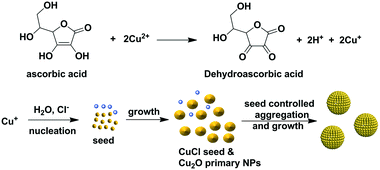
One-pot synthesis of monodisperse Cu2O nanoparticle aggregates through an in situ seed generation process†
Jianwei
Jiang
,
Sanghyuk
Park
 * and
Longhai
Piao
* and
Longhai
Piao
 *
*
Department of Chemistry, Kongju National University, Chungnam, 32588, Korea. E-mail: spark0920@kongju.ac.kr; piaolh@kongju.ac.kr
First published on 12th November 2019
Abstract
Monodisperse nanoparticle aggregates (NPAs) have great potential for applications in biomedical detection, light harvesting, and photocatalysis, but the synthesis of these materials using a one-pot method remains challenging due to the difficulty of the separation of the nucleation and growth processes. In this paper, monodisperse spherical Cu2O NPAs were synthesized using a simple one-pot strategy. The size of the Cu2O NPAs was controlled from 294 nm to 443 nm, and the size distribution was as narrow as δ = 8.1% and 5.6%. The key to the formation of NPAs with a narrow size distribution can be attributed to the in situ generated CuCl acting as seeds to control the aggregation process of the primary Cu2O NPs. This method is extremely simple and scalable, and the reaction was conducted in air and aqueous solution at room temperature.
Introduction
Inorganic NPs are of tremendous interest in the science and technology fields due to their unique size-dependent optical and electronic properties. Recently, the research interest in NPs has shifted to the construction of secondary structures of NPs in order to obtain materials having novel integrated functions and potential applications.1–5 Among the various types of secondary structures of NPs, spherical NPAs composed of packed primary NPs have attracted considerable interest. These NPAs show collective magnetic, optical, or electrical characteristics that are different from those of either individual NPs in solution or NPs self-assembled onto a substrate. Additionally, NPAs can be highly dispersed in solution enabling their easy post-synthetic modification and resulting in their extended applicability.NPAs with a narrow size distribution have been of particular interest in fundamental science and technological applications.1–3 Generally, only NPAs with a narrow size distribution can be further assembled into ordered 2D or 3D hierarchical structures that have demonstrated a wide range of potential applications, including biomedical detection, surface enhanced Raman scattering enhancement, light harvesting and photocatalysis.1 Uniform NPAs are also an excellent model system for the study of the catalytic reaction mechanisms.6
There are two general methods for the synthesis of NPAs. The first is the two-step method in which a stable NP suspension is first formed, and then the NPs are assembled into NPAs by the modification of the interparticle forces using an external stimulus.1–3 However, this method is time-consuming and difficult to scale up for mass production. The other method is the one-pot method in which the NP formation and assembly processes are achieved in a single process without necessitating either the isolation or purification of the primary NPs. This method is more facile and rapid than the two-step method and is also scalable for large-scale NPA production. However, to date, only limited classes of monodisperse NPAs using the one-pot method have been reported such as Fe3O4, ZnO, In2O3, and PdS NPAs,3 which can be ascribed to the following reasons: (i) no clear separation of the nucleation and growth processes, which leads to an uncontrollable and random aggregation process of primary NPs resulting in polydisperse NPAs; (ii) the given complicated reaction system in which the formation and aggregation processes of the primary NPs are conducted, leading to the formation mechanism of secondary structures remaining unclear; (iii) fewer control parameters that can be used to obtain monodisperse NPAs in the one-pot method, causing the synthetic procedures to be described mostly as “art” and their design not to be based on a rational approach.
