Excess electrons bound to H2S trimer and tetramer clusters
Abstract
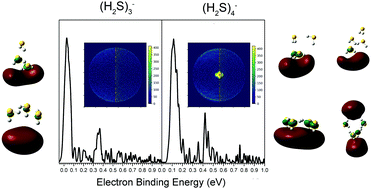
We have prepared the hydrogen sulfide trimer and tetramer anions, (H2S)3− and (H2S)4−, measured their anion photoelectron spectra, and applied high-level quantum chemical calculations to interpret the results. The sharp peaks at low electron binding energies in their photoelectron spectra and their diffuse Dyson orbitals are evidence for them both being dipole-bound anions. While the dipole moments of the neutral (H2S)3 and (H2S)4 clusters are small, the excess electron induces structural distortions that enhance the charge-dipolar attraction and facilitate the binding of diffuse electrons.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...