Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
DOI: 10.1039/D0CC90020F
(Correction)
Chem. Commun., 2020, 56, 2211-2213
Correction: Diastereoselective synthesis of 1,3-disubstituted isoindolines and sultams via bronsted acid catalysis
Ye
Tao
and
Scott R.
Gilbertson
*
Department of Chemistry, University of Houston, Houston, TX 77204, USA. E-mail: srgilbertson@uh.edu
Received
7th January 2020
, Accepted 7th January 2020
First published on 10th February 2020
Abstract
Correction for ‘Diastereoselective synthesis of 1,3-disubstituted isoindolines and sultams via bronsted acid catalysis’ by Ye Tao et al., Chem. Commun., 2018, 54, 11292–11295.
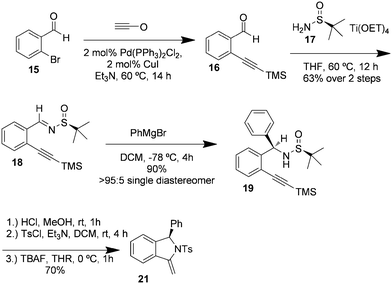
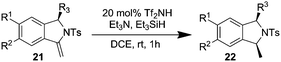
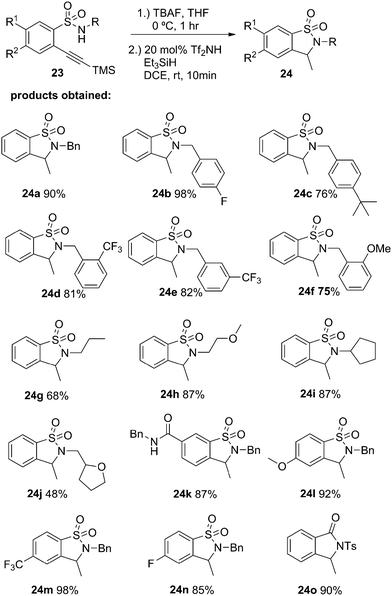
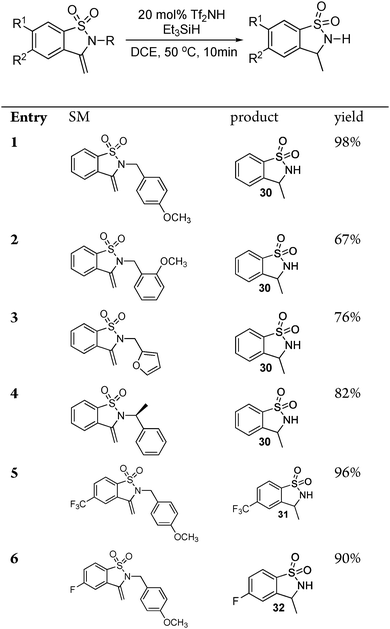
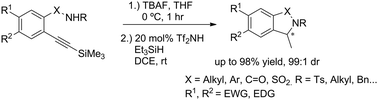
The authors regret that the intermediates identified as 21 and 23, 25, 26, 27, 28 and 29 in Schemes 3–5 and Table 2 are not alkynes as indicated in the original article, but rather cyclized 5-member rings with an exocyclic double bond. The subsequent reaction with triethylsilane then provides the title compounds by diastereoselective reduction. Corrected versions of Table 2 and Schemes 3–5 are shown below. The structures have also been corrected in the ESI of the original article, which is available online.
Table 2 Cyclization with loss of nitrogen group
In addition, a corrected version of the graphical abstract is shown below.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2020 |