Cu2O, a p-type semiconductor with a band gap of 2.17 eV, has been widely used in solar energy conversion,7 water splitting,8 lithium-ion batteries,9 and low temperature CO oxidation.10 To date, only a few studies of monodisperse Cu2O NPAs have been reported. For example, Wu et al. recently synthesized uniform Cu2O NPAs in ethylene diglycol using a hot-injection (180 °C) step for seed generation and a heating-up (25–150 °C) step for further growth and applied the synthesized NPAs to construct photonic crystal structures.11 Li et al. reported the synthesis of monodisperse Cu2O NPAs from the reduction of Cu(CH3COO)2 with NaBH4 in N,N-dimethyl formamide at 90 °C through the rapid formation of supersaturated primary Cu2O NPs followed by their regular spherical aggregation.12 Zeng et al. also synthesized uniform Cu2O NPAs by reducing Cu(OH)42− in 2-propanol using a polyvinylpyrrolidone (PVP) surfactant through the dropwise addition of N2H4·H2O and constructed highly ordered self-assemblies of Cu2O using its monodisperse properties.13 The syntheses described in the above reports are complicated, and they were performed under relatively harsh conditions such as using toxic reductants or elevated temperature. Notably, all of these syntheses were performed in organic solvents that are not environmentally friendly. Therefore, the development of a green and facile synthetic approach for obtaining monodisperse Cu2O NPAs is desirable and significant.
Previously, we reported a facile synthesis of Cu2O NPAs by reducing Cu(CH3COO)2 with ascorbic acid in water with the help of a surfactant such as polyacrylamide (PAM) or PVP.14–18 This method was extremely simple and scalable, and the reaction was conducted under mild conditions in several minutes. However, the size distribution of NPAs was relatively broad with a deviation of δ = 13–50%.
Inspired by the reports that ionic compounds play an essential role in the morphology control and the growth process of NPs, we tested the effect of ionic compounds on the Cu2O NPA synthesis.19–22 Here, the report by the group of Lou, where it was shown that insoluble CuCl can be generated in situ in the interfacial reaction between an aqueous solution of SnCl4 and solid Cu2O crystals, was of particular importance.19 In this study, monodisperse Cu2O NPAs were synthesized through modification of our previous method, which involved the simple addition of NaCl into the reaction system. The key to the formation of monodisperse NPAs can be attributed to the separation of nucleation and growth processes due to the in situ generated CuCl seeds.
Experimental section
Materials and methods
Cu(Ac)2·H2O, L(+)-ascorbic acid, PVP (Mw = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 29
000, 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 40
000, 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 or 55
000 or 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1), PAM (Mw = 1500 g mol−1), polyethylene glycol (4000 g mol−1), and hydroxypropyl cellulose (Mw = 80
000 g mol−1), PAM (Mw = 1500 g mol−1), polyethylene glycol (4000 g mol−1), and hydroxypropyl cellulose (Mw = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) were purchased from Sigma-Aldrich. NaCl (extra pure reagent) was supplied by TCI. Ethanol (95%) was obtained from Samchun Chemical. The morphology and composition of the samples were characterized using a Hitachi Model S-4800 field-emission scanning electron microscopy (FESEM) system at an accelerating voltage of 10.0 kV and an energy-dispersive X-ray (EDX) spectrometer. The powder samples were mounted on double sided carbon tape for FESEM–EDX measurements. The structure of the prepared samples was determined by transmission electron microscopy (TEM) operated at 300 kV and using the obtained selected area electron diffraction (SAED) pattern. The sample for TEM–SAED measurements was prepared by placing 3 drops of a dilute (0.5 mg mL−1) product solution in ethanol onto a carbon-coated copper grid; the sample was dried in a vacuum at room temperature for several hours prior to the measurement. The phase structures of the products were characterized by using an X-ray diffractometer with Cu Kα radiation (λ = 1.5418 Å) at a voltage of 40 kV with a scanning rate of 0.02° s−1 in the 2θ range of 10–80°. The hydrodynamic size and zeta potential of particles were measured using a Malvern Zetasizer ZS90 instrument. The temperature was held at 25 °C with an external thermostat. The UV-vis diffuse reflectance spectra (DRS) of the powder samples were obtained using a Shimadzu SolidSpec-3700 DUV spectrophotometer. The size distribution of the particles (δ) is defined as the ratio of the standard deviation (SD) to the average diameter of the particles (Ā).22–24 The sample SD is calculated by the formula
