Systematic comparison of racemic and enantiopure multicomponent crystals of phenylsuccinic acid—the role of chirality†
Abstract
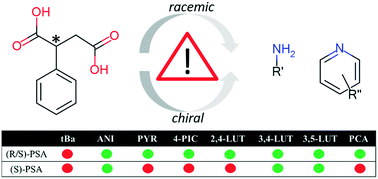
The effect of chirality was investigated by designing, synthesizing and analysing a series of racemic multicomponent crystals and their homochiral counterparts. Eleven new crystalline materials were synthesised by combining phenylsuccinic acid (racemate or the S-enantiomer) with primary amines (tert-butylamine, aniline), pyridine or its derivatives (4-picoline, 2,4-lutidine, 3,4-lutidine and 3,5-lutidine) and a structurally related drug, pyrazinecarboxamide (PCA). The carboxylic acids were combined with the coformers to ensure the formation of the multicomponent crystals via acid–base heterosynthons. The various positions of the functional groups on the pyridine derivatives gave rise to the fine tuning of the packing of the crystals. Differential scanning calorimetry, thermogravimetry, Fourier transform infrared spectroscopy, powder and single crystal X-ray diffraction analysis were carried out. These crystallisation experiments resulted in a variety of different crystal classes. The analysis of the crystal packing (Z and related parameters), the formed synthons and the thermal behaviour of the crystals highlighted the complexity of the packing and the differences between the racemic multicomponent crystals and their homochiral counterparts. It was concluded that the inclusion of chiral building blocks may lead to the formation of rare classes of multicomponent crystals, occasionally with increased Z′′ and Zr values, and allow the observation of compounds at different protonation stages in the same crystal.


 Please wait while we load your content...
Please wait while we load your content...