about 1.0% for lithium, about 1.4% for sodium, and about 1.9% for potassium), which means good cycling performance. These results indicate that T-graphene is expected to replace graphene and become a very attractive anode material for AIBs.


 Please wait while we load your content...
Please wait while we load your content...