000 g mol−1) were purchased from Sigma-Aldrich. NaCl (extra pure reagent) was supplied by TCI. Ethanol (95%) was obtained from Samchun Chemical. The morphology and composition of the samples were characterized using a Hitachi Model S-4800 field-emission scanning electron microscopy (FESEM) system at an accelerating voltage of 10.0 kV and an energy-dispersive X-ray (EDX) spectrometer. The powder samples were mounted on double sided carbon tape for FESEM–EDX measurements. The structure of the prepared samples was determined by transmission electron microscopy (TEM) operated at 300 kV and using the obtained selected area electron diffraction (SAED) pattern. The sample for TEM–SAED measurements was prepared by placing 3 drops of a dilute (0.5 mg mL−1) product solution in ethanol onto a carbon-coated copper grid; the sample was dried in a vacuum at room temperature for several hours prior to the measurement. The phase structures of the products were characterized by using an X-ray diffractometer with Cu Kα radiation (λ = 1.5418 Å) at a voltage of 40 kV with a scanning rate of 0.02° s−1 in the 2θ range of 10–80°. The hydrodynamic size and zeta potential of particles were measured using a Malvern Zetasizer ZS90 instrument. The temperature was held at 25 °C with an external thermostat. The UV-vis diffuse reflectance spectra (DRS) of the powder samples were obtained using a Shimadzu SolidSpec-3700 DUV spectrophotometer. The size distribution of the particles (δ) is defined as the ratio of the standard deviation (SD) to the average diameter of the particles (Ā).22–24 The sample SD is calculated by the formula 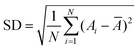 , where A1, A2, …, AN are the measured values of the samples and N is the number of the measured samples. Ā was randomly measured using the ImageJ software by the analysis of 150 particles from the SEM images.
, where A1, A2, …, AN are the measured values of the samples and N is the number of the measured samples. Ā was randomly measured using the ImageJ software by the analysis of 150 particles from the SEM images.
Synthesis of the uniform Cu2O NPAs
The uniform Cu2O NPAs were synthesized according to our previously reported method with a slight modification.16 In a typical procedure, PVP-10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (3.375 g), Cu(CH3COO)2·H2O (1.125 g) and H2O (51.5 mL) were added into a 100 mL round flask containing a magnetic stirring bar at room temperature. After the dissolution of PVP and Cu(CH3COO)2·H2O within 2 h, an NaCl aqueous solution (2.250 g, 10 mg g−1) was added to the mixture solution. Subsequently, an ascorbic acid solution (1.125 g in 2.5 mL H2O) was quickly poured into the above solution and allowed to react for 5 minutes at a stirring rate of 200 rpm. After the reaction, the suspension was centrifuged (3000 rpm, 3 minutes) and washed twice with distilled water. The product (0.317 g) was obtained after drying in a vacuum at 45 °C with an 82% yield.
000 (3.375 g), Cu(CH3COO)2·H2O (1.125 g) and H2O (51.5 mL) were added into a 100 mL round flask containing a magnetic stirring bar at room temperature. After the dissolution of PVP and Cu(CH3COO)2·H2O within 2 h, an NaCl aqueous solution (2.250 g, 10 mg g−1) was added to the mixture solution. Subsequently, an ascorbic acid solution (1.125 g in 2.5 mL H2O) was quickly poured into the above solution and allowed to react for 5 minutes at a stirring rate of 200 rpm. After the reaction, the suspension was centrifuged (3000 rpm, 3 minutes) and washed twice with distilled water. The product (0.317 g) was obtained after drying in a vacuum at 45 °C with an 82% yield.
