Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Can aromaticity be a kinetic trap? Example of mechanically interlocked aromatic [2-5]catenanes built from cyclo[18]carbon
Nikita
Fedik
a,
Maksim
Kulichenko
a,
Dmitriy
Steglenko
b and
Alexander I.
Boldyrev
*ab
aDepartment of Chemistry and Biochemistry, Utah State University, 0300 Old Main Hill, Logan, UT 84322-0300, USA. E-mail: a.i.boldyrev@usu.edu
bInstitute of Physical and Organic Chemistry, Southern Federal University, Rostov-on-Don 344090, Russia
First published on 27th March 2020
Abstract
Correction for ‘Can aromaticity be a kinetic trap? Example of mechanically interlocked aromatic [2-5]catenanes built from cyclo[18]carbon’ by Nikita Fedik et al., Chem. Commun., 2020, 56, 2711–2714.
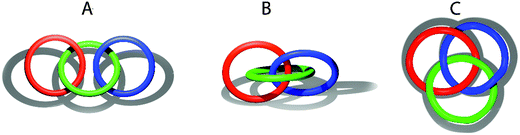
The authors are grateful to the attentive reader who found that the structure built of three C18 rings in Fig. 4D in the original article was improperly referred to as Borromean rings. To correct the terminology, we suggest the following examples, depicted in Fig. 1 below.
Example A in Fig. 1 topologically corresponds to a linear [3]catenane (Fig. 4A in the original article). The red and blue rings are linked through the intermediate green ring. In example B, all three rings are interlocked with each other. If we remove one of the rings, the two other rings will be still linked. Thus, such an arrangement would be correctly described as a cyclic [3]catenane (Fig. 4D in the original article). Apparently, topology B does not correspond to molecular Borromean rings. Borromean rings are constructed in such a way that the removal of any ring would break the whole arrangement because none of the rings are connected directly. Example C clearly illustrates this principle.
We would like to apologize for this terminology error and suggest considering the structure in Fig. 4D in the original article as a cyclic [3]catenane. Therefore, any mention of Borromean rings in both the main text and the ESI should be replaced by cyclic [3]catenene. All qualitative and quantitative results reported in the work are unaffected.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2020 |