Results and discussion
As found in our previous work,16 Cu2O NPAs were produced by reducing Cu(CH3COO)2 with ascorbic acid using a PVP surfactant at room temperature in aqueous solution within 5 minutes. After pouring the ascorbic acid solution into the Cu(CH3COO)2 solution containing PVP, the mixed solution immediately changed from blue-green to dark-green and then rapidly turned orange. Highly inhomogeneous and micrometer-sized particles (∼1.5 μm) were typically obtained in our various experiments that included the use of various surfactant types, chain lengths, surfactant concentrations, temperatures, and solvents (Fig. 1a and S1–S5†).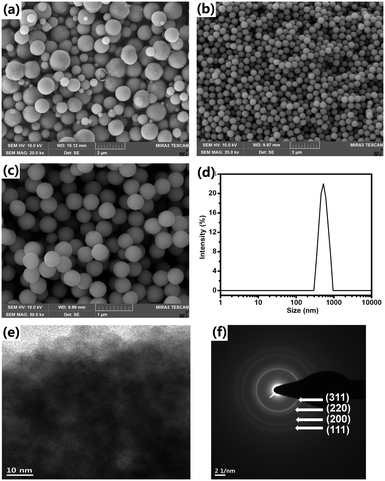 | ||
| Fig. 1 Characterization of Cu2O NPAs produced without NaCl (a) and with 3 wt% NaCl (b and c) via SEM, DLS (d), TEM (e), and SAED (f). | ||
In our revised method, NaCl was first added into the Cu(CH3COO)2 solution containing PVP. Then, the ascorbic acid solution was poured into the mixture at a stirring speed of 200 rpm. After adding the ascorbic acid solution, before we observe the abovementioned color changes, a milk-like color appeared rapidly and subsequently disappeared. The FESEM images confirm that the synthesized particles have a narrow size distribution and spherical shape (Fig. 1b and c and S6†). The size and size distribution of the NPAs are 443 nm and δ = 5.6%, respectively, according to the results obtained by measuring 150 particles in the given SEM images. The DLS results show a single peak and a small PDI value of 0.031 ± 0.005, further demonstrating the narrow size distribution of the NPAs (Fig. 1d). DLS also confirmed that the size of the NPAs is 540 ± 16 nm, which is larger than that measured from the FESEM images, because DLS detects the hydrodynamic diameter of the Cu2O NPAs. A close inspection of the TEM image revealed that the particles were composed of packed primary NPs (Fig. 1e). The SAED pattern shows highly diffusive and weak diffraction rings revealing the small sizes and the irregular assemblies of the primary NPs (Fig. 1f).
Some other ionic additives were also tested to synthesize the Cu2O NPAs under the same reaction conditions. For example, when NaNO3 was used, non-uniform NPAs were formed (Fig. S7†). In the presence of KCl, uniform NPAs can also be prepared with a similar product quality to that of NaCl (Fig. S8†). Based on the above results and reaction phenomenon, it is therefore deduced that the Cl− anion acts as the promoter for the nucleation agent formation at the initial stage of the reaction. The Cl element was observed from the EDX spectrum of the Cu2O NPAs, but no Na element was detected (Fig. S9†), further indicating that the Cl− anion plays a key role in the formation of monodisperse Cu2O NPAs.
The effect of the NaCl amount on the size and size distribution of the Cu2O NPAs was studied. To obtain NPAs with a relatively narrow size distribution, the NaCl amount should be controlled in a certain range. For 1% NaCl, the NPA size distribution was 15.9% (Fig. 2a). When 2% NaCl amount was used, a relatively narrow size distribution of NPAs was obtained (10.9%, Fig. 2b). As the NaCl amount increased (3% and 4%), the NPA size distribution was further narrowed, giving a size distribution of 5.6% and 8.1%, respectively (Fig. 1c and 2c). DLS also demonstrated that the size distribution narrowed with increasing amount of NaCl, and the PDI values were 0.162 ± 0.056, 0.107 ± 0.013, 0.031 ± 0.005, and 0.062 ± 0.015 for Cu2O with 1%, 2%, 3%, and 4% NaCl, respectively. Murphy's group also reported that the size distribution of Au NPs decreased from 58% to 30% as the concentration of Au seeds increased from 8.9 × 1012 to 1.7 × 1014 (number of seeds per L) due to the reduction in further nucleation during the growth process.24 However, the size distribution of the NPAs broadened when 10% NaCl was added (Fig. S10†).
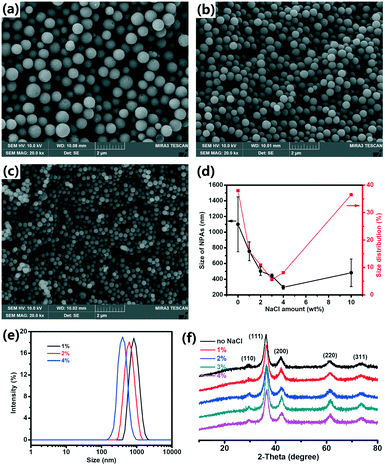 | ||
| Fig. 2 Characterization of Cu2O NPAs with SEM (a–c), DLS (e) and XRD (f). (d) Size and size distribution of Cu2O NPAs obtained with various amounts of NaCl. | ||
To analyze the effect of the NaCl amount on the NPA size, the NPA size was plotted as a function of the NaCl amount, as shown in Fig. 2d(black line). The NPA size was reduced as the NaCl amount increased from 0% to 4%. The diameters were 756 ± 120 nm, 503 ± 55 nm, and 297 ± 24 nm for Cu2O with 1%, 2%, and 4% NaCl, respectively. This trend in the variation of NPA sizes was also demonstrated by the DLS data (Fig. 2e). Generally, in a given reaction system, more seed particles result in a higher number of aggregates, thus leading to smaller NPAs; conversely, fewer seed particles lead to larger NPAs. For example, Weller's group reported that in the synthesis of CoPt3 NPs, slower nucleation rates result in low nuclei concentrations and consequently, larger NPs, whereas fast nucleation yields smaller NPs due to the high nuclei concentration.25 This result is similar to the results found in polymer chemistry such as for living anionic polymerization. These results further proved that NaCl plays a key role in the control of the seed formation.
XRD measurements were performed to study the effect of the NaCl amount on the phase structures of NPAs. The peaks of the NPAs synthesized with different amounts of NaCl were similar and each sample showed only Cu2O diffraction peaks (JCPDS 05-0667, Fig. 2f). The primary NP size in the NPAs was calculated from the half-width of the intense (111) peak using the Scherrer equation. We found that the primary NPs in the NPAs maintain a similar size (5.0 nm for 1100 nm NPAs, 5.3 nm for 753 nm NPAs, 4.8 nm for 503 nm NPAs, 5.8 nm for 443 nm NPAs, and 4.9 nm for 297 nm NPAs). These results suggested that NaCl has no effect on the phase structure and the size of the primary NPs.
The influence of the stirring speed on the size distribution of the products was investigated. Without stirring, uniform NPAs could not be obtained even when the amount of NaCl was tuned from 2% to 6% due to the highly inhomogeneous diffusion of the reactants (Fig. S11†). Specifically, after pouring the ascorbic acid solution into the reaction system, the primary Cu2O NPs first formed and aggregated in the upper layer of the solution, and this time, the lower layer maintained the blue color of the Cu2+ ion solution. With the diffusion of ascorbic acid and products into the lower layer, the newly formed primary NPs not only attach to the existing aggregates but also form new aggregates, resulting in polydisperse particles. Under a relatively high stirring speed (600 rpm), uniform spherical particles could not be obtained due to the failure in controlling the aggregation process of the primary NPs (Fig. S12†). To our delight, monodisperse Cu2O NPAs were well obtained at the optimal stirring speed of 200 rpm, which can be attributed to the seed-mediated aggregation of the primary NPs.
The anti-oxidation behavior of monodisperse Cu2O was studied. After storing the dried Cu2O (4% NaCl) for one month in air at 4 °C, the color of the product remains yellow. To our surprise, only the diffraction peaks of Cu2O were observed in the XRD pattern after storage for one month (Fig. S13†). This may be ascribed to the protection of Cu2O by a thin layer of PVP, as revealed in the TEM image of the product (Fig. S14†).
To evaluate the surface charge of Cu2O in the aqueous solution, the zeta potential of Cu2O (0–4% NaCl) was obtained from the DLS results under neutral conditions. For example, the pure Cu2O (no NaCl) shows a zeta potential of +17.8 mV. The positive zeta potential of Cu2O is due to the protection of PVP macromolecules, leading to the electron donation of their carbonyl groups onto Cu2O.26 In comparison, the zeta potential values are +15.3 mV, +8.2 mV, +11.9 mV, and +11.3 mV for Cu2O obtained with 1%, 2%, 3%, and 4% NaCl, respectively. The slight difference in the zeta potential values between the different Cu2O samples may be caused by the introduction of chloride to Cu2O.
Based on the above results and our previous reports, a plausible mechanism for uniform Cu2O synthesis was proposed and is shown in Scheme 1. First, Cu2+ is rapidly reduced by ascorbic acid to give Cu+. Then, Cu+ reacts with H2O to produce Cu(OH), followed by the conversion to Cu2O via the condensation process due to its thermodynamic instability.27 Subsequently, the primary Cu2O NPs aggregated into Cu2O NPAs with the synergism of the secondary surfactant (e.g., PAM or PVP). However, the resulting Cu2O NPAs showed a broad size distribution, probably due to the lack of control in the nucleation stage. Upon the addition of NaCl, the generated Cu+, prior to the formation of Cu2O NPs, reacted with Cl− to form CuCl, which can act as nuclei due to the limited solubility of CuCl in water (Ksp = 1.72 × 10−7, solubility: 47 mg L−1 at 20 °C). The appearance of the milk-like color after adding ascorbic acid at the initial stage of the reaction further demonstrated that the reaction of Cu+ and Cl− resulting in CuCl occurred. It is therefore reasonable to deduce that the formed CuCl serves as the nucleation agent in the initial stage of the reaction.
It has been previously reported that the separation of the nucleation and growth stages is critical for controlling the size distribution of colloidal particles. Generally, a rapid nucleation stage or the intentional addition of seeds and a relatively slow growth stage facilitate the monodispersity of the product, as demonstrated in the syntheses of polymeric beads and NPs and their assemblies.2,3,11,28,29 Notably, Wu et al. recently demonstrated that the seed formation plays the key role in the synthesis of monodisperse Cu2O NPAs.11 Therefore, in our case, the production of Cu2O NPAs with a narrow size distribution can be mainly ascribed to the generated CuCl in situ, which serves as aggregation centers (seeds) to control the growth of a secondary structure. However, when excess NaCl was used (e.g., 10%), many Cu+ ions react with Cl− to form excess CuCl, resulting in the failure in controlling the formation of NPAs with a narrow size distribution.
This method performs well with a 10-fold increase in reactant concentrations at a stirring rate of 300 rpm due to the in situ generated seed-mediated aggregation of the primary NPs. From the SEM images, spherical particles with a size and size distribution of 383 nm and 7.6% were revealed, respectively (Fig. S15†). We believe that this strategy, which is based on the in situ seed generation, can be applied to other systems for the synthesis of monodisperse particles.
To evaluate the bandgap of the prepared Cu2O, we measured the UV-vis DRS curves of the powder samples. Fig. 3a shows the DRS curves of the pure Cu2O (no NaCl) and Cu2O obtained with 4% NaCl. For pure Cu2O, the spectrum shows strong absorption at approximately 600 nm due to a fast decrease in the reflectance for wavelengths shorter than 600 nm. Compared with the pure product, Cu2O obtained with 4% NaCl shows lower light absorption at approximately 600 nm. The bandgaps of the samples were evaluated using Tauc plots according to the relation [F(R) × hν]2 = A(hν − Eg), where F(R) was calculated by the Kubelka–Munk function 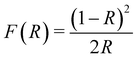 (R is the measured reflectance), and h, ν, A, and Eg are Planck's constant, the light frequency, a constant, and the band gap, respectively.30–32 The bandgaps of the samples were obtained from the curve of [F(R) × hν]2versus hν (Fig. 3b). For the pure Cu2O, the bandgap was found to be 2.35 eV, slightly larger than the typical value (2.17 eV) of Cu2O, due to the smaller size of the primary Cu2O NPs (∼5 nm) and the quantum confinement effect.33,34 Cu2O with 4% NaCl exhibits a larger bandgap (2.60 eV), most likely due to the Cl doping effect.
(R is the measured reflectance), and h, ν, A, and Eg are Planck's constant, the light frequency, a constant, and the band gap, respectively.30–32 The bandgaps of the samples were obtained from the curve of [F(R) × hν]2versus hν (Fig. 3b). For the pure Cu2O, the bandgap was found to be 2.35 eV, slightly larger than the typical value (2.17 eV) of Cu2O, due to the smaller size of the primary Cu2O NPs (∼5 nm) and the quantum confinement effect.33,34 Cu2O with 4% NaCl exhibits a larger bandgap (2.60 eV), most likely due to the Cl doping effect.
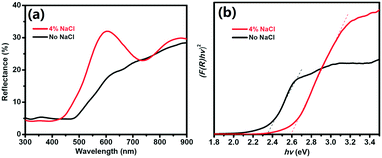 | ||
| Fig. 3 UV-vis DRS (a) and [F(R) × hν]2versus hν curves (b) for Cu2O obtained without and with 4% NaCl. | ||
Conclusions
We have developed a facile method for the preparation of highly uniform Cu2O NPAs. The size distribution width of the Cu2O NPAs was as low as δ = 5.6% and 8.1%, corresponding to the NPA size of 443 nm and 294 nm, respectively. The key point of the method is the introduction of NaCl for in situ seed generation; subsequently, the seeds serve as aggregation centers to control the primary NP aggregation process. Compared with previously reported methods for the preparation of uniform Cu2O NPAs, our method has the following merits: (1) the use of an aqueous solvent and environmentally benign reducing agent; (2) a reaction that was carried out at room temperature in air; (3) a simple one-pot reaction; (4) high yields and scalable production in a short reaction time. The availability of this facile method for synthesis of uniform Cu2O NPAs may broaden their potential applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D1A3B03035300). We acknowledged the Energy Technology Development Program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) financially supported by the Ministry of Trade, Industry & Energy, Republic of Korea (No. 20163010012200) and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D1A3B03033045).References
- J. K. Stolarczyk, A. Deak and D. F. Brougham, Adv. Mater., 2016, 28, 5400–5424 CrossRef CAS PubMed.
- Y. Xia and Z. Tang, Chem. Commun., 2012, 48, 6320–6336 RSC.
- Z. Lu and Y. Yin, Chem. Soc. Rev., 2012, 41, 6874–6887 RSC.
- S. Wintzheimer, T. Granath, M. Oppmann, T. Kister, T. Thai, T. Kraus, N. Vogel and K. Mandel, ACS Nano, 2018, 12, 5093–5120 CrossRef CAS PubMed.
- V. N. Manoharan, Science, 2015, 349, 1253751 CrossRef.
- C. Chen, C. Nan, D. Wang, Q. Su, H. Duan, X. Liu, L. Zhang, D. Chu, W. Song, Q. Peng and Y. Li, Angew. Chem., Int. Ed., 2011, 50, 3725–3729 CrossRef CAS.
- R. N. Briskman, Sol. Energy Mater. Sol. Cells, 1992, 27, 361–368 CrossRef CAS.
- A. Paracchino, V. Laporte, K. Sivula, M. Grätzel and E. Thimsen, Nat. Mater., 2011, 10, 456–461 CrossRef CAS.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 2000, 407, 496–499 CrossRef CAS.
- B. White, M. Yin, A. Hall, D. Le, S. Stolbov, T. Rahman, N. Turro and S. O'Brien, Nano Lett., 2006, 6, 2095–2098 CrossRef CAS.
- X. Su, J. Chang, S. Wu, B. Tang and S. Zhang, Nanoscale, 2016, 8, 6155–6161 RSC.
- J. Zhang, J. Liu, Q. Peng, X. Wang and Y. Li, Chem. Mater., 2006, 18, 867–871 CrossRef CAS.
- M. Pang and H. C. Zeng, Langmuir, 2010, 26, 5963–5970 CrossRef CAS PubMed.
- W.-r. Lee, L. Piao, C.-H. Park, Y. S. Lim, Y. R. Do, S. Yoon and S.-H. Kim, J. Colloid Interface Sci., 2010, 342, 198–201 CrossRef CAS PubMed.
- W.-r. Lee, Y. S. Lim, S. Kim, J. Jung, Y.-K. Han, S. Yoon, L. Piao and S.-H. Kim, J. Mater. Chem., 2011, 21, 6928–6933 RSC.
- J. Jiang, S.-H. Kim and L. Piao, Nanoscale, 2015, 7, 8299–8303 RSC.
- J. Jiang, Y. Soo Lim, S. Park, S.-H. Kim, S. Yoon and L. Piao, Nanoscale, 2017, 9, 3873–3880 RSC.
- J. Jiang, G. H. Gunasekar, S. Park, S.-H. Kim, S. Yoon and L. Piao, Mater. Res. Bull., 2018, 100, 184–190 CrossRef CAS.
- Z. Wang, D. Luan, F. Y. C. Boey and X. W. Lou, J. Am. Chem. Soc., 2011, 133, 4738–4741 CrossRef CAS.
- M. H. Kim, B. Lim, E. P. Lee and Y. Xia, J. Mater. Chem., 2008, 18, 4069–4073 RSC.
- H. Zhao, J. Jiang, Y. S. Lim, S.-H. Kim and L. Piao, RSC Adv., 2014, 4, 41927–41933 RSC.
- M. V. Kovalenko, M. I. Bodnarchuk, R. T. Lechner, G. Hesser, F. Schäffler and W. Heiss, J. Am. Chem. Soc., 2007, 129, 6352–6353 CrossRef CAS PubMed.
- Y. Xia, T. D. Nguyen, M. Yang, B. Lee, A. Santos, P. Podsiadlo, Z. Tang, S. C. Glotzer and N. A. Kotov, Nat. Nanotechnol., 2011, 6, 580–587 CrossRef CAS PubMed.
- N. R. Jana, L. Gearheart and C. J. Murphy, Langmuir, 2001, 17, 6782–6786 CrossRef CAS.
- E. V. Shevchenko, D. V. Talapin, H. Schnablegger, A. Kornowski, Ö. Festin, P. Svedlindh, M. Haase and H. Weller, J. Am. Chem. Soc., 2003, 125, 9090–9101 CrossRef CAS.
- Q. Fu, H. Zhu and J. Ge, Adv. Funct. Mater., 2018, 28, 1804628 CrossRef.
- Y. Shang, Y.-M. Shao, D.-F. Zhang and L. Guo, Angew. Chem., Int. Ed., 2014, 53, 11514–11518 CrossRef CAS.
- J.-S. Song, F. Tronc and M. A. Winnik, J. Am. Chem. Soc., 2004, 126, 6562–6563 CrossRef CAS.
- Y. Xia, Y. Xiong, B. Lim and S. E. Skrabalak, Angew. Chem., Int. Ed., 2009, 48, 60–103 CrossRef CAS PubMed.
- Y. Yang, D. Xu, Q. Wu and P. Diao, Sci. Rep., 2016, 6, 35158 CrossRef CAS.
- D. Gupta, S. R. Meher, N. Illyaskutty and Z. C. Alex, J. Alloys Compd., 2018, 743, 737–745 CrossRef CAS.
- X. Bai, J. Wei, B. Tian, Y. Liu, T. Reiss, N. Guiblin, P. Gemeiner, B. Dkhil and I. C. Infante, J. Phys. Chem. C, 2016, 120, 3595–3601 CrossRef CAS.
- N. A. M. Shanid and M. A. Khadar, Thin Solid Films, 2008, 516, 6245–6252 CrossRef CAS.
- X. Meng, G. Tian, Y. Chen, Y. Qu, J. Zhou, K. Pan, W. Zhou, G. Zhang and H. Fu, RSC Adv., 2012, 2, 2875–2881 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ce01279f |
| This journal is © The Royal Society of Chemistry 2